Abstract
A series of N-(2-amino-6-trifluoromethyl-pyridin-3-ylmethyl) 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamides were designed combining previously identified pharmacophoric elements and evaluated as hTRPV1 antagonists. The SAR analysis indicated that specific hydrophobic interactions of the 2-amino substituents in the C-region of the ligand were critical for high hTRPV1binding potency. In particular, compound 49S was an excellent TRPV1 antagonist (Ki(CAP) = 0.2 nM; IC50(pH) = 6.3 nM) and was thus ca. 100- and 20-fold more potent, respectively, than the parent compounds 2 and 3 for capsaicin antagonism. Furthermore, it demonstrated strong analgesic activity in the rat neuropathic model superior to 2 with almost no side effects. Compound 49S antagonized capsaicin induced hypothermia in mice, but showed TRPV1-related hyperthermia. The basis for the high potency of 49S compared to 2 is suggested by docking analysis with our hTRPV1 homology model in which the 4-methylpiperidinyl group in the C-region of 49S made additional hydrophobic interactions with the hydrophobic region.
INTRODUCTION
The transient receptor potential V1 (TRPV1) receptor is a molecular integrator of nociceptive stimuli, located predominantly in primary sensory neurons.1 The receptor functions a ligand-gated and non-selective cation channel with high Ca2+ permeability, activated by endogenous agonists including protons,2 noxious heat,3 inflammatory lipid mediators such as anandamide4 and lipoxygenase products,5 as well as by natural products such as capsaicin (CAP)6 and resiniferatoxin (RTX)7.
The increase in intracellular Ca2+ upon TRPV1 activation causes excitation of the primary sensory neurons and the consequent central perception of pain. TRPV1 antagonists inhibit this transmission of nociceptive signaling from the periphery to the CNS as well as block other pathological states associated with this receptor. In recent years a number of TRPV1 antagonists have been developed as novel analgesic and antiinflammatory agents, particularly for the treatment of chronic pain and inflammatory hyperalgesia.8 The clinical development and therapeutic potential of TRPV1 antagonists have been extensively reviewed.9–13
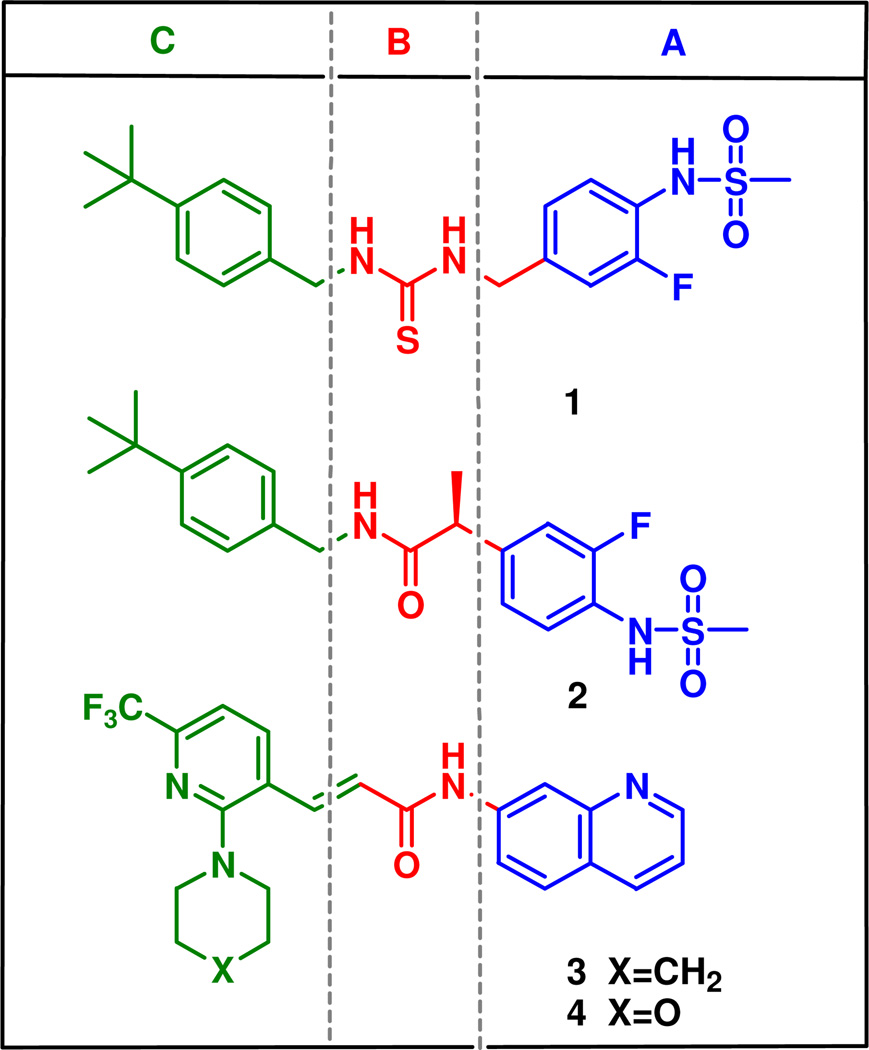
Previously, we identified a potent and stereospecific antagonist, (S)-N-(4-t-butylbenzyl) 2-(3-fluoro-4-methanesulfonylaminophenyl) propanamide (2),14 which exhibited better binding affinity and more potent antagonism for both rTRPV1 and hTRPV1 in CHO cells compared to the prototype thiourea antagonist 115 (Figure 1). Its activity was stereospecific, with marked selectivity for the (S)-configuration; whereas the (S)-isomer was ca. two-fold more potent than the racemate, the (R)-isomer was 30- to 40-fold weaker. A docking study of 2 with our hTRPV1 homology model demonstrated a novel aspect of its binding to the receptor and identified crucial hydrogen bonds between the ligand and the receptor contributing to its stereospecific potency.14
Figure 1. Lead TRPV1 antagonists.
