Abstract
Articular cartilage experiences significant mechanical loads during daily activities. Healthy cartilage provides the capacity for load bearing and regulates the mechanobiological processes for tissue development, maintenance, and repair. Experimental studies at multiple scales have provided a fundamental understanding of macroscopic mechanical function, evaluation of the micromechanical environment of chondrocytes, and the foundations for mechanobiological response. In addition, computational models of cartilage have offered a concise description of experimental data at many spatial levels under healthy and diseased conditions, and have served to generate hypotheses for the mechanical and biological function. Further, modeling and simulation provides a platform for predictive risk assessment, management of dysfunction, as well as a means to relate multiple spatial scales. Simulation-based investigation of cartilage comes with many challenges including both the computational burden and often insufficient availability of data for model development and validation. This review outlines recent modeling and simulation approaches to understand cartilage function from a mechanical systems perspective, and illustrates pathways to associate mechanics with biological function. Computational representations at single scales are provided from the body down to the microstructure, along with attempts to explore multiscale mechanisms of load sharing that dictate the mechanical environment of the cartilage and chondrocytes.
Keywords: mechanical system, musculoskeletal, joint loading, chondrocyte, chondron, extracellular matrix, pericellular matrix, collagen, proteoglycan, continuum mechanics, microstructure, mechanobiology, locomotion, finite element analysis
Introduction
Overview of Cartilage Function and Composition
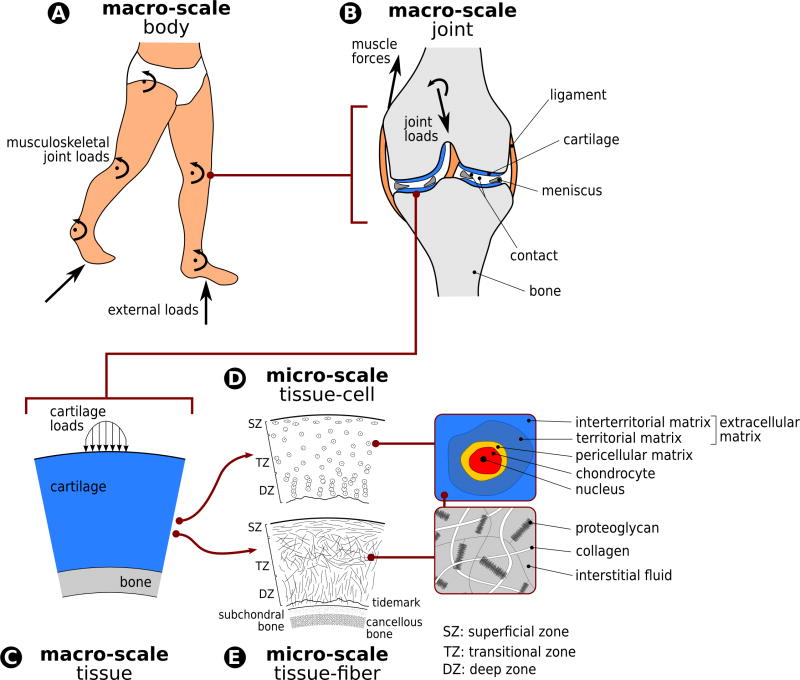
The diarthrodial joint is one of nature’s marvels, providing a low-friction bearing surface that, under normal conditions, can withstand millions of cycles of extreme loading, which can exceed multiples of body weight (Figure 1-A). The mechanisms by which mammalian joints perform these functions involve an intricate combination of biomechanical and biological factors, acting at a variety of spatial and temporal scales. In particular, the load-bearing function of the joint is predominantly due to the properties of the articular cartilage, the thin layer of soft-tissue lining the ends of long bones within the joint (Figure 1-B & 1-C).
Figure 1.
Anatomical architecture of musculoskeletal joints dictates the distribution of muscle forces and external loading on the body (A) to individual passive structures of the joint, e.g. ligaments, menisci, and cartilage (B). Cartilage’s regional anatomy, e.g. thickness and curvature, and its interaction with opposing tissues, e.g. cartilage or meniscus, has mechanical implications (C). The tissue also exhibits a structural composition, incorporating zone-dependent chondrocyte distributions and shapes (D) and orientation of collagen fibers (E). This zonal and cellular architecture is also known to be dependent on the region of the cartilage within the tissue. For a given gross cartilage deformation, this architecture dictates deformation of individual cellular, pericellular, and extracellular regions within the zones. The composition of the extracellular matrix and its multiphysics mechanics dictate the loads in individual fiber architecture that can have implications for fiber level damage.
The unique mechanical properties of cartilage are attributed to the composition and organization of its extracellular matrix, which by weight consists primarily of water with dissolved ions (~75–80% by wet weight). The solid matrix of articular cartilage consists of a dense fibrous network of collagen (primarily type II) and a concentrated solution of aggregating proteoglycans, as well as other proteins, lipids, and cells 72 (Figure 1-E). The tissue, as a bulk material, exhibits viscoelastic behavior, which arises predominantly from frictional drag due to relative motion between the solid and fluid phases 111,112.
The cartilage extracellular matrix is maintained in a slow state of turnover by a balance of anabolic and catabolic activities of the chondrocytes, the sole cell type found in cartilage (Figure 1-D). Chondrocyte activity is regulated by a complex interplay between genetic, environmental, and biomechanical factors 54. Soluble mediators (e.g., growth factors, cytokines) and extracellular matrix composition dictate the environment of these cells. Biomechanical factors are a result of mechanical loads distributed on chondrocytes and the extracellular matrix. These include but are not limited to tension, compression, shear, osmolarity, fluid pressure, and associated electrokinetic effects 59,138,145.
Mechanical Load Sharing at Multiple Scales
The local loads on cartilage and its substructures are dictated by the transmission and sharing of body level mechanical loads (Figure 1). External and inertial loading on the extremities are accommodated by joint forces and torques to achieve stability and to meet the constraints of locomotion (Figure 1-A). This dynamic equilibrium is accomplished through a combination of active force generation such as muscle contraction, and reaction forces carried by passive joint structures. Within the passive structures, cartilage contributes to the load sharing among other tissues such as the ligaments and menisci of the knee (Figure 1-B). Within a local region of the cartilage, macroscopic tissue level loads are distributed among different superficial, transitional, and deep zones dictated by the differences in microstructural properties (Figure 1-D & 1-E). Within these zones, collagen fiber architecture in the extracellular matrix and the pericellular matrix, and chondrocyte shape and distribution provide the basis for cellular deformations and fiber loading.
Under physiologic conditions, mechanical loading is necessary for joint health 54,73. Furthermore, clinical data indicate that a variety of exercises are safe and effective for individuals with osteoarthritis 163 yet the efficacy of exercise can be dependent on disease progression 115. Clinical and animal studies have also provided strong evidence that altered joint loads can lead to potentially undesirable alterations in the composition, structure, metabolism, and mechanical properties of articular cartilage and other joint tissues 9,80. Abnormal loading may be caused by a variety of factors such as congenital or developmental deformity, obesity, immobilization, joint instability, overuse, disease or trauma 10,22. However, the specific sequence of biomechanical and biochemical events through which mechanical loads at the whole body and joint level are converted to mechanical, chemical, and electrical “signals” at the cellular and intracellular level are not fully understood. This information has utmost importance to understand the mechanical homeostasis of cartilage and the regulation of the aforementioned changes in its properties.
Experimentation of Cartilage Mechanics
A number of experimental studies have been performed to determine the relationship between body/joint/tissue (Figure 1) level loading and the mechanics of cartilage, as well as chondrocyte mechanical and biological response. For example, joint loads, presumably an indicator of cartilage loading, have been quantified in vivo for healthy subjects and patient populations using instrumented implants 50,76 and/or gait analysis 17,88. Indicators of cartilage contact have also been quantified for live subjects using several different imaging modalities. For example, magnetic resonance imaging was utilized to establish cartilage deformations and contact under simplified load bearing scenarios 32. Dual x-ray planar fluoroscopy has been used to characterize cartilage-to-cartilage contact mechanics during locomotion 20.
A more detailed understanding of the in situ role of cartilage in joint mechanics has been possible through experimentation. In this respect, basic relationships between joint loading (and sometimes muscular loading) and cartilage contact pressures have been established 19,39, albeit somewhat removed from in vivo loading scenarios. At the spatial scale of tissue and cells, and from the perspective of load transfer from higher scales, contemporary work has pointed out the significance of joint shape, combined loading and motion, and interfaces for tissue engineering of cartilage 148. More recently, in situ tibiofemoral experimentation has quantified zonal strains as a function of physiological boundary conditions, which indicated different deformation modes for the opposing cartilage surfaces 156. In animal studies, the deformation of chondrocytes in the tibiofemoral joint was documented as a function of quadriceps force for the first time 1.
While experimental studies at the body and joint level have explored functional mechanics of the cartilage from a load sharing perspective, tissue and cell level studies commonly targeted the basic mechanical properties of chondrocytes and chondrons 120 and the biomechanics of the cartilage in relation to its micro-mechanical and chemical environment 140. Much of the information currently available on chondrocyte response to mechanical stress has been determined using tissue explant culture experiments, which provide a more controlled mechanical and biochemical environment than the in vivo condition (e.g.84,127 and reviewed in 59). The general consensus is that static compression at the joint or tissue level suppresses chondrocyte activity, while cyclic or intermittent compression at certain frequencies and magnitudes generally increases biosynthetic activity 54. High stresses or strain rates have been shown to be injurious to chondrocytes, resulting in cell death and increased pro-inflammatory and catabolic responses 142,143. To examine the biomechanical relationships between compression at the tissue and cellular/subcellular level, a number of studies have examined cellular and intracellular deformation under controlled loading conditions 23,31,56,81. These studies have shown that chondrocyte and nuclear deformation are integrally linked to the deformation of the extracellular matrix.
Role of Computational Modeling and Simulation in Cartilage Mechanics
Experimental studies, while providing fundamental information on cartilage biomechanics, are unable to measure a number of biomechanical parameters. At the higher spatial levels of the joint, cartilage contact forces and pressures have been difficult to quantify. Likewise, at the lower spatial scales, variables such as stress, fluid flow, and pressure within the cartilage and among chondrocytes and the extracellular matrix fiber architecture cannot be measured easily, if at all. In this regard, simulation-based investigations of cartilage and its underlying structures have become the tool of choice for interpreting the biomechanical and biophysical basis of experimental results and as an independent investigative approach when experimental investigation is difficult or impossible 53,60,109,139.
