Abstract
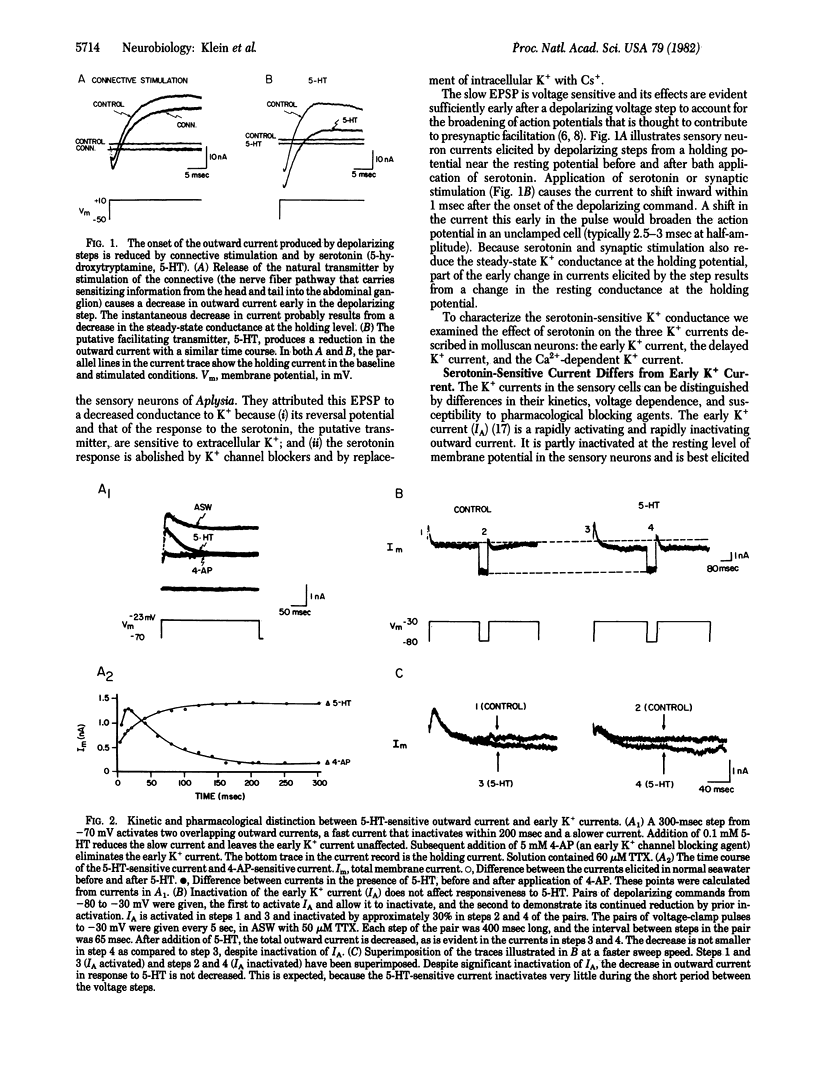
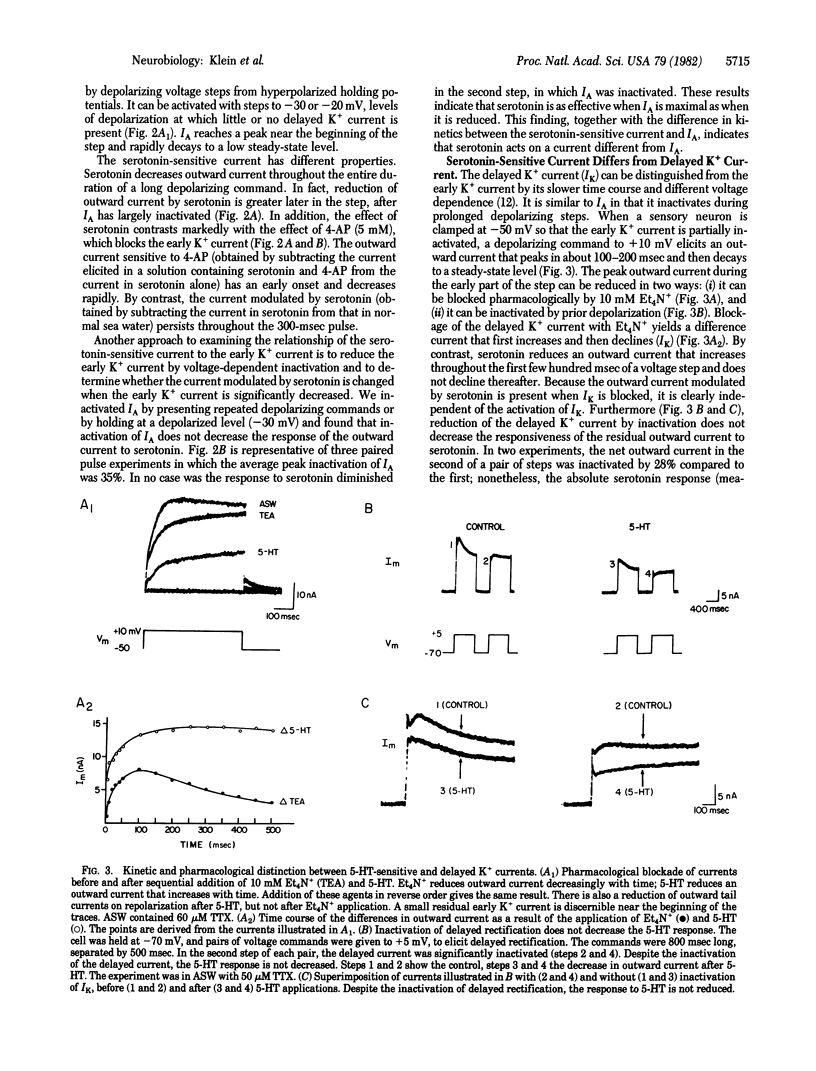
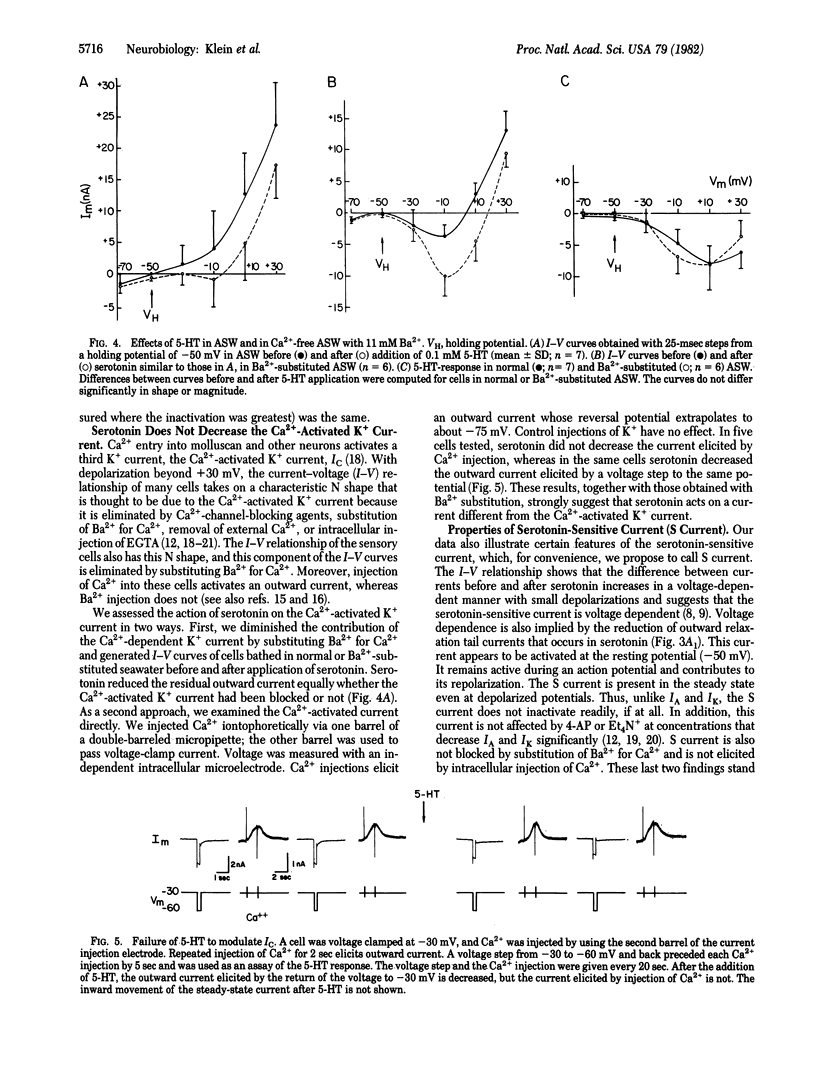
Serotonin exerts a long-lasting excitatory action on sensory neurons of Aplysia californica by decreasing outward K+ current. The depression of outward current delays repolarization of the action potential and extends the duration of Ca2+ influx into the presynaptic terminals, thereby contributing to the facilitation of transmitter release that underlies behavioral sensitization. We have extended the analysis of serotonin's action and find that it acts on a specific serotonin-sensitive K+ current (S current), which is different from the early K+ current (IA), the delayed K+ current (IK), the Ca2+-dependent K+ current (IC), and the muscarine-sensitive M current. The serotonin-sensitive current in these cells persists when IA and IK are reduced by conditioning depolarization or channel-blocking agents. The S current is not activated by intracellular injection of Ca2+, nor is it affected by substitution of Ba2+ for Ca2+, a treatment that reduces IC. Moreover, intracellular injection of cyclic AMP exerts an effect indistinguishable from that of serotonin. This observation and the insensitivity of the current to Ba2+ distinguishes the S current from M current. S current is activated at the resting potential and does not inactivate with steady-state depolarization. It is active sufficiently early during an action potential to contribute to the repolarization of the action potential and therefore accounts for the physiological effects of serotonin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Byrne J., Castellucci V., Kandel E. R. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974 Sep;37(5):1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. Role of cyclic AMP in a serotonin-evoked slow inward current in snail neurones. Nature. 1981 Apr 30;290(5809):783–785. doi: 10.1038/290783a0. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Potassium conductance and internal calcium accumulation in a molluscan neurone. J Physiol. 1980 Nov;308:287–313. doi: 10.1113/jphysiol.1980.sp013472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Mechanisms of slow postsynaptic potentials. Nature. 1981 Jun 18;291(5816):539–544. doi: 10.1038/291539a0. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Marty A. Certain slow synaptic responses: their properties and possible underlying mechanisms. Annu Rev Biophys Bioeng. 1980;9:437–465. doi: 10.1146/annurev.bb.09.060180.002253. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Shapiro E., Kandel E. R. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980 Dec;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Deterre P., Gerschenfeld H. M. Relationship between two voltage-dependent serotonin responses of molluscan neurones. Brain Res. 1981 Jul 27;217(1):201–206. doi: 10.1016/0006-8993(81)90201-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderMaelen C. P., Aghajanian G. K. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980 Sep 25;287(5780):346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]