To further optimize the antagonistic acitivity of the lead 2, structural modifications were performed based on the three principal pharmacophores (A-region: (4-methylsulfonylamino)phenyl, B-region: propanamide, C-region: 4-t-butylbenzyl), respectively. However, none of the compounds was found to be better than 2 in terms of both binding affinity and antagonism to capsaicin activation for rTRPV1 in CHO cells.14,16
A series of N-aryl cinnamides had been reported by the Amgen group17 as potent antagonists for rat-human chimeric TRPV1 expressed in CHO cells. In that series, compounds 3 and 4 (Figure 1), which had 2-(piperidin-1-yl and morpholino)-6-(trifluoromethyl)pyridin-3-yl moieties respectively, exhibited potent antagonism of activation of TRPV1 by capsaicin and acid and further displayed good oral bioavailability.
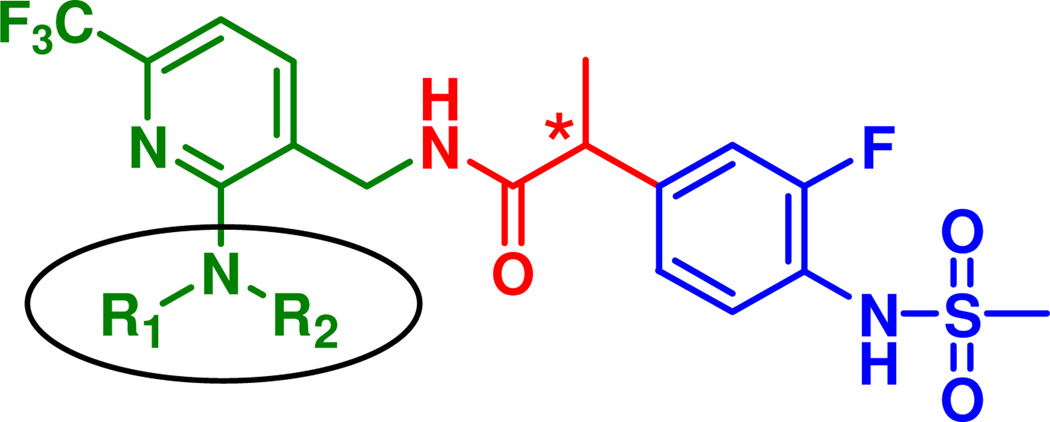
Combining the 2-(3-fluoro-4-methylsulfonaminophenyl) propanamide (the A and B regions in 2) and 2-(piperidinyl or morpholinyl)-6-trifluoromethyl-pyridin-3-ylmethyl groups (C-region of 3 and 4) provided the novel designed compounds 45 and 97. Their syntheses and biological evaluation indicated that they exhibited highly potent antagonism toward both capsaicin and pH for hTRPV1 in CHO cells, in which compound 45 and 97 showed 46- and 15-folds enhanced potency in capsaicin antagonism compared to parent 2, respectively. This preliminary result prompted us to investigate extensively the structure activity relationship of this template.
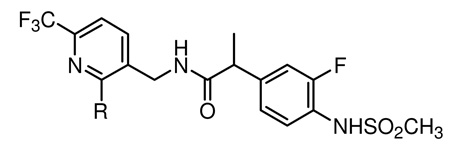
In this study, we investigated the structure activity relationships for 2-amino substituted analogues (12–99) of the template N-(6-trifluoromethyl-pyridin-3-ylmethyl) 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide (Figure 2) as hTRPV1 antagonists and we further characterized analgesic activity in a neuropathic pain model and performed molecular modeling with our hTRPV1 homology model for the most potent antagonist in the series.