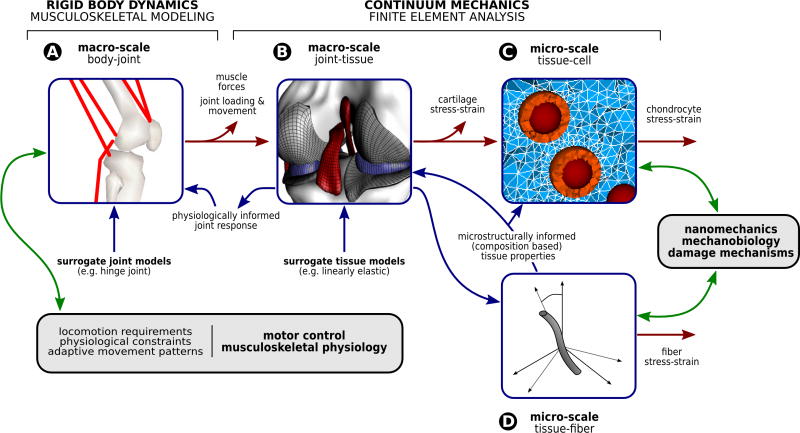
At the higher spatial scales, body-level modeling approaches seek to apply the principles of rigid body statics and dynamics to predict motion and/or joint reaction forces and torques 41 (Figure 2-A). At the lower spatial scales of tissues and cells, past and contemporary studies relied predominantly on continuum mechanics to describe and predict the mechanical function of articular cartilage in the context of intact joints 107,108 and of chondrocytes in the context of tissue structure 60 (Figure 2-B & 2-C). Through decades, modeling and simulation studies focused on understanding the structure and function of the normal tissue mechanics, and attempted to interpret the alterations that occur to the biomechanics and biophysics of the tissue due to disease and injury. Regardless, a clear understanding of the multiscale relationships between the ultrastructural organization of the extracellular matrix, chondrocyte mechanobiology, the macroscopic material behavior, and the joint mechanics is severely lacking. Such knowledge could elucidate fundamental structure-function relationships for the tissue, the mechanical effects of injury at macroscopic and cellular levels, and the phenotype of both acquired and inherited disease states. When conducted in a multiscale fashion, modeling and simulation may resolve the challenging task to relate joint level descriptors of cartilage mechanics (potentially accessible in a clinical setting) to tissue-cell level indicators of cartilage mechanobiological function. The lack of progress in this area can be attributed to the difficulty and lack of strategies for coupling the physics between different structural and physical levels.
Figure 2.
The load sharing pathway, to identify the mechanical environment of the cartilage, can be decomposed by utilizing computational models at different spatial scales. Simulations of the musculoskeletal system (based on rigid body dynamics) can provide net loads acting on the passive structures of the joint during locomotion (A). Musculoskeletal models commonly utilize surrogate joint models to manage computational cost. Finite element analysis of the whole joint can provide net forces acting on the cartilage, contact pressures, and the macroscopic stress-strain state within the tissue (B). Representations at the interface of joint-tissue commonly rely on the simplification of tissue mechanics, i.e. utilize surrogate tissue models. Microstructural finite element analysis of chondrocytes within their in situ environment help establish their mechanical environment (C). Supported by models representative of the fiber architecture and multiphase physics (D), a detailed quantification of cartilage mechanics from macro- to micro-scales is a possibility, simply through a post-processing based feedforward coupling. This load sharing pathway can provide multiscale indicators of cartilage damage and mechanical stimuli on chondrocytes. Nonetheless, if feedback coupling is established, surrogate models can be replaced by physiologically informed models. While such an approach establishes the potential for investigating the influence of lower scale response on the higher scale function, it comes with a dramatically increased cost when iterative solutions at multiple scales cannot be avoided. This mechanical modeling framework can further be coupled to models of relevant biological systems that are driven (or at least influenced) by cartilage mechanics. Explorations of motor control and musculoskeletal physiology in one end, and nanomechanics, cell mechanobiology on the other end, all coupled to cartilage mechanics, may be a possibility.
Review Focus
This review aims to summarize simulation-based explorations of cartilage from the perspective of different spatial scales, stand-alone or as they are coupled to each other. The intent is not to provide a complete literature review on cartilage mechanics. Rather, the goal of this paper is to emphasize state-of-the-art modeling approaches for quantification of the mechanical environment of cartilage at a multitude of scales, ranging from the whole body to that at the cellular and collagen fiber level. Within that context, specific focus is given to identification of joint loads and gross cartilage contact mechanics (body and joint level), cartilage stresses and strains (tissue level), chondrocyte and extracellular matrix mechanics (cell level), and fiber stresses (fiber level) (Figure 2). The emerging area of molecular modeling of extracellular mechanics is also briefly discussed. Several cases that need to employ coupled simulation strategies are outlined and a brief discussion on the feasibility of such investigations within the context of current technological capacity is provided. We also outline pathways to establish confidence in simulation results, as well as the potential to couple representations of cartilage in musculoskeletal, joint, and microstructural models to other spatial scales and models of biological response.
Single Scale Approaches
Cartilage Mechanics from Musculoskeletal Modeling Perspective
Synopsis
At the interface of body, organ, and joint mechanics, joint kinematics and kinetics (Figure 2-A, joint loading) as well as variables to identify overall tissue loading (Figure 2-A, joint response, e.g. contact forces), have been used to infer the underlying mechanics of articular cartilage (Figures 1v and 2-A, macro-scale body). An understanding of joint mechanics, as it functions as part of the musculoskeletal system, has significant value as the system is a common target for mechanical intervention, e.g., knee bracing, and its descriptors are easy to relate to the mechanical requirements of daily activities. As many joint level mechanical variables can be acquired in the field, they possess diagnostic value for assessment of cartilage’s healthy function.
Current Approaches
Body level modeling approaches based on rigid body dynamics have been used for prediction of joint loading (Figure 2-A). In their simplest form, simulations incorporate inverse dynamic analysis to predict joint torques 41. At more advanced levels, optimization approaches coupled with inverse and forward dynamics are utilized 41. Musculoskeletal modeling and movement simulations relying on such methods have been employed to predict neuromuscular control, and in turn muscular load sharing and net joint loading due to passive structures 41. All of these variables have implications for the underlying state of cartilage mechanics. In clinical movement analysis for example, studies relying on motion data and external force measurements utilized generic or specimen-specific models to predict joint torques. In cases where muscle force prediction was also performed, the load sharing between active and passive components of the joint was also included 8,33,104,134.
Due to complexity and computational expense, musculoskeletal simulations often utilize simplified mechanical representation of joints (Figure 2-A, surrogate joint models), such as a hinge for the knee and a ball and socket for the hip. Along with these assumptions, segment moments and/or joint reactions, as predicted by the musculoskeletal simulation, are relied on to infer the state of cartilage loading. In spite of these simplifications, musculoskeletal modeling has been critical in establishing a correlation between ambulatory knee loading and the incidence of osteoarthritis 17,113,126,133,155. More specifically, results of computational studies have led to interventions aimed at reducing the peak knee adduction moment in patients with early onset and/or advanced osteoarthritis. The correlation of post-intervention measures of clinical outcome with model predictions has proven to be a promising indicator of model efficacy, realizing an apparent reduction in the affected medial compartment loads and potentially in the disease progression 42.
Multiscale Context
Musculoskeletal modeling also has the potential to provide insight into neuromuscular control strategies along with tissue loading, and to help improve the diagnosis and management of neurologic and orthopedic conditions 41. This body level approach is valuable but, as mentioned earlier, it generally lacks the fidelity to explore mechanisms of disease onset and progression at lower spatial scales. For example, the mechanical environment of the cartilage at the tissue level is implied, not explicitly quantified. Nonetheless, the joint variables are readily related to the requirements of locomotion and provide a conceptualization of body mechanics in the clinical field. Computational cost is tractable, particularly when an inverse dynamics approach or simplified muscle load sharing algorithms are selected 41. Yet, the reliability of predicted muscle and passive joint loading may need to be established on a case-specific basis. Common validation procedures simply have relied on comparison of measured muscle activity to time history of muscle forces 41.
If musculoskeletal models are intended to be used for cartilage mechanics in a multiscale context, the requirements for their validity may change. Lower scale joint level models, i.e. macroscale continuum representations (Figure 2-B) will likely be driven by muscle forces or joint loading, which should be predicted by a musculoskeletal model reliably. Simplified joint representations may not be adequate for this purpose. Joint contact forces can be obtained directly from musculoskeletal movement simulations to infer cartilage mechanics. Nonetheless, the appropriateness of these methods to accurately quantify variables of interest is still questionable 50. Ongoing research aimed at establishing validity will likely promote greater confidence in such predictions 50. Advances in scientifically adequate and computationally cost-effective modeling of neuromuscular and musculoskeletal interactions would definitely benefit multiscale simulations to evaluate cartilage mechanics in relation to body level loading. Not only could pervading simplifications be addressed (e.g., the knee as a hinge) but neuromuscular control would be informed by realistic joint and underlying tissue representation.
Cartilage Mechanics from Joint-Tissue Modeling Perspective
Synopsis
At the tissue level, predictions of accurate cartilage mechanics may offer insight not afforded by a higher level approach. To understand the effect of joint loading on the cartilage deformation, a model incorporating joint and tissue level representations is necessary (Figure 2-B). Simulations spanning the scale of the joint-tissue interface can provide localized tissue deformations and contact stress distributions, establishing a more detailed understanding of the regional cartilage stress-strain and fluid pressure state for a set of specific joint loads. Such analyses have translational value as they can provide an evaluation of mechanical interventions targeted at the cartilage itself, e.g. osteochondral grafting 35,69. The local stress concentrations in a healthy or diseased state can also indicate potential damage risk 122,136.
Current Approaches
Finite element analysis has become an accepted and widely applied tool to investigate cartilage stresses, strains, and fluid pressures (Figure 2-B), subject to simplified 62 or elaborate 160 joint loading. In these macro-scale models of the joint and tissue, the macroscopic anatomy is represented, providing the capacity for a regional analysis. Specifically, one might predict the load sharing between medial and lateral compartments of the knee, and other regions within the joint 2,135,162. This computational framework has aided investigations of the specific effects of joint mechanics on cartilage function. For example, relative tibiofemoral alignment has been found to significantly influence cartilage stress distribution and magnitude 159–161. Supporting the results of investigations conducted at a higher scale, e.g. musculoskeletal simulation (refer to previous section), these studies found that joint malalignment results in high adduction moments and may lead to a potentially adverse cartilage stress state.
Driven by clinical findings of the relatively high incidence of osteoarthritis in patients with full or partial meniscectomy 114, finite element studies have also highlighted the role the meniscus plays in distribution of joint contact forces 123,124,146,164. Quantifying the effects of modeling assumptions has been one particular challenge in this area but the adoption of probabilistic techniques has shown promise. For instance, a recent study demonstrated bounded predictions of cartilage contact mechanics based on soft tissue material uncertainties 34. Analyzing the tissue-level mechanical environment has not been limited to the knee joint, and the hip has also garnered attention as it is a common site for osteoarthritis. Specimen-specific and simplified representations of human hips have been analyzed to establish discrepancies in predicting cartilage contact stress, with geometry demonstrating a dramatic effect on simulation results 5,6,28,70.
When the computational expense of finite element analysis is prohibitive, internal tissue mechanics can be neglected in favor of a surface based technique such as discrete element analysis 27,38,106. This approach simplifies the surrogate tissue models used in macro-scale simulations through replacement of the continuum representation (Figure 2-A & 2-B). The realized computational gains have afforded population based studies 7 and an in-depth assessment of uncertainty 48, while still retaining three-dimensional generic or specimen-specific representation of cartilage topology.