Figure 2. General structure of designed compounds.
RESULTS AND DISCUSSION
Chemistry
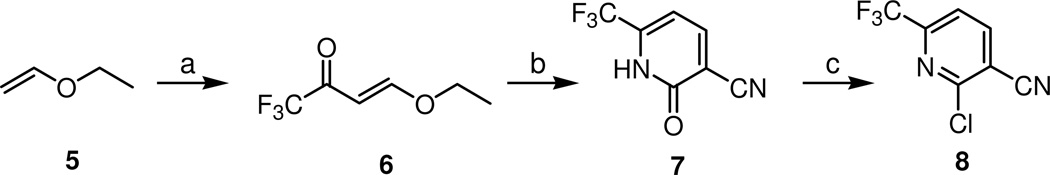
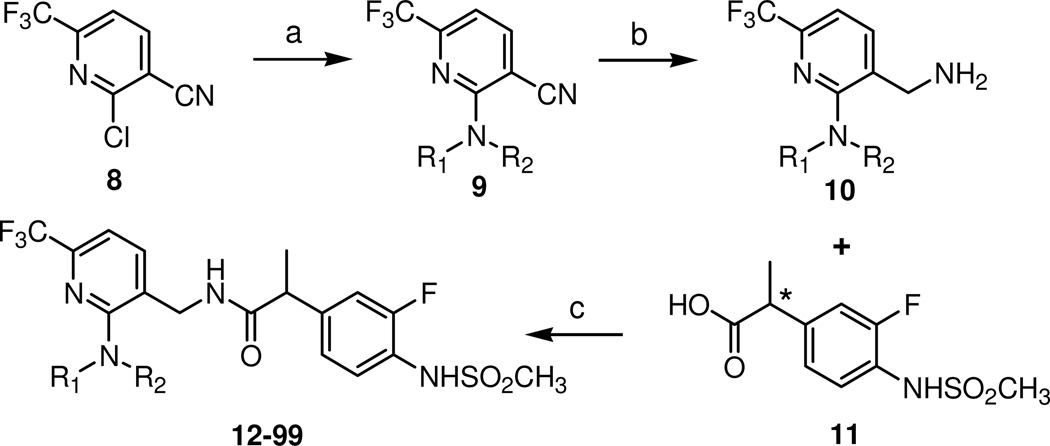
The key intermediate of the C-region 8 was synthesized starting from ethylvinyl ether 5 in three steps by a modification of the previously report procedure,18 as shown in Scheme 1. Trifluoromethylacetylation of 5 followed by condensation with cyanoacetamide provided pyridone 7, which was readily converted to 2-chloropyridine by POCl3. The syntheses of final compounds are represented in Scheme 2. A library of amines was reacted with 8 to afford 2-amino substituted pyridines 9 and then their nitrile groups were reduced to yield the corresponding primary amines 10.17 The amines were coupled with racemic or chiral 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide 11 to give the final compounds 12–99, respectively.
Scheme 1. Synthesis of 2-chloro-3-cyano-6-trifluoromethylpyridine.
Reagents and conditions: (a) (CF3CO)2O, pyridine, CHCl3; (b) NCCH2CONH2, K2CO3, toluene; (c) POCl3
Scheme 2. Syntheses of 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide analogues.
Reagents and conditions: (a) [Method A] neat NR1R2: [Method B] NR1R2, Pd(OAc)2, dppf, Na2CO3, toluene-THF (7:1): [Method C] NR1R2, K2CO3, 18-C-6, CH3CN: [Method D] DBU, CH3CN; (b) [Method A] 2M BH3-SMe2 in THF: [Method B] NaBH4, NiCl2-6H2O (or CoCl2-6H2O), MeOH: [Method C] H2, 10% Pd-C, c-HCl, MeOH; (c) EDC, HOBt, TEA, CH3CN
Structure-Activity Relationship (SAR) Analysis
The synthesized TRPV1 ligands were evaluated in vitro for antagonism as measured by inhibition of activation by four separate stimuli - capsaicin (CAP), pH, heat and N-acetyldopamine (NADA) as indicated. The assays were conducted using a fluorometric imaging plate reader (FLIPR) with human TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells. The results are summarized in Tables 1–5, together with the potencies of the previously reported parent antagonists 1–4.
Table 1.