Of particular importance in joint level modeling, simulation of the joint contact interface(s) can be a challenging issue. Related to computational cost and robustness, most studies assume frictionless or near frictionless contact 71,122. In reality, the coefficient of friction of cartilage is dependent on the pressurization of the cartilage interstitial fluid, and thus a product of loading rate, magnitude, and duration 11. For example, an experiment measuring the coefficient of friction between a cartilage disk and glass under a static load subjected to reciprocal sliding reported coefficient of friction values ranging from nearly frictionless at initial loading to 0.15 at steady state 99. While the frictionless assumption may be valid for studies involving fast loading of cartilage, modeling mechanical behavior over longer time intervals may require more advanced friction contact implementations which can model these documented time and load dependent values. Accounting for fluid exchange across contact interfaces, as has already been performed in a frictionless model 15, will ultimately result in more accurate time and load dependent contact modeling in joints.
Multiscale Context
Continuum mechanics based models of the macroscale, simulating response at the joint level, have generally focused on the macroscopic tissue level mechanical response of the cartilage; the underlying physics of the tissue was largely simplified (Figure 2-B, surrogate tissue models). Cartilage was often modeled as an elastic material, although this assumption is only valid at certain loading and boundary conditions 14. While these boundary conditions may encompass common many common activities (e.g. gait, stair climbing, etc.), if the distribution of internal deformation is desired, a more accurate biphasic representation may be necessary. Recent advances, not intimidated by the multiphase complexity of cartilage physics, have integrated more realistic representations of cartilage constitutive behavior at the joint level 107,108. A similar investigation explored the role of biphasic response of the tissue within a realistic joint anatomy with collagen fiber representation, yet under simplified loads 62,87. Finite element analysis software, e.g. FEBio (http://www.febio.org), implementing features necessary for realistic representation of cartilage mechanics 12,15,103, will likely popularize such investigations. Regardless, the computational cost and convergence sensitivity of this considerably more advanced finite element analysis can be prohibitive, especially when considered in a multiscale application. Even under simple material representations of the cartilage, large forces acting on the joint, and large deformations coupled with significant rigid body movements, may slow down or prevent the convergence of such models. The use of a biphasic model results in increased computational cost due to the large number of additional unknowns that are necessary to describe the mechanics of porous media 62.
Another challenge for finite element analysis based predictions of cartilage mechanics relates to the reliability of findings, particularly for lifelike loads and when using patient-specific models. In vitro testing can provide confirmation of model results 5,47 but obtaining accurate in vivo data remains an area of ongoing work. Recent studies based on magnetic resonance imaging 24 and CT arthrography 70, which can be applied in vivo, and current in vivo studies utilizing biplanar fluoroscopy 20, are promising exceptions. Focused studies that not only address the reliability of simulations results but also evaluate the balance between model fidelity and computational burden are of particular importance for advancement at this spatial scale.
Cartilage Mechanics from Cell-Microstructural Modeling Perspective
Synopsis
Modeling of cartilage at the cellular and microstructural levels provides the ability to predict chondrocyte deformations as a function of macroscopic tissue strains, to establish indicators of microstructural loading, and provides an understanding of the role of matrix composition on cellular and extracellular mechanics. Two modeling modalities are commonly utilized: one focusing on the micromechanics as dictated by the cartilage’s composition (Figure 2-D); another exploring the mechanical environment of chondrocytes, commonly relying on finite element analysis and also informed by the former micromechanical modeling modality (Figure 2-C).
Current Approaches – Microstructural Architecture
A wide range of modeling studies have explored the mechanical properties of the cartilage as a function of its microstructural architecture 152. Cartilage mechanical properties at the tissue level are primarily determined by the combination of the mechanical contributions of proteoglycans and collagen 110 (Figure 1-E). Proteoglycans, via their fixed negative charges, induce a Donnan osmotic swelling pressure; this swelling is counteracted by tensile stresses in the collagen network. The combination of the swelling and the restriction against swelling determines the mechanical properties of the cartilage tissue 144,154. Cartilage mechanical models have attempted to capture these effects of swelling with triphasic representations (solid, fluid, and ionic) 79,100,147, fitting parameters to tissue-level consolidation experiments, or into computationally less expensive biphasic swelling models that assume ion flux is significantly faster than water flow 149. To better capture the nature of the tissue, models with fiber-reinforcement were developed in the last decade 101,150,151. In fact, the contributions of swelling and fiber-reinforcement are products of the primary biochemical constituents of the tissue, i.e., proteoglycans and collagens. Current developments start from the premise that given this tissue composition, including the spatial distributions of the constituents and their structural organizations, the global and local mechanical properties of the cartilage can be determined. Hence, with knowledge of the properties of cartilage constituents, biochemically and histologically measurable data, rather than a set of fitted parameters, could ultimately be used as inputs for the computational model. Microscale composition could then be used to inform the tissue level response, alleviating the need to obtain site- or specimen-specific bulk mechanical parameters (commonly performed using inverse analysis). First steps toward such approaches are already present in the literature 83,144,153,154. When coupled with chondrocyte mechanics, this capacity will also allow dynamic changes to the composition of the material during a simulation, simulating temporal adaptations to a given stimuli. An example of such an approach was shown at the cellular level, where a cell hypertrophies in response to measured changes in pericellular tissue composition 37.
Current Approaches – Chondrocyte Mechanics
Continuum mechanics based models also provide quantification of cartilage mechanics at the spatial scale of chondrocytes to understand damage risk to the chondrocytes and to explore mechanical thresholds for cellular mechanobiological response. Many models of cell-matrix interactions, particularly utilizing finite element analysis (Figure 2-C), have shown that the combined solid and fluid environment of chondrocytes are highly dependent on the relative mechanical properties of the cell, its pericellular matrix, and the extracellular matrix 60,63,90,96. The pericellular and extracellular matrices have been shown to have a significant effect on the mechanical environment of the chondrocytes, serving to either amplify or reduce cell-level strains, depending on chondrocyte location as well as the magnitude of tissue strain 3,31. Thus, alterations in pericellular matrix properties, as may occur with osteoarthritis 4, may have significant effects on the mechanical environment of the chondrocytes. Recent models of cell-matrix interactions have taken into account the compositions of pericellular matrix, using models that also accommodate tissue swelling as well as fiber reinforcement. These studies have shown the importance of the composition of the pericellular matrix, and of the difference in composition between pericellular and extracellular matrices, on the mechanical environment of the chondrocyte 37,82,98.
Multiscale Context
At the spatial scale of cells and cartilage microstructure, models commonly represent cartilage constitutive behavior and the chondrocyte environment in a detailed manner. Implicitly multiscale by nature, novel modeling approaches incorporating biphasic and/or triphasic response 13,100, informed by anatomical fiber distribution 43, and accommodating zone-specific chondron shape 67 are actively being developed. Simulations are commonly used for hypothesis generation on healthy and diseased chondrocyte, pericellular matrix, and extracellular matrix function. Yet, multicellular representations, which may provide the possibility to explore cell-to-cell mechanical and biochemical signaling, are absent in the literature. In contrast to the minimal computational cost of single-cell analysis, commonly approximated through axisymmetric modeling 60, multiphysics simulations with three-dimensional anatomical representations of chondrocytes may be prohibitive. It can also be difficult to relate cell level variables to in vivo joint mechanics. Reliability of the prediction of in situ deformations of chondrocytes is commonly implied but not established, and is generally based on laboratory testing of simplified and isolated conditions 26,31. In summary, computational cost, confirmation of simulation results, and establishing the relationship of this scale to higher spatial scales are issues of great potential and importance to the future of the simulation of cartilage mechanics.
Cartilage Mechanics from Molecular Modeling Perspective
While the focus of this review falls short of a discussion on explicit modeling of mechanics beyond the microscale, examples of the emerging area of molecular level modeling can pinpoint research directions to understand the nanomechanical basis of cartilage function. Molecular modeling has been commonly conducted on aggrecan, the predominant proteoglycan in cartilage. Continuum Poisson-Boltzman cell models have been employed to determine charge distribution over a continuum with consideration of molecular interaction and mobility 18. More recently, a statistical thermodynamic approach that considered nonuniform and dynamic charge distribution along the aggrecan molecule and the effects of solution salt content and pH on molecular structure and interaction has been developed 116. Through manipulation of charge distribution and molecular structure, this model provided the capability to explain how charged proteoglycans can aggregate into clusters. Potential advancements in molecular modeling based on such studies and increases in computational capacity will likely facilitate the development of tissue-scale constitutive models founded on nanomechanics, which in turn establish coupling of tissue mechanics to the molecular scale.
Multiscale Coupling
Multiscale Simulations at the Interface of Musculoskeletal and Joint-Tissue Representations
Premise
Movement goals, and anatomical and physics-based constraints dictate neuromuscular response for muscular force generation. Joint movements, generated by musculoskeletal loading, result in cartilage deformations (Figure 1). Moving beyond the inference of tissue loading using musculoskeletal simulation alone, it is recognized that a computational framework able to capture interactions between tissue and movement is beneficial 41. While from a computational perspective this is a challenging prospect, simulations at both spatial scales would benefit, where the interplay between tissue level deformations and body level loading is explicitly included. Instead of estimating lower spatial scale tissue stress from body level predicted loads, as in a post-processing sense, cartilage geometry and behavior could be concurrently represented in the computational framework (Figure 2-A & 2-B, physiologically informed joint response). One can imagine predicting compensatory movement patterns able to target specific tissue mechanics, reduce potential pain, and quantify internal cartilage mechanics during common activities. Awareness of the challenges and advances in this field would surely benefit studies with an interest in the relationship between cartilage mechanics and activities of daily living.
Current Approaches
Specific to cartilage but not incorporated in a concurrent multiscale framework, a variety of studies have utilized loading predicted from musculoskeletal models, both generic and specimen-specific, to drive tissue level models 45,46,160. These post-processing studies have offered significant value in understanding joint mechanics but the validity of utilizing a simplified joint representation, e.g. a hinge as a surrogate for knee function, during muscle force prediction and subsequent application to a detailed joint level model is yet to be established.
To address the limitations of a post-processing approach, and of particular interest to cartilage, multiple recent studies have attempted explicit coupling of musculoskeletal loading and tissue deformations. Explorations of the joints included the knee 55,102, the patellofemoral joint 19, and the jaw 94. Of particular interest, studies which included geometric representation of cartilage 19,55 utilized a single leg musculoskeletal representation of either a static or flexion-extension task, a simplification when compared to typical musculoskeletal simulations 41. Similar simplifications were employed at the musculoskeletal modeling and/or continuum mechanics of the cartilage to avoid the computational burden of incorporating two different simulation modalities within the same model.
An alternative method is the nested coupling of musculoskeletal movement simulations with finite element analysis at the tissue level 64,65. During each solution step of musculoskeletal movements simulations with a continuum mechanics model of the tissue of interest are conducted to predict tissue stress-strain state along with movement trajectories and muscle forces. While not performed for the cartilage, such an approach showed promise to predict foot deformations during jumping 65 and walking 64, in particular, to identify adaptive walking strategies for reduction of strains within the foot 64. Similar approaches can be applied to the cartilage as well.