In vitro hTRPV1 Antagonistic Activities for 2-Acylic Amino Derivatives
 | |||||||
|---|---|---|---|---|---|---|---|
| R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
||
| 1 | 26 | NE | 21 | 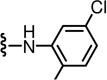 |
14 | 140 | |
| 2 | 20 | 1210 | 22 | 15.8 | 203 | ||
| 3 | 4.4 | NE | 23 | 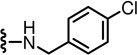 |
65.7 | 414 | |
| 4 | 1.2 | 116 | 24 | 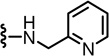 |
WE | WE | |
| 12 | H | WE | NE | 25 | NE | NE | |
| 13 | 9.1 | 293 | 26 | WE | WE | ||
| 14 | 20.4 | 260 | 27 | 19.1 | 2390 | ||
| 15 | 27.2 | 1130 | 28 | 3.5 | 148 | ||
| 16 | 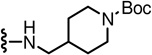 |
19.3 | 1140 | 29 | 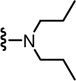 |
0.2 | 14.7 |
| 17 | 59.6 | 1400 | 30 | 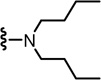 |
0.6 | 10.5 | |
| 18 | 39.2 | WE | 31 | 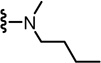 |
0.8 | 21.3 | |
| 19 | WE | WE | 32 |  |
0.9 | 57.4 | |
| 20 | 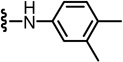 |
39.3 | 1160 | ||||
Table 5.
In vitro hTRPV1 Antagonistic Activities for 2 and 49S to multiple activators
| Activators | 2 (nM) |
49S (nM) |
|---|---|---|
| CAP (f)Ki | 28 | 0.2 |
| pH (IC50) | 1281 | 6.3 |
| Heat 45°C (IC50) | 174 | 0.8 |
| NADA (f)Ki | 6.6 | 0.01 |
The 2-unsubstituted analogue 12 was initially prepared but was found to be a weak antagonist, indicating that a hydrophobic substituent at the 2-position was necessary for substantial activity. The SAR for derivatives incorporating an amino group into the 2-position of the pyridine C-region was investigated starting from 2-acyclic amino derivatives (Table 1). The secondary amino derivatives, incorporating alkylamino 13–16, arylamino 17–21 and benzylamino 22–26 groups, showed reasonable potencies for CAP antagonism, with values for Ki(CAP) ranging from 9.1 to 65.7 nM. Exceptions were the 4-chloroanilino 19 and pyridinylmethylamino 24–26 analogues, which were devoid of activity.
Tertiary acyclic amino analogues 27–32 were also examined. The activity was enhanced as the number of carbons in the chain increased, and the derivatives exhibited much better potencies than the corresponding secondary amino surrogates. (for example, 13 vs 28 and 14 vs 29). In particular, the dipropylamino analogue 29 and the dibutylamino analogue 30 displayed excellent antagonism toward both CAP and pH (29: Ki(CAP) = 0.2 nM, IC50(pH) = 14.7 nM; 30: Ki(CAP) = 0.6 nM, IC50(pH) = 10.5 nM). The methylbutylamino analogue 31 (5-carbons) showed a potency intermediate between 28 (4-carbons) and 29 (6-carbons), suggesting that the lipophilicity is a contributor to the activity. The methylphenylamino analogue 32 showed a significant increase in potency (75-fold for CAP, 25-fold for pH) compared to the phenylamino analogue 17, indicating that an NH in a secondary amino group was detrimental to antagonism.
Next, we sought to evaluate the SAR for mono-azacyclic rings. The introduction of unsubstituted azacyclic rings, including 1-pyrrolidinyl 34, 1-piperidinyl 45, 1-azepanyl 79 and 1-azocanyl 80 analogues, provided potent antagonists with subnanomolar activities (Ki(CAP) = 0.43–1.3 nM) regardless of ring size.
In the SAR of substituted 1-pyrrolidinyl analogues (Table 2), we restricted our syntheses to the chiral propionic acid 11S at the condensation step because the substituted pyrrolidines were themselves chiral. The 2-methylpyrrolidine analogue 34 showed enhanced antagonism to CAP but markedly reduced antagonism to pH compared to the pyrrolidine analogue 33. The structural modification of the 2- or 3-positions of pyrrolidine all resulted in a similar SAR pattern in which the hydrophilic substituents (35–36, 38, 40–41) led to the loss of activity whereas the hydrophobic ones (37, 39, 42–44) retained potency. The stereochemistry of the substituents did not affect the antagonism (40 vs 41 and 42 vs 43).
Table 2.
In vitro hTRPV1 Antagonistic Activities for 2-Pyrrolidinyl Derivatives
| R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
||
|---|---|---|---|---|---|---|---|
| 33 | 0.6 | 211 | 39* | 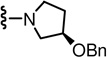 |
1.4 | 50 | |
| 34* | 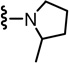 |
0.3 | WE | 40* | 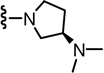 |
101 | WE |
| 35* | 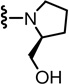 |
WE | NE | 41* | 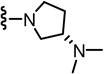 |
103 | WE |
| 36* | 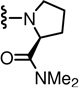 |
WE | NE | 42* | 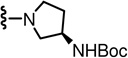 |
11 | 757 |
| 37* | 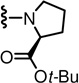 |
2.6 | 209 | 43* | 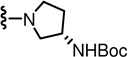 |
7.1 | 737 |
| 38* | 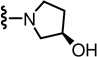 |
WE | WE | 44 | 5 | 192 |
(S)-propanamide
The SAR of six-membered azacyclic analogues was investigated next (Table 3). The 1-piperidinyl analogue 45 exhibited excellent antagonism; its activity was stereospecific, with the (S)-isomer 45S (Ki(CAP) = 0.3 nM) representing the active configuration. The potency of 45S was ca. 15-fold higher than that of the lead 3, which has the same C-region, indicating that the 2-(3-fluoro-4-methylsulfonaminophenyl) propanamide template for the A and B-regions was superior to the arylcinnamide for antagonism. The tetrahydropyridinyl analogue 46 was highly potent like 45. The methylpiperidinyl derivatives 47–49 were examined and the 4-methyl-1-piperidinyl analogue 49 exhibited stereospecific, potent antagonism toward both CAP and pH. The active isomer 49S was found to be the most potent antagonist in this study with Ki(CAP) = 0.2 nM and IC50(pH) = 6.3 nM. Its potency was thus 100-fold and 200-fold better than the reference propamide 2 for CAP and pH antagonism, respectively. The structural analysis comparing 2 and 49S indicated that the additional 4-methylpiperidine moiety in 49S provided a new hydrophobic interaction with the receptor, which could explain the enhanced potency of 49S. The docking analysis using our hTRPV1 homology model will be described in the next section.
Table 3.
In vitro hTRPV1 Antagonistic Activities for 2-Piperidinyl Derivatives
| R | Ki[CAP] (nM) |
IC50 [pH] (nM) |
R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
||
|---|---|---|---|---|---|---|---|
| 45 | 0.43 | 218 | 60 | 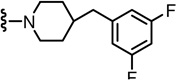 |
0.4 | 24 | |
| 45S | 0.3 | 46.7 | 61 | 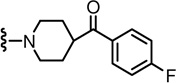 |
3.9 | 990 | |
| 46 | 0.3 | 12.1 | 62 | 0.5 | 19.8 | ||
| 47 | 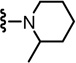 |
2.9 | WE | 63 | 0.6 | 31 | |
| 48 | 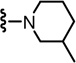 |
0.8 | WE | 64 | 91 | WE | |
| 49 | 0.3 | 8.4 | 65 | NE | NE | ||
| 49S | 0.2 | 6.3 | 66 | 2.4 | 280 | ||
| 49R | 9.8 | 254 | 67 | 1.3 | 612 | ||
| 50 | 1.5 | 97.3 | 68 | 1.4 | 31 | ||
| 51 | 0.3 | 8 | 69 | 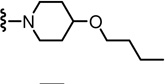 |
3.5 | 28.2 | |
| 52 | 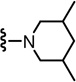 |
0.7 | 25.6 | 70 | 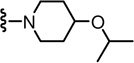 |
3 | WE |
| 53 | 0.6 | 25 | 71 | 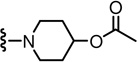 |
4 | 139 | |
| 53S | 0.3 | 17.4 | 72 | 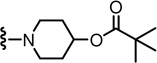 |
2 | 45.2 | |
| 53R | 7.5 | 2350 | 73 | 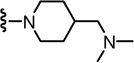 |
2.1 | 426 | |
| 54 | 1.7 | 43 | 74 | 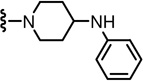 |
4.4 | 480 | |
| 55 | 4.2 | 81 | 75 | WE | WE | ||
| 56 | 2.4 | 159 | 76 | WE | WE | ||
| 57 | 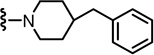 |
0.2 | 40 | 77 | 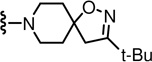 |
3.8 | 281 |
| 58 | 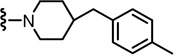 |
0.2 | 17 | 78 | 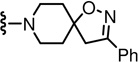 |
6.1 | 289 |
| 59 | 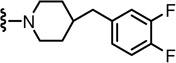 |
0.2 | 30 |
The impressive potency of 49 prompted us to investigate a variety of 4-substituted piperidinyl analogues, 50–78. As demonstrated in the SAR of the pyrrolidines, most hydrophobic 4-substituents provided significant antagonism. Conversely, incorporation of hydrophilic substituents, such as the 4-keto 64, the 4-hydroxyl 65, the 4-pyrrolidinyl 75 and the 4-piperidinyl 76, caused a dramatic loss of activity. Interestingly, a series of 4-benzyl-1-piperidinyl analogues, 57–60, exhibited great potencies, suggesting that there is a large hydrophobic pocket in TRPV1 sufficient to accommodate the benzyl group.