Challenges
When using a post-processing approach, the representation of joints (or tissues) in different modeling modalities should be mechanically consistent, i.e. they provide the same average response, and the assumptions of temporal and spatial separation for this loose coupling needs to be justified. If muscle forces and/or joint kinematic/kinetic variables passed from the musculoskeletal model depend on the complexity of the surrogate joint model (Figure 2-A), non-physiologic inputs to the continuum mechanical representation of the joint (Figure 2-B) may be possible. This in turn may cause potentially erroneous calculation of cartilage stress-strain.
For explicit or nested coupling of musculoskeletal and joint-tissue representations, a major challenge is the computational disparity between the domains of tissue mechanics and musculoskeletal-based movement prediction, especially when nonlinear analysis is a necessity. Generally requiring an iterative approach, musculoskeletal modeling (Figure 2-A) typically solves an optimization problem to produce the desired movement pattern. Even one quasi-static solution of deformable tissue mechanics (commonly performed with finite-element analysis) may have a similar computation cost as a complete musculoskeletal movement prediction, particularly when realistic anatomy and nonlinear mechanics are represented (Figure 2-B). One extreme example demonstrates that a probabilistic approach of joint injury required literally thousands of muscle driven movement simulations 104. Each of those forward dynamic simulations required around 200 time points where the solution of internal tissue mechanics, had they been modeled, would have been required. Obviously, the nested computational expense associated with concurrent coupling of these domains, even for the most basic approach, is an issue that needs to be addressed. Although not specific to cartilage, this coupling problem has been addressed in previous studies, which did simulate muscle driven movement patterns (maximum height jumping and gait) along with finite element analysis of foot mechanics 64,65. For the maximum height jumping simulation 65, this was possible through an adaptive surrogate modeling scheme, during which finite element analysis was avoided when an approximation using a database of previous solutions was deemed adequate. Nonetheless, the cited models were two-dimensional and the solutions still required weeks of computational time (on an IntelR Xeon 3.4 GHz machine with 6GB of memory). While these promising studies have demonstrated great potential, the discipline is still at a relatively exploratory stage and when internal tissue deformation is of interest, investigations reportedly suffer from high computational cost.
Multiscale Simulations at the Interface of Joint-Tissue and Cell-Microstructural Representations
Premise
Mechanical factors play a significant role in chondrocyte activity, viability, and differentiation 57. In musculoskeletal biomechanics, joint loading results in chondrocyte deformations in cartilage (Figure 1). Much like interfacing musculoskeletal simulations to joint-tissue representations, joint-tissue level models (Figure 2-B) may also be related to lower spatial scales, and in this context to cell and/or fiber microstructural simulations (Figure 2-C & 2-D). As a function of the surrounding extracellular matrix, and with the potential to represent the native cartilage geometry and/or a joint as a construct, simulations connecting these spatial scales have the ability to quantify the complex biomechanical and biochemical environment of the chondrocyte and the extracellular matrix. Developing this capability, computational or otherwise, will lead to a better understanding of natural cartilage function and health. Through this understanding, work in this field also has implications for development of interventions aimed at maintaining and promoting favorable conditions for chondrocyte function. Connecting these spatial scales is not only advantageous to develop interventions and to quantify the mechanical environment of the cell under physiological joint loads, but also serves as a critical step in relating macro-scale cartilage function with the underlying microstructure. Informing macro-scale models through accurate representation of the underlying constituents may elucidate important relationships between microstructural composition and the resulting mechanobiology, and vice versa.
Current Approaches–Tissue to Cell
The use of dual-scale (macro- to micro-) models of chondrocytes in their mechanical environment, driven by macro-scale tissue strains, is widely spread. These investigations looked at the spatial and/or temporal effects of tissue level loading and the architecture of pericellular and extracellular matrices on chondrocyte behavior. Some studies have focused on the role of the pericellular matrix as a strain transducer, which has been shown to shield or amplify tissue level loading 3,31,58,60,90,91,105. This phenomenon is more pronounced as the loading magnitude increases, depends on the relative material properties of the extracellular matrix and the pericellular matrix, and results in relatively consistent chondrocyte strain throughout the depth of the cartilage layer. Other studies have aimed to understand cartilage behavior as a construct of depth dependent collagen fiber alignment and chondron shape 43,44,67,144,154,157,158. These largely theoretical approaches have also incorporated tissue poroelasticity and are particularly suited for efficient parametric exploration of tissue level mechanics as informed by the complex cartilage microstructure. A recent study based on this approach supports the depth dependent sensitivity of cell deformations, much like previous studies, and also concludes that in situ chondrocyte stiffness may be much higher than those found in previous experimentation conducted on isolated cells 68. Additional numerical explorations using such a coupled framework have been undertaken to evaluate the effects of tissue level osmotic loads on chondron shape 95–98. Depth dependent collagen network alignment and proteoglycan content (to induce a fixed charge density) as a function of cartilage tissue depth was modeled and chondron shape predictions correlated well with experimental results 95. Through these studies, osmotic loading has been shown to be a significant contributor to chondrocyte mechanics and shape, especially when considered in concert with the surrounding collagen fiber microstructure. The previously cited studies have largely been based on explant samples with a closed-form definable geometry, due to the controlled mechanical and biochemical environment.
Current Approaches–Joint to Cell, Microstructural Architecture
Another approach in multiscale mechanical simulations of cartilage incorporated anatomically realistic joint representation with explicit definitions of microstructural architecture. Using a three-dimensional knee joint model with collagen fiber representation, Shirazi and Shirazi-Adl 136 looked at the effect of osteochondral defects on cartilage contact mechanics. They found that the localized tibial bone damage and the removal of deep vertical fibers influenced joint compliance and contact pressure results. Another study incorporated depth-dependent and split-line fiber alignment along with tissue poroelasticity and found split-line fiber alignment to have an especially important influence on the joint level weight-bearing properties of cartilage 108. Phenomenological modeling approaches 125 to represent cartilage morphology and patient-specific material response will likely encourage development of new three-dimensional models of the joints capable of predicting collagen fiber tracking.
Current Approaches–Joint to Cell, Chondrocyte Mechanics
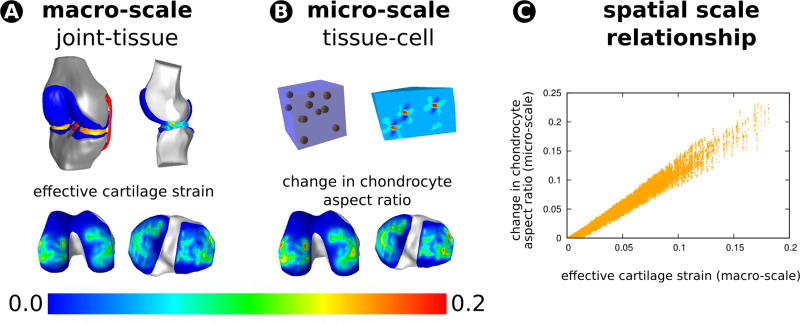
Chondrocyte behavior as a result of joint level loading, including multiscale interactions between joint, tissue, and cells, is also of interest. A recent study confirmed the feasibility of a post-processing approach to obtain chondrocyte deformations in the tibiofemoral joint while subjected to one body weight of compressive loading (Figure 3, 137). This study also focused on the potential differences between a single-cell microstructural model versus a physiologically based 11 cell model 137. As dictated by joint level loading, it was found that the cells in the 11 cell microstructural model experienced less deformation overall and a wide range of strains, at least compared to the single cell case and for simplified hyperelastic materials. Despite the mechanical simplifications in the models of this post-processing approach, the analysis still required approximately 8,000 simulations of the cell model to provide chondrocyte deformation metrics throughout a coarsely modeled transitional zone of tibial and femoral cartilage. Parallel processing on supercomputing facilities enabled acquisition of results within a day. If these three-dimensional models are further developed using the discoveries from previous experimental and computational studies 3,44,58,95, this type of a modeling framework has the potential to elucidate the relationship between macro- and microscale mechanics in cartilage for lifelike loading scenarios and supported by physiologically sound micromechanical, composition-based response.
Figure 3.
Finite element analysis of joints can help quantify load sharing between passive structures and in turn help establish the macroscopic stress-strain state within the cartilage as a function of joint forces and moments. Microscale models can be used to post-process the macroscopic cartilage tissue deformations to obtain stress-strain state within the chondrocytes, their pericellular environment, and the extracellular matrix. Sibole and Erdemir 137 illustrated that this post processing approach is attainable for large regions of cartilage in an automated fashion. Their multiscale modeling pipeline incorporated anatomically realistic joint and tissue geometry to obtain cartilage deformation at the macro-scale (A). Sibole and Erdemir 137 then utilized these deformations to solve micro-scale models incorporating anatomically realistic cell shapes and distributions (B) with the ultimate goal to relate chondrocyte deformations and macro-scale cartilage deformations (C). These multiscale explorations can help establish the relationship between macroscopic joint loads, macroscopic cartilage strains, and chondrocyte deformations. This analysis can be extended to incorporate more detailed mechanics of the cartilage, i.e. biphasic response, fiber-based extracellular matrix architecture, and the simulations can be driven by joint loads that represent lifelike conditions. Musculoskeletal simulations and/or body and joint level experimentation can provide lifelike joint loads that can drive this pipeline. The resulting information may help to understand damage mechanisms and mechanobiological stimuli as they relate to functional loading of the joint.
Challenges
There are a number of difficulties associated in the aforementioned types of multiscale biomechanical computational frameworks, and the development of mechanical models that span the tissue to the cell require considerations on many levels of complexity and abstraction. At both spatial scales, appropriate definitions of loading, material properties, temporal response, and structure are required, as framed by the question of interest. As mentioned before, cartilage is a composite avascular tissue that demonstrates viscoelastic depth-dependent behavior 29,75. The involved constituents of the microstructure (collagen fibers, fluid, solutes, proteoglycans, and cells) and their individual properties and interactions dictate structural function (Figure 1-D & 1-E). Accounting for this balance when translating between lumped macroscopic tissue scale and the detailed microscale presents technical challenges. Proper scale separation, the relative sizes of the microstructural (the representative volume element) and tissue level models, needs to be addressed 52.
Coupling of microstructural and/or cell level models to macroscopic tissue models can be conducted through nested solutions of lower scale models to provide constitutive response to the finite element analysis at the macroscale 52 (Figure 2, microstructurally informed tissue properties). Computational homogenization serves this purpose, by providing a mechanically consistent translation between the scales that may not be achievable in a post-processing approach. Nonetheless, this will likely raise computational cost due to high number of numerical iterations 52. Scale separation, whether the heterogeneous microstructural model is sufficiently small compared to the homogeneous representation of the macroscale (i.e. the characteristic sizes of the micro- and macrostructural models), is one important issue to highlight when using computational homogenization 52. Given the size, prevalence, and biphasic nature of chondrocytes in cartilage, it is anticipated that assumptions related to scale separation may be important to address in multiscale simulations of chondrocyte mechanics.
Capturing the phenomenon of interest may also span many fields of study, e.g. microbiology to continuum mechanics, and accounting for the necessary complexity largely depends on the available expertise and the study goal(s). There seems to be a discrepancy between the detail of continuum mechanical models used in joint simulations (Figure 2-B) and those utilized at the microscale (Figure 2-C). Except a few recent examples 107,108, the majority of macroscopic tissue representations used in joint models relied on linear/nonlinear elasticity. On the other hand, for microscale or cell level tissue mechanics, incorporation of biphasic mechanical behavior is a common practice 60,139. The mechanical coupling between the analysis of these spatial scales may be challenging. For example, three-dimensional biphasic predictions of chondrocyte mechanics, which may need to be solved iteratively in joint mechanics models, can easily require hours to achieve convergence. Not to be forgotten, validity of the outputs of multiscale analysis need to rely on the availability of sound experimental data, and promising work on this front is emerging 1.
Potential Directions for Multiscale Modeling of Cartilage Mechanics
Simulation Framework
To establish multiscale interactions for healthy cartilage, in osteoarthritis, and as a function of aging, it will be necessary to build a data integrated modeling and simulation framework combining musculoskeletal, joint, tissue, cell, and microstructural response. Even conducted in a post-processing manner, different modeling modalities, driven by data collected at multiple scales, can identify body level musculoskeletal indicators of cartilage stress-strain and chondrocyte deformations. From a clinical perspective, such a framework can aid in personalized assessment of cartilage mechanics in relation to musculoskeletal function at multiple scales. The current unidirectional down scale approach of post-processing can be expanded to a concurrent multiscale simulation platform where the lower spatial scales provide constitutive response in a bidirectional feedback manner (Figure 2). This will be important for phenomena where adaptive simulations at lower spatial scales are necessary, e.g. growth, remodeling, failure. This can also help quantify the influence of lower scale response on movement adaptations at a higher level. While this promise is enticing, many scientific and technological hurdles, sometimes specific to the scale of interest and the modeling modality, need to be addressed. These range from computational burden (associated with coupling of complicated models) to laborious model development efforts, the need for data to build and confirm models, and uncertainty estimation to support clinical decision-making.
Coupling to Subcellular and Molecular Scales
Expansion of the multiscale modeling framework to lower spatial scales is certainly possible (Figure 2, nanomechanics). For example, the mechanical information on the cartilage microstructure may further drive models at the biomolecular level to establish the nanomechanical operational principles of the extracellular matrix 66. Molecular modeling will likely provide the pathway for such simulations. As briefly described above, such models can relate composition and structure of molecules to tissue scale properties.
An interesting direction for multiscale mechanics of the cartilage also includes subcellular levels. Understanding the mechanical interactions of chondrocytes with the extracellular matrix requires interface modeling. The assumption of a perfectly adhesive interface between the chondrocyte membrane and the surrounding collagen matrix is coarse. In reality, the actin cytoskeleton is attached to the surrounding scaffold at focal adhesion points via integrins. Therefore, loads are concentrated at these binding sites rather than being distributed over the cell membrane area. This load-focusing characteristic has been hypothesized to be the avenue for the communication of tissue scale traction into specific mechanotransduced cellular processes 30. Structural complexity is further compounded through the consideration of the mechanical properties of the cytoskeletal network as well as intracellular organelles, particularly the chondrocyte nucleus 61,121, and computational modeling of these components is an emerging area.
Through active processes, the cell also creates ion gradients between the cytoplasm and the interstitial space. The resulting osmotic pressure, in addition to, the hydrostatic pressure resist traction induced volume change, while also in the presence of viscous effects. The lumped behavior of the cell is, therefore, the sum of a multitude of structures experiencing tension, compression, shear, and volumetric tractions. Future research at this scale should enhance our understanding of the intracellular mechanical environment. Extending multiscale modeling to this scale will, hopefully, elicit a better understanding of how the mechanics of daily life ultimately affect cellular processes.
Coupling of Cartilage Mechanics to Biological Systems
The mechanical information acquired through simulations of cartilage mechanics can be utilized as driving stimuli for mechanobiological function (Figure 2). Avenues for mechanotransduction such as flows and pressures, streaming potentials, changes in pH and osmolarity with sustained compression, and mechanosensitive ion channels, are essential components of biological function. As described previously, mechanotransduction also requires information on cell attachment (focal adhesion points), and intracellular organization of actin filaments (and dynamics thereof), to understand the mechanical signal acting on the cell. Many of these potential effects are nicely reviewed by Hopewell and Urban 77. The (relative) importance of most (if not all) of these factors is really unknown. For example, after a mechanical signal enters the cells, a whole cascade of biological events may occur, which are beyond the scope of this review. Nonetheless, it is fair to say that for a thorough understanding of mechanotransduction from the tissue level to the chondrocytes, and to describe how cells respond to the mechanical stimuli they receive, it is insufficient to consider tissue stress and strain only. Many of the potential signaling mechanisms may depend upon other physical and chemical factors that are related to the stress/strain environment, such as fluid pressure, fluid flow, electrokinetic potentials, and osmotic changes 59. Further complicating this paradigm, such interactions may even occur at the interplay between tissues. For instance, growing cartilage induces a physical stimulus (strain) to periosteum cells, which respond by expressing cytokines that diffuse into the cartilage, where they limit cartilage growth 49. Further down the spatial scale, a thorough understanding requires models that also link extracellular mechanics to cell metabolism at the level of nutrient, cytokine and matrix-molecule transport, and account for the effects of cell activity and extracellular tissue adaptation or degradation. A driving force behind models of this kind in the recent past has arisen from the study of normal cartilage development as well as tissue engineering. When trying to engineer articular cartilage, researchers encountered the problem that a combination of mechanical and biochemical stimuli is required to optimize the formation of cartilage; and different stimuli may lead to a completely dissimilar extracellular matrix 93. Conceptual frameworks for integrating mechanics with matrix synthesis, transport and assembly were published by several groups 16,25,51,92,117,119,131,132. Sengers 128, for example, used a model to determine whether or not cyclic deformation of tissue engineered cartilage improved the transport of nutrients and oxygen, necessary components for tissue development. Similar models 36,130 were used to study the effect of changes in diffusion properties of a developing extracellular matrix on newly formed extracellular matrix distribution. An approach purely based on transport and utilization of nutrients was used by Sengers et al. 129 to study the growth of tissue engineered cartilage in different medium concentrations. Similarly, effects of paracrine signaling between chondrocytes or between different cell types has been accounted for in computational models 21. While many of the aforementioned studies commonly focused on tissue engineering applications25,36,89 some of these domain coupling methods may also be valuable for use in a multiscale approach to understand the effect of joint loading on cartilage growth, maintenance, and degeneration.
On the other end of the spatial spectrum and biological function, it will be useful to couple joint mechanics to neurological function, the somatosensory system, and muscle physiology (Figure 2). While these domains may not be directly associated with cartilage mechanobiology, they implicitly change (or are influenced by) the mechanical environment of the cartilage. Any alterations in joint mechanics will change the feedback signals for control of locomotion, subchondral pain may cause adaptive movement patterns, and muscle strengthening (or weakening) may influence the capacity to match movement constraints to maintain the stability of the joints. As a result, the mechanical pathway for cartilage and chondrocyte load sharing will be altered. If one is interested in the explicit delineation of the role of these systems in healthy and diseased cartilage mechanics, their coupling to the mechanics of cartilage is unavoidable.
Outlook
Cartilage is a prominent load bearing tissue in musculoskeletal joints. Its function is a multisystem (biological, chemical, mechanical), multiphysics (solid, fluid, charged particles), and multiscale (joint, tissue, cell, fiber) phenomenon. As a result, pathological and age related changes within the cartilage occur in multiple systems, scales, and physics. The mechanical environment of the cartilage is also affected by changes to the other body systems as well. Such alterations have been documented, i.e., for aging. The elderly have been shown to display higher neuromuscular activity, potentially indicating the differences in the way their joints are loaded 85. The properties of the passive structures of the joint are altered, as seen in changes in diminished range of motion 118. Functional joint movement and loading patterns, i.e., increased knee joint moments during stair ascent 86, were different, indicating adaptations in daily loading exerted on the cartilage. Gross anatomy of cartilage changes; such as tissue thinning in the elderly 78, consequentially modify the deformations and contact loading of the cartilage. Chondrocytes, their properties and distribution, and the extracellular matrix architecture also adapt with age 141, possibly altering the way tissue level stresses are distributed among the cells and within the matrix itself. While a wide range of experimentation (individually conducted at different spatial scales), and modeling at single scales have significantly contributed to the understanding of cartilage mechanics, a multiscale simulation-based approach is needed to establish the causal relationships between systems, scales, and physics of cartilage function in health and disease. Such simulations can be utilized in a descriptive fashion to explain the significance of in vivo measurements on the evaluation of cartilage mechanics, which potentially mine variables for diagnostic value. Such knowledge may provide subject-specific risk assessment for cartilage damage and degeneration. Multiscale simulations of the mechanics of healthy and osteoarthritic knees may provide multiscale coupling between mechanical risk factors. For example, confidence levels on joint level biomechanical markers may be established, based on how joint level mechanical loads are reflected as cartilage, chondrocyte, and even fiber deformations. Modeling and simulation platforms also possess the predictive capacity, which has the potential to facilitate intervention design. In a multiscale framework, one may evaluate the effect of a joint level mechanical intervention on the mechanical environment of cartilage and chondrocytes. The knowledge of cartilage, cell and fiber mechanics during activities of daily living will also establish the baseline mechanical stimuli necessary for tissue engineering approaches.
Simulation-based exploration of cartilage mechanics can be challenging even at a single spatial scale. Useful information of cartilage mechanics requires models with adequate representations of anatomy and constitutive relationships, driven by lifelike loading scenarios, and supported by verification and validation studies. Modeling and simulation constraints associated with computational costs, development labor, and uncertainties multiply as investigations span multiple scales. Adhering to sound modeling and reporting principles 40,74 and open access to data, models, and simulation software will promote reproducibility, reusability and accountability and encourage community-driven development of models. While progress has been realized, resolution of many technical, scientific, and social challenges remain. Nonetheless, multiscale modeling and simulation promise a pathway for patient-oriented simulation-based medicine to assess cartilage function and manage its dysfunction.
Acknowledgments
This study was supported by the National Institutes of Health grants R01EB009643 (A. Erdemir), R01AG15768 (F. Guilak), R01AR48182 (F. Guilak), R01AR48852 (F. Guilak), P01AR50245 (F. Guilak), R01GM083925 (J.A. Weiss), R01AR047369 (J.A. Weiss), and R01AR053344 (J.A. Weiss). The authors would also like to acknowledge Simbios, NIH Center for Biomedical Computation at Stanford, for hosting the project site for collaborative research.