As piperidine surrogates, the 4-substituted piperazinyl 81–96 and morpholinyl 97–99 analogues were examined (Table 4). The 1-piperazinyl analogue 81 proved to be a weak antagonist; the 4-methylpiperidyl analogue 82 showed slightly better activity. The introduction of a more lipophilic group into the 4-position of piperazine led to an improvement in the antagonism, except in the case of the 4-pyridyl analogue 92, confirming that a hydrophobic substituent at the 4-position is a determinant for high potency. Among the morpholinyl analogues, the 1-morpholinyl analogue 97 and the 2,6-dimethyl morpholinyl analogue 98 exhibited potent antagonism; the 4,4-dioxothiomorpholinyl analogue 99 showed only weak potency reflecting its polar sulfonyl group.
Table 4.
In vitro hTRPV1 Antagonistic Activities for 2-Piperazinyl and 2-Morpholinyl Pyridines
| R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
R | Ki [CAP] (nM) |
IC50 [pH] (nM) |
||
|---|---|---|---|---|---|---|---|
| 79 | 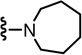 |
0.8 | 25.1 | 90 | 2.5 | 44.5 | |
| 80 |  |
0.7 | 27.8 | 91 | 1.4 | 108 | |
| 81 | WE | WE | 92 | WE | WE | ||
| 82 | 146 | WE | 93 | 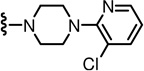 |
2.1 | 117 | |
| 83 | 21.6 | WE | 94 | 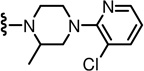 |
4.1 | WE | |
| 84 | 0.7 | 30.7 | 95 | 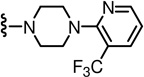 |
2.8 | WE | |
| 85 | 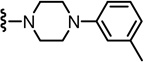 |
3.4 | 39.2 | 96 | 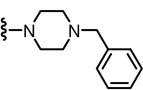 |
8.8 | 634 |
| 86 | 3.3 | 38.3 | 97 | 1.3 | 169 | ||
| 87 | 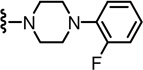 |
2.4 | 34 | 98 | 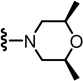 |
1.43 | 1090 |
| 88 | 1.1 | 23.1 | 99 | WE | WE | ||
| 89 | 2.3 | 117 |
Detailed in vitro activity of 49S, the most potent antagonist in this study, was investigated for multiple TRPV1 activators including capsaicin, pH, heat (45°C) and NADA, and compared to the activity of lead 2 (Table 5). Compound 49S showed excellent antagonism of all four TRPV1 activators and was ca. 140–660 fold more potent than 2.
Selectivity of compound 49S was assessed at a concentration of 10 µM against a panel of 135 other receptors and enzymes (CEREP). Even at this concentration 4 orders of magnitude higher than its Ki for capsaicin, 49S was negative for all but 7 targets and gave greater than 50% inhibition for only 3. While detailed mechanistic studies were not carried out, we confirmed that 49S inhibited [3H]resiniferatoxin binding to human TRPV1 (data not shown), as has been repeated observed for structurally related TRPV1 antagonists. [3H]Resiniferatoxin binding provides a convenient measure for ligand interaction at the capsaicin binding site on TRPV1. We conclude that 49S is exerting its antagonistic activity, as fully expected, at the capsaicin binding site rather than as a channel blocker.
In vivo Activity
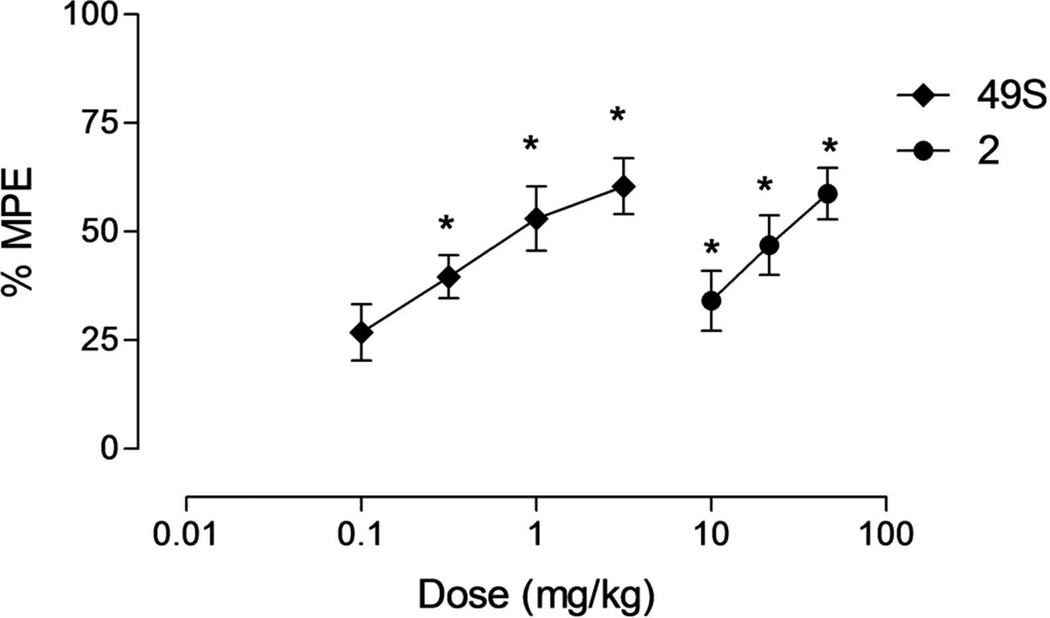
As part of the initial in vivo characterization of 49S, analgesic activity of 49S was evaluated orally in the rat Bennett model19 as a neuropathic pain model and its activity was compared to that of parent 2 (Figure 3). The analgesic potency of 49S demonstrated dose-dependent efficacy with ED50 = 0.9 mg/Kg po (max 60% at 3.16 mg/Kg) and was superior to 2. Side effects like sedation or decreased locomotion were not observed.