References
- 1.Abusara Z, Seerattan R, Leumann A, Thompson R, Herzog W. A novel method for determining articular cartilage chondrocyte mechanics in vivo. J Biomech. 2011;44:930–934. doi: 10.1016/j.jbiomech.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Adouni M, Shirazi-Adl A. Knee joint biomechanics in closed-kinetic-chain exercises. Comput Methods Biomech Biomed Engin. 2009;12:661–670. doi: 10.1080/10255840902828375. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38:509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Anderson AE, Ellis BJ, Maas SA, Peters CL, Weiss JA. Validation of finite element predictions of cartilage contact pressure in the human hip joint. J Biomech Eng. 2008;130:051008. doi: 10.1115/1.2953472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AE, Ellis BJ, Maas SA, Weiss JA. Effects of idealized joint geometry on finite element predictions of cartilage contact stresses in the hip. J Biomech. 2010;43:1351–1357. doi: 10.1016/j.jbiomech.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DD, Iyer KS, Segal NA, Lynch JA, Brown TD. Implementation of discrete element analysis for subject-specific, population-wide investigations of habitual contact stress exposure. J Appl Biomech. 2010;26:215–223. doi: 10.1123/jab.26.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17:159–169. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 9.Arokoski JP, Hyttinen MM, Lapveteläinen T, Takács P, Kosztáczky B, Módis L, Kovanen V, Helminen H. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996;55:253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 11.Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ateshian GA, Albro MB, Maas S, Weiss JA. Finite element implementation of mechanochemical phenomena in neutral deformable porous media under finite deformation. J Biomech Eng. 2011;133:081005. doi: 10.1115/1.4004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ateshian GA, Chahine NO, Basalo IM, Hung CT. The correspondence between equilibrium biphasic and triphasic material properties in mixture models of articular cartilage. J Biomech. 2004;37:391–400. doi: 10.1016/s0021-9290(03)00252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ateshian GA, Ellis BJ, Weiss JA. Equivalence between short-time biphasic and incompressible elastic material responses. J Biomech Eng. 2007;129:405–412. doi: 10.1115/1.2720918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ateshian GA, Maas S, Weiss JA. Finite element algorithm for frictionless contact of porous permeable media under finite deformation and sliding. J Biomech Eng. 2010;132:061006. doi: 10.1115/1.4001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ateshian GA, Ricken T. Multigenerational interstitial growth of biological tissues. Biomech Model Mechanobiol. 2010;9:689–702. doi: 10.1007/s10237-010-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, Andriacchi TP. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthr Cartil. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 18.Bathe M, Grodzinsky AJ, Tidor B, Rutledge GC. Optimal linearized Poisson-Boltzmann theory applied to the simulation of flexible polyelectrolytes in solution. J Chem Phys. 2004;121:7557–7561. doi: 10.1063/1.1808411. [DOI] [PubMed] [Google Scholar]
- 19.Besier TF, Gold GE, Beaupré GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37:1924–1930. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]
- 20.Bischof JE, Spritzer CE, Caputo AM, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo cartilage contact strains in patients with lateral ankle instability. J Biomech. 2010;43:2561–2566. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouwers JEM, van Donkelaar CC, Sengers BG, Huiskes R. Can the growth factors PTHrP, Ihh and VEGF, together regulate the development of a long bone? J Biomech. 2006;39:2774–2782. doi: 10.1016/j.jbiomech.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl. 1995;43:13–15. [PubMed] [Google Scholar]
- 23.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 24.Butz KD, Chan DD, Nauman EA, Neu CP. Stress distributions and material properties determined in articular cartilage from MRI-based finite strains. J Biomech. 2011;44:2667–2672. doi: 10.1016/j.jbiomech.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Catt CJ, Schuurman W, Sengers BG, van Weeren PR, Dhert WJA, Please CP, Malda J. Mathematical modelling of tissue formation in chondrocyte filter cultures. Eur Cell Mater. 2011;22:377–392. doi: 10.22203/ecm.v022a28. [DOI] [PubMed] [Google Scholar]
- 26.Chahine NO, Hung CT, Ateshian GA. In-situ measurements of chondrocyte deformation under transient loading. Eur Cell Mater. 2007;13:100–111. doi: 10.22203/ecm.v013a11. discussion 111. [DOI] [PubMed] [Google Scholar]
- 27.Chao EYS, Volokh KY, Yoshida H, Shiba N, Ide T. Discrete element analysis in musculoskeletal biomechanics. Mol Cell Biomech. 2010;7:175–192. [PubMed] [Google Scholar]
- 28.Chegini S, Beck M, Ferguson SJ. The effects of impingement and dysplasia on stress distributions in the hip joint during sitting and walking: a finite element analysis. J Orthop Res. 2009;27:195–201. doi: 10.1002/jor.20747. [DOI] [PubMed] [Google Scholar]
- 29.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 30.Chen CS, Ingber DE. Tensegrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthr Cartil. 1999;7:81–94. doi: 10.1053/joca.1998.0164. [DOI] [PubMed] [Google Scholar]
- 31.Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connolly KD, Ronsky JL, Westover LM, Küpper JC, Frayne R. Differences in patellofemoral contact mechanics associated with patellofemoral pain syndrome. J Biomech. 2009;42:2802–2807. doi: 10.1016/j.jbiomech.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 34.Dhaher YY, Kwon TH, Barry M. The effect of connective tissue material uncertainties on knee joint mechanics under isolated loading conditions. J Biomech. 2010;43:3118–3125. doi: 10.1016/j.jbiomech.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Lima DD, Chen PC, Colwell CW., Jr Osteochondral grafting: effect of graft alignment, material properties, and articular geometry. Open Orthop J. 2009;3:61–68. doi: 10.2174/1874325000903010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Donkelaar CC, Chao G, Bader DL, Oomens CWJ. A reaction-diffusion model to predict the influence of neo-matrix on the subsequent development of tissue-engineered cartilage. Comput Methods Biomech Biomed Engin. 2011;14:425–432. doi: 10.1080/10255842.2011.554409. [DOI] [PubMed] [Google Scholar]
- 37.van Donkelaar CC, Wilson W. Mechanics of chondrocyte hypertrophy. Biomech Model Mechanobiol. 2011 doi: 10.1007/s10237-011-0340-0. [DOI] [PubMed] [Google Scholar]
- 38.Elias JJ, Cosgarea AJ. Computational modeling: an alternative approach for investigating patellofemoral mechanics. Sports Med Arthrosc. 2007;15:89–94. doi: 10.1097/JSA.0b013e31804bbe4d. [DOI] [PubMed] [Google Scholar]
- 39.Elias JJ, Kirkpatrick MS, Saranathan A, Mani S, Smith LG, Tanaka MJ. Hamstrings loading contributes to lateral patellofemoral malalignment and elevated cartilage pressures: an in vitro study. Clin Biomech (Bristol, Avon) 2011;26:841–846. doi: 10.1016/j.clinbiomech.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdemir A, Guess TM, Halloran J, Tadepalli SC, Morrison TM. Considerations for reporting finite element analysis studies in biomechanics. Journal of Biomechanics. 2012 doi: 10.1016/j.jbiomech.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdemir A, McLean S, Herzog W, van den Bogert AJ. Model-based estimation of muscle forces exerted during movements. Clin Biomech (Bristol, Avon) 2007;22:131–154. doi: 10.1016/j.clinbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Erhart-Hledik JC, Elspas B, Giori NJ, Andriacchi TP. Effect of variable-stiffness walking shoes on knee adduction moment, pain, and function in subjects with medial compartment knee osteoarthritis after 1 year. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2011 doi: 10.1002/jor.21563. [DOI] [PubMed] [Google Scholar]
- 43.Federico S, Grillo A, La Rosa G, Giaquinta G, Herzog W. A transversely isotropic, transversely homogeneous microstructural-statistical model of articular cartilage. J Biomech. 2005;38:2008–2018. doi: 10.1016/j.jbiomech.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Federico S, Herzog W. Towards an analytical model of soft biological tissues. J Biomech. 2008;41:3309–3313. doi: 10.1016/j.jbiomech.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez JW, Akbarshahi M, Crossley KM, Shelburne KB, Pandy MG. Model predictions of increased knee joint loading in regions of thinner articular cartilage after patellar tendon adhesion. J Orthop Res. 2011;29:1168–1177. doi: 10.1002/jor.21345. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez JW, Pandy MG. Integrating modelling and experiments to assess dynamic musculoskeletal function in humans. Exp Physiol. 2006;91:371–382. doi: 10.1113/expphysiol.2005.031047. [DOI] [PubMed] [Google Scholar]
- 47.Fischer KJ, Johnson JE, Waller AJ, McIff TE, Toby EB, Bilgen M. MRI-based modeling for radiocarpal joint mechanics: validation criteria and results for four specimen-specific models. J Biomech Eng. 2011;133:101004. doi: 10.1115/1.4005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzpatrick CK, Baldwin MA, Laz PJ, FitzPatrick DP, Lerner AL, Rullkoetter PJ. Development of a statistical shape model of the patellofemoral joint for investigating relationships between shape and function. J Biomech. 2011;44:2446–2452. doi: 10.1016/j.jbiomech.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Foolen J, van Donkelaar CC, Ito K. Intracellular tension in periosteum/perichondrium cells regulates long bone growth. J Orthop Res. 2011;29:84–91. doi: 10.1002/jor.21224. [DOI] [PubMed] [Google Scholar]
- 50.Fregly BJ, Besier TF, Lloyd DG, Delp SL, Banks SA, Pandy MG, D’Lima DD. Grand challenge competition to predict in vivo knee loads. J Orthop Res. 2012;30:503–513. doi: 10.1002/jor.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galban CJ, Locke BR. Analysis of cell growth in a polymer scaffold using a moving boundary approach. Biotechnol Bioeng. 1997;56:422–432. doi: 10.1002/(SICI)1097-0290(19971120)56:4<422::AID-BIT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Geers MGD, V, Kouznetsova G, Brekelmans WAM. Multi-scale computational homogenization: Trends and challenges. Journal of Computational and Applied Mathematics. 2010;234:2175–2182. [Google Scholar]
- 53.Goldsmith AA, Hayes A, Clift SE. Application of finite elements to the stress analysis of articular cartilage. Med Eng Phys. 1996;18:89–98. doi: 10.1016/1350-4533(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 54.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 55.Guess TM, Liu H, Bhashyam S, Thiagarajan G. A multibody knee model with discrete cartilage prediction of tibio-femoral contact mechanics. Computer Methods in Biomechanics and Biomedical Engineering. 2011 doi: 10.1080/10255842.2011.617004. [DOI] [PubMed] [Google Scholar]
- 56.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 57.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 59.Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 259–300. [Google Scholar]
- 60.Guilak F, V, Mow C. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 61.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 62.Gu KB, Li LP. A human knee joint model considering fluid pressure and fiber orientation in cartilages and menisci. Med Eng Phys. 2011;33:497–503. doi: 10.1016/j.medengphy.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Haider MA, Guilak F. Application of a Three-Dimensional Poroelastic BEM to Modeling the Biphasic Mechanics of Cell-Matrix Interactions in Articular Cartilage (REVISION) Comput Methods Appl Mech Eng. 2007;196:2999–3010. doi: 10.1016/j.cma.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halloran JP, Ackermann M, Erdemir A, van den Bogert AJ. Concurrent musculoskeletal dynamics and finite element analysis predicts altered gait patterns to reduce foot tissue loading. J Biomech. 2010;43:2810–2815. doi: 10.1016/j.jbiomech.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halloran JP, Erdemir A, van den Bogert AJ. Adaptive surrogate modeling for efficient coupling of musculoskeletal control and tissue deformation models. J Biomech Eng. 2009;131:011014. doi: 10.1115/1.3005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han L, Grodzinsky AJ, Ortiz C. Nanomechanics of the Cartilage Extracellular Matrix. Annual Review of Materials Research. 2011;41:133–168. doi: 10.1146/annurev-matsci-062910-100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han SK, Federico S, Grillo A, Giaquinta G, Herzog W. The mechanical behaviour of chondrocytes predicted with a micro-structural model of articular cartilage. Biomech Model Mechanobiol. 2007;6:139–150. doi: 10.1007/s10237-006-0016-3. [DOI] [PubMed] [Google Scholar]
- 68.Han SK, Federico S, Herzog W. A depth-dependent model of the pericellular microenvironment of chondrocytes in articular cartilage. Comput Methods Biomech Biomed Engin. 2011;14:657–664. doi: 10.1080/10255842.2010.493512. [DOI] [PubMed] [Google Scholar]
- 69.Harris JD, Solak KK, Siston RA, Litsky A, Richards J, Flanigan DC. Contact pressure comparison of proud osteochondral autograft plugs versus proud synthetic plugs. Orthopedics. 2011;34:97. doi: 10.3928/01477447-20101221-06. [DOI] [PubMed] [Google Scholar]
- 70.Harris MD, Anderson AE, Henak CR, Ellis BJ, Peters CL, Weiss JA. Finite element prediction of cartilage contact stresses in normal human hips. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2011 doi: 10.1002/jor.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haut Donahue TL, Hull ML, Rashid MM, Jacobs CR. How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. J Biomech. 2003;36:19–34. doi: 10.1016/s0021-9290(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 72.Heinegård D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- 73.Helminen HJ, Hyttinen MM, Lammi MJ, Arokoski JP, Lapveteläinen T, Jurvelin J, Kiviranta I, Tammi MI. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J Bone Miner Metab. 2000;18:245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- 74.Henninger HB, Reese SP, Anderson AE, Weiss JA. Validation of computational models in biomechanics. Proc Inst Mech Eng H. 2010;224:801–812. doi: 10.1243/09544119JEIM649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herzog W, Federico S. Considerations on joint and articular cartilage mechanics. Biomech Model Mechanobiol. 2006;5:64–81. doi: 10.1007/s10237-006-0029-y. [DOI] [PubMed] [Google Scholar]
- 76.Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA. 1986;83:2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hopewell B, Urban JPG. Adaptation of articular chondrocytes to changes in osmolality. Biorheology. 2003;40:73–77. [PubMed] [Google Scholar]
- 78.Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, Eckstein F. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 79.Huyghe JM, Wilson W, Malakpoor K. On the thermodynamical admissibility of the triphasic theory of charged hydrated tissues. J Biomech Eng. 2009;131:044504. doi: 10.1115/1.3049531. [DOI] [PubMed] [Google Scholar]
- 80.Hyttinen MM, Arokoski JP, Parkkinen JJ, Lammi MJ, Lapveteläinen T, Mauranen K, Király K, Tammi MI, Helminen HJ. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthr Cartil. 2001;9:694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- 81.Idowu BD, Knight MM, Bader DL, Lee DA. Confocal analysis of cytoskeletal organisation within isolated chondrocyte sub-populations cultured in agarose. Histochem J. 2000;32:165–174. doi: 10.1023/a:1004095207330. [DOI] [PubMed] [Google Scholar]
- 82.Julkunen P, Wilson W, Jurvelin JS, Korhonen RK. Composition of the pericellular matrix modulates the deformation behaviour of chondrocytes in articular cartilage under static loading. Med Biol Eng Comput. 2009;47:1281–1290. doi: 10.1007/s11517-009-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Julkunen P, Wilson W, Jurvelin JS, Rieppo J, Qu CJ, Lammi MJ, Korhonen RK. Stress-relaxation of human patellar articular cartilage in unconfined compression: prediction of mechanical response by tissue composition and structure. J Biomech. 2008;41:1978–1986. doi: 10.1016/j.jbiomech.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 84.Jurvelin J, Buschmann M, Hunziker E. Optical and mechanical determination of Poisson’s ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 85.Kang HG, Dingwell JB. Dynamics and stability of muscle activations during walking in healthy young and older adults. J Biomech. 2009;42:2231–2237. doi: 10.1016/j.jbiomech.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karamanidis K, Arampatzis A. Evidence of mechanical load redistribution at the knee joint in the elderly when ascending stairs and ramps. Ann Biomed Eng. 2009;37:467–476. doi: 10.1007/s10439-008-9624-7. [DOI] [PubMed] [Google Scholar]
- 87.Kazemi M, Li LP, Buschmann MD, Savard P. Partial meniscectomy changes fluid pressurization in articular cartilage in human knees. J Biomech Eng. 2012;134:021001. doi: 10.1115/1.4005764. [DOI] [PubMed] [Google Scholar]
- 88.Kerrigan DC, Riley PO, Nieto TJ, Della Croce U. Knee joint torques: a comparison between women and men during barefoot walking. Arch Phys Med Rehabil. 2000;81:1162–1165. doi: 10.1053/apmr.2000.7172. [DOI] [PubMed] [Google Scholar]
- 89.Khoshgoftar M, van Donkelaar CC, Ito K. Mechanical stimulation to stimulate formation of a physiological collagen architecture in tissue-engineered cartilage: a numerical study. Comput Methods Biomech Biomed Engin. 2011;14:135–144. doi: 10.1080/10255842.2010.519335. [DOI] [PubMed] [Google Scholar]
- 90.Kim E, Guilak F, Haider MA. The dynamic mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions under cyclic compressive loading. J Biomech Eng. 2008;130:061009. doi: 10.1115/1.2978991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim E, Guilak F, Haider MA. An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. J Biomech Eng. 2010;132:031011. doi: 10.1115/1.4000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klisch SM, Asanbaeva A, Oungoulian SR, Masuda K, Thonar EJM, Davol A, Sah RL. A cartilage growth mixture model with collagen remodeling: validation protocols. J Biomech Eng. 2008;130:031006. doi: 10.1115/1.2907754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kock LM, Ravetto A, van Donkelaar CC, Foolen J, Emans PJ, Ito K. Tuning the differentiation of periosteum-derived cartilage using biochemical and mechanical stimulations. Osteoarthr Cartil. 2010;18:1528–1535. doi: 10.1016/j.joca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Koolstra JH, van Eijden TMGJ. Combined finite-element and rigid-body analysis of human jaw joint dynamics. J Biomech. 2005;38:2431–2439. doi: 10.1016/j.jbiomech.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Korhonen RK, Han SK, Herzog W. Osmotic loading of in situ chondrocytes in their native environment. Mol Cell Biomech. 2010;7:125–134. [PubMed] [Google Scholar]
- 96.Korhonen RK, Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J Biomech. 2008;41:480–485. doi: 10.1016/j.jbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Korhonen RK, Julkunen P, Rieppo J, Lappalainen R, Konttinen YT, Jurvelin JS. Collagen network of articular cartilage modulates fluid flow and mechanical stresses in chondrocyte. Biomech Model Mechanobiol. 2006;5:150–159. doi: 10.1007/s10237-006-0021-6. [DOI] [PubMed] [Google Scholar]
- 98.Korhonen RK, Julkunen P, Wilson W, Herzog W. Importance of collagen orientation and depth-dependent fixed charge densities of cartilage on mechanical behavior of chondrocytes. J Biomech Eng. 2008;130:021003. doi: 10.1115/1.2898725. [DOI] [PubMed] [Google Scholar]
- 99.Krishnan R, Mariner EN, Ateshian GA. Effect of dynamic loading on the frictional response of bovine articular cartilage. J Biomech. 2005;38:1665–1673. doi: 10.1016/j.jbiomech.2004.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 101.Li LP, Cheung JTM, Herzog W. Three-dimensional fibril-reinforced finite element model of articular cartilage. Med Biol Eng Comput. 2009;47:607–615. doi: 10.1007/s11517-009-0469-5. [DOI] [PubMed] [Google Scholar]
- 102.Lin YC, Walter JP, Banks SA, Pandy MG, Fregly BJ. Simultaneous prediction of muscle and contact forces in the knee during gait. J Biomech. 2010;43:945–952. doi: 10.1016/j.jbiomech.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 103.Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: Finite Elements for Biomechanics. J Biomech Eng. 2012;134:011005. doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McLean SG, Su A, van den Bogert AJ. Development and validation of a 3-D model to predict knee joint loading during dynamic movement. J Biomech Eng. 2003;125:864–874. doi: 10.1115/1.1634282. [DOI] [PubMed] [Google Scholar]
- 105.Michalek AJ, Iatridis JC. A numerical study to determine pericellular matrix modulus and evaluate its effects on the micromechanical environment of chondrocytes. J Biomech. 2007;40:1405–1409. doi: 10.1016/j.jbiomech.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller EJ, Riemer RF, Haut Donahue TL, Kaufman KR. Experimental validation of a tibiofemoral model for analyzing joint force distribution. J Biomech. 2009;42:1355–1359. doi: 10.1016/j.jbiomech.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mononen ME, Julkunen P, Töyräs J, Jurvelin JS, Kiviranta I, Korhonen RK. Alterations in structure and properties of collagen network of osteoarthritic and repaired cartilage modify knee joint stresses. Biomech Model Mechanobiol. 2011;10:357–369. doi: 10.1007/s10237-010-0239-1. [DOI] [PubMed] [Google Scholar]
- 108.Mononen ME, Mikkola MT, Julkunen P, Ojala R, Nieminen MT, Jurvelin JS, Korhonen RK. Effect of superficial collagen patterns and fibrillation of femoral articular cartilage on knee joint mechanics-A 3D finite element analysis. J Biomech. 2011 doi: 10.1016/j.jbiomech.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Mow VC, Ateshian GA, Spilker RL. Biomechanics of diarthrodial joints: a review of twenty years of progress. J Biomech Eng. 1993;115:460–467. doi: 10.1115/1.2895525. [DOI] [PubMed] [Google Scholar]
- 110.Mow VC, Gu WY, Chen FH. Structure and Function of Articular Cartilage and Meniscus. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 181–258. [Google Scholar]