Figure 3.
Effect of compound 2 (30 min after po administration) and compound 49S (45 min after po administration) on CCI-induced cold allodynia in rats. Data are presented as mean ± SEM, * p<0.05 vs vehicle.
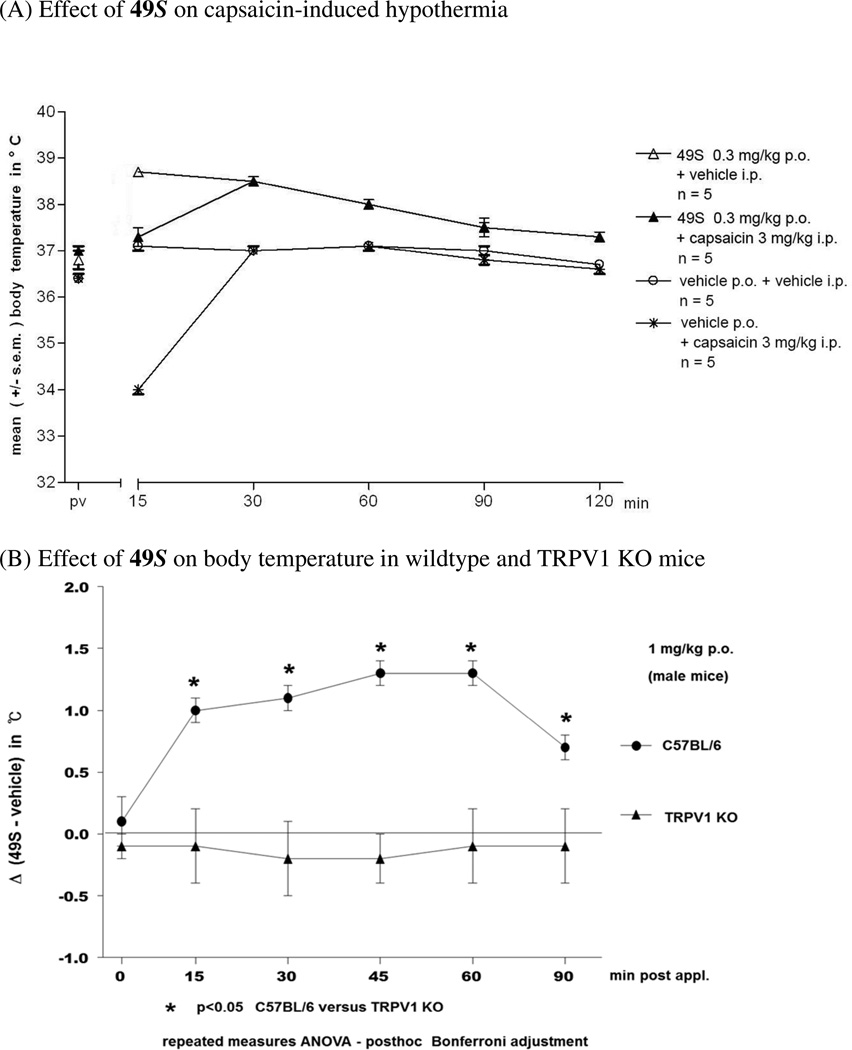
Consistent with its in vitro mechanism of action, in vivo 49S also blocked response to capsaicin (Figure 4A). The intraperitoneal injection of 3 mg/kg capsaicin resulted in a decrease of body temperature as expected,20,21 with a reduction from 37.1 ± 0.1 to 34.0 ± 0.1 °C (15 min post capsaicin injection; p < 0.05). By 30 min body temperature had returned to normal. The oral administration of 0.3 mg/kg 49S (15 min before capsaicin injection) completely inhibited the effect of capsaicin on body temperature (Fig. 4A).