- 111.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 112.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 113.Mündermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis Rheum. 2004;50:1172–1178. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- 114.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthr Cartil. 2011;19:1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 115.Nam J, Perera P, Liu J, Wu LC, Rath B, Butterfield TA, Agarwal S. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum. 2011;63:1613–1625. doi: 10.1002/art.30311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nap RJ, Szleifer I. Structure and interactions of aggrecans: statistical thermodynamic approach. Biophys J. 2008;95:4570–4583. doi: 10.1529/biophysj.108.133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nikolaev NI, Obradovic B, Versteeg HK, Lemon G, Williams DJ. A validated model of GAG deposition, cell distribution, and growth of tissue engineered cartilage cultured in a rotating bioreactor. Biotechnol Bioeng. 2010;105:842–853. doi: 10.1002/bit.22581. [DOI] [PubMed] [Google Scholar]
- 118.Nonaka H, Mita K, Watakabe M, Akataki K, Suzuki N, Okuwa T, Yabe K. Age-related changes in the interactive mobility of the hip and knee joints: a geometrical analysis. Gait Posture. 2002;15:236–243. doi: 10.1016/s0966-6362(01)00191-6. [DOI] [PubMed] [Google Scholar]
- 119.Obradovic B, Meldon JH, Freed LE, Vunjak-Novakovic G. Glycosaminoglycan deposition in engineered cartilage: Experiments and mathematical model. AIChE Journal. 2000;46:1860–1871. [Google Scholar]
- 120.Ofek G, Athanasiou K. Micromechanical properties of chondrocytes and chondrons: relevance to articular cartilage tissue engineering. Journal of Mechanics of Materials and Structures. 2007;2:1059–1086. [Google Scholar]
- 121.Ofek G, Natoli RM, Athanasiou KA. In situ mechanical properties of the chondrocyte cytoplasm and nucleus. J Biomech. 2009;42:873–877. doi: 10.1016/j.jbiomech.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peña E, Calvo B, Martínez MA, Doblaré M. Effect of the size and location of osteochondral defects in degenerative arthritis. A finite element simulation. Computers in Biology and Medicine. 2007;37:376–387. doi: 10.1016/j.compbiomed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 123.Peña E, Calvo B, Martínez MA, Doblaré M. Computer simulation of damage on distal femoral articular cartilage after meniscectomies. Comput Biol Med. 2008;38:69–81. doi: 10.1016/j.compbiomed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Peña E, Calvo B, Martinez MA, Palanca D, Doblaré M. Why lateral meniscectomy is more dangerous than medial meniscectomy. A finite element study. J Orthop Res. 2006;24:1001–1010. doi: 10.1002/jor.20037. [DOI] [PubMed] [Google Scholar]
- 125.Pierce DM, Trobin W, Trattnig S, Bischof H, Holzapfel GA. A phenomenological approach toward patient-specific computational modeling of articular cartilage including collagen fiber tracking. J Biomech Eng. 2009;131:091006. doi: 10.1115/1.3148471. [DOI] [PubMed] [Google Scholar]
- 126.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188–1194. [PubMed] [Google Scholar]
- 127.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 128.Sengers BG, Van Donkelaar CC, Oomens CWJ, Baaijens FPT. The local matrix distribution and the functional development of tissue engineered cartilage, a finite element study. Ann Biomed Eng. 2004;32:1718–1727. doi: 10.1007/s10439-004-7824-3. [DOI] [PubMed] [Google Scholar]
- 129.Sengers BG, van Donkelaar CC, Oomens CWJ, Baaijens FPT. Computational study of culture conditions and nutrient supply in cartilage tissue engineering. Biotechnol Prog. 2005;21:1252–1261. doi: 10.1021/bp0500157. [DOI] [PubMed] [Google Scholar]
- 130.Sengers BG, Heywood HK, Lee DA, Oomens CWJ, Bader DL. Nutrient utilization by bovine articular chondrocytes: a combined experimental and theoretical approach. J Biomech Eng. 2005;127:758–766. doi: 10.1115/1.1993664. [DOI] [PubMed] [Google Scholar]
- 131.Sengers BG, Oomens CW, Baaijens FP. An integrated finite-element approach to mechanics, transport and biosynthesis in tissue engineering. J Biomech Eng. 2004;126:82–91. doi: 10.1115/1.1645526. [DOI] [PubMed] [Google Scholar]
- 132.Sengers BG, Oomens CWJ, Nguyen TQD, Bader DL. Computational modeling to predict the temporal regulation of chondrocyte metabolism in response to various dynamic compression regimens. Biomech Model Mechanobiol. 2006;5:111–122. doi: 10.1007/s10237-006-0023-4. [DOI] [PubMed] [Google Scholar]
- 133.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, Schnitzer TJ, Kirwan-Mellis G, Andriacchi TP. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 134.Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37:1948–1956. doi: 10.1249/01.mss.0000180404.86078.ff. [DOI] [PubMed] [Google Scholar]
- 135.Shirazi-Adl A, Moglo KE. Effect of changes in cruciate ligaments pretensions on knee joint laxity and ligament forces. Comput Methods Biomech Biomed Engin. 2005;8:17–24. doi: 10.1080/10255840500062922. [DOI] [PubMed] [Google Scholar]
- 136.Shirazi R, Shirazi-Adl A. Computational biomechanics of articular cartilage of human knee joint: effect of osteochondral defects. J Biomech. 2009;42:2458–2465. doi: 10.1016/j.jbiomech.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 137.Sibole SC, Erdemir A. Chondrocyte deformations as a function of tibiofemoral joint loading predicted by a generalized high-throughput pipeline of multi-scale simulations. PLoS ONE. 2012 doi: 10.1371/journal.pone.0037538. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 139.Soulhat J, Buschmann MD, Shirazi-Adl A. A fibril-network-reinforced biphasic model of cartilage in unconfined compression. J Biomech Eng. 1999;121:340–347. doi: 10.1115/1.2798330. [DOI] [PubMed] [Google Scholar]
- 140.Stolz M, Raiteri R, Daniels AU, VanLandingham MR, Baschong W, Aebi U. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 2004;86:3269–3283. doi: 10.1016/S0006-3495(04)74375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, Sah RL. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthr Cartil. 2007;15:1042–1052. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 142.Torzilli PA, Bhargava M, Park S, Chen CTC. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthr Cartil. 2010;18:97–105. doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Torzilli PA, Deng XH, Ramcharan M. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol. 2006;5:123–132. doi: 10.1007/s10237-006-0030-5. [DOI] [PubMed] [Google Scholar]
- 144.van Turnhout MC, Kranenbarg S, van Leeuwen JL. Contribution of postnatal collagen reorientation to depth-dependent mechanical properties of articular cartilage. Biomech Model Mechanobiol. 2011;10:269–279. doi: 10.1007/s10237-010-0233-7. [DOI] [PubMed] [Google Scholar]
- 145.Urban JP. Present perspectives on cartilage and chondrocyte mechanobiology. Biorheology. 2000;37:185–190. [PubMed] [Google Scholar]
- 146.Vaziri A, Nayeb-Hashemi H, Singh A, Tafti BA. Influence of meniscectomy and meniscus replacement on the stress distribution in human knee joint. Ann Biomed Eng. 2008;36:1335–1344. doi: 10.1007/s10439-008-9515-y. [DOI] [PubMed] [Google Scholar]
- 147.Wan LQ, Guo XE, Mow VC. A triphasic orthotropic laminate model for cartilage curling behavior: fixed charge density versus mechanical properties inhomogeneity. J Biomech Eng. 2010;132:024504. doi: 10.1115/1.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Williams GM, Chan EF, Temple-Wong MM, Bae WC, Masuda K, Bugbee WD, Sah RL. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J Biomech. 2010;43:156–165. doi: 10.1016/j.jbiomech.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wilson W, van Donkelaar CC, Huyghe JM. A comparison between mechano-electrochemical and biphasic swelling theories for soft hydrated tissues. J Biomech Eng. 2005;127:158–165. doi: 10.1115/1.1835361. [DOI] [PubMed] [Google Scholar]
- 150.Wilson W, van Donkelaar CC, van Rietbergen B, Huiskes R. A fibril-reinforced poroviscoelastic swelling model for articular cartilage. J Biomech. 2005;38:1195–1204. doi: 10.1016/j.jbiomech.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 151.Wilson W, van Donkelaar CC, van Rietbergen B, Ito K, Huiskes R. Stresses in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study. J Biomech. 2004;37:357–366. doi: 10.1016/s0021-9290(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 152.Wilson W, van Donkelaar CC, van Rietbergen R, Huiskes R. The role of computational models in the search for the mechanical behavior and damage mechanisms of articular cartilage. Med Eng Phys. 2005;27:810–826. doi: 10.1016/j.medengphy.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 153.Wilson W, Huyghe JM, van Donkelaar CC. A composition-based cartilage model for the assessment of compositional changes during cartilage damage and adaptation. Osteoarthr Cartil. 2006;14:554–560. doi: 10.1016/j.joca.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 154.Wilson W, Huyghe JM, van Donkelaar CC. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomech Model Mechanobiol. 2007;6:43–53. doi: 10.1007/s10237-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 155.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 156.Wong BL, Sah RL. Mechanical asymmetry during articulation of tibial and femoral cartilages: local and overall compressive and shear deformation and properties. J Biomech. 2010;43:1689–1695. doi: 10.1016/j.jbiomech.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu JZ, Herzog W. Analysis of the mechanical behavior of chondrocytes in unconfined compression tests for cyclic loading. J Biomech. 2006;39:603–616. doi: 10.1016/j.jbiomech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 158.Wu JZ, Herzog W, Epstein M. Modelling of location- and time-dependent deformation of chondrocytes during cartilage loading. J Biomech. 1999;32:563–572. doi: 10.1016/s0021-9290(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 159.Yang NH, Canavan PK, Nayeb-Hashemi H. The effect of the frontal plane tibiofemoral angle and varus knee moment on the contact stress and strain at the knee cartilage. J Appl Biomech. 2010;26:432–443. doi: 10.1123/jab.26.4.432. [DOI] [PubMed] [Google Scholar]
- 160.Yang NH, Nayeb-Hashemi H, Canavan PK, Vaziri A. Effect of frontal plane tibiofemoral angle on the stress and strain at the knee cartilage during the stance phase of gait. J Orthop Res. 2010;28:1539–1547. doi: 10.1002/jor.21174. [DOI] [PubMed] [Google Scholar]
- 161.Yang N, Nayeb-Hashemi H, Canavan PK. The combined effect of frontal plane tibiofemoral knee angle and meniscectomy on the cartilage contact stresses and strains. Ann Biomed Eng. 2009;37:2360–2372. doi: 10.1007/s10439-009-9781-3. [DOI] [PubMed] [Google Scholar]
- 162.Yao J, Salo AD, Lee J, Lerner AL. Sensitivity of tibio-menisco-femoral joint contact behavior to variations in knee kinematics. Journal of Biomechanics. 2008;41:390–398. doi: 10.1016/j.jbiomech.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 163.Ytterberg SR, Mahowald ML, Krug HE. Exercise for arthritis. Baillieres Clin Rheumatol. 1994;8:161–189. doi: 10.1016/s0950-3579(05)80230-4. [DOI] [PubMed] [Google Scholar]
- 164.Zielinska B, Donahue TLH. 3D finite element model of meniscectomy: changes in joint contact behavior. J Biomech Eng. 2006;128:115–123. doi: 10.1115/1.2132370. [DOI] [PubMed] [Google Scholar]