Figure 4.
Effect of compound 49S on body temperature
Furthermore, we could demonstrate that 49S targeted TRPV1 in vivo. Although TRPV1 knockout mice show normal body temperature,22 induction of modest hyperthermia is a common acute side effect of administration of TRPV1 antagonists.23 We therefore examined the effect on body temperature of administration of 49S. As illustrated in Figure 4A/B, oral administration of 1 mg/kg 49S in C57BL/6J wild type mice induced an body temperature increase of about 1.3 ± 0.1 °C 45 min and 60 min post substance administration. In TRPV1 knockout mice (Figure 4B) this hyperthermic response was completely absent.
Molecular Modeling
Compound 49S was identified as a highly potent antagonist showing ca. 100-fold greater potency compared to prototype 2, with this enhancement in potency being attributable to an additional 4-methylpiperidinyl group in 49S. To clarify the basis for this enhanced activity, we used our hTRPV1 homology model to compare the binding interactions of 49S and 2.
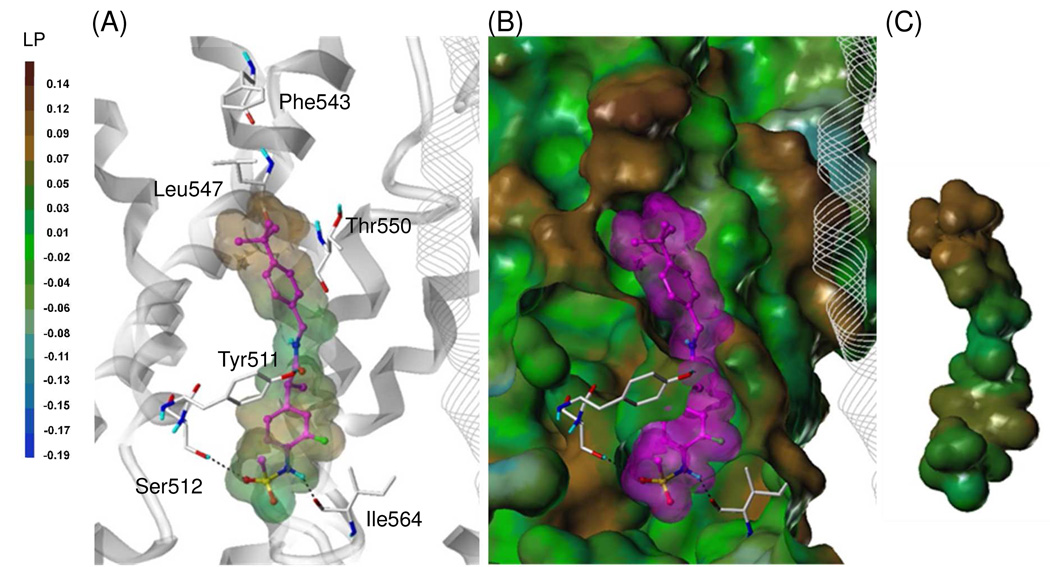
We constructed the tetramer homology model of human TRPV1 (hTRPV1) based on our rat TRPV1 model24 through in silico mutation and refinement by energy minimization. Using our hTRPV1 model, we then performed the flexible docking study of 2 and 49S to investigate their binding interactions and found compound 2 fitted well into the binding site (Figure 5). The sulfonylaminobenzyl group (A-region) occupied the deep bottom hole and was involved in a hydrophobic interaction with Tyr511. An oxygen atom and NH of the sulfonamide group participated in hydrogen bonding with Ser512 and Ile564. The amide group (B region) made a hydrogen bond with Tyr511 and also contributed to the appropriate positioning of the C-region for the hydrophobic interaction. The hydrophobic 4-t-butylbenzyl group (C-region) oriented toward the upper hydrophobic region of the binding site and formed the hydrophobic interaction with Leu547.
Figure 5.
Flexible docking of 2 in the hTRPV1 model.
(A) Binding mode of 2. The key residues are marked and displayed as capped-stick with carbon atoms in white. The helices are colored in gray and the helices of the adjacent monomer are displayed in line ribbon. The ligand is depicted as ball-and-stick with carbon atoms in magenta. The van der Waals surface of the ligand is presented with its lipophilic potential property. Hydrogen bonds are shown as black dashed lines and non-polor hydrogens are undisplayed for clarity. (B) Surface representations of the docked ligand and hTRPV1. The Fast Connolly surface of hTRPV1 was generated by MOLCAD and colored by the lipophilic potential property. The surface of hTRPV1 is Z-clipped and that of the ligand is in its carbon color for clarity. (C) Van dar Waals surface of the ligand colored by its lipophilic potential property.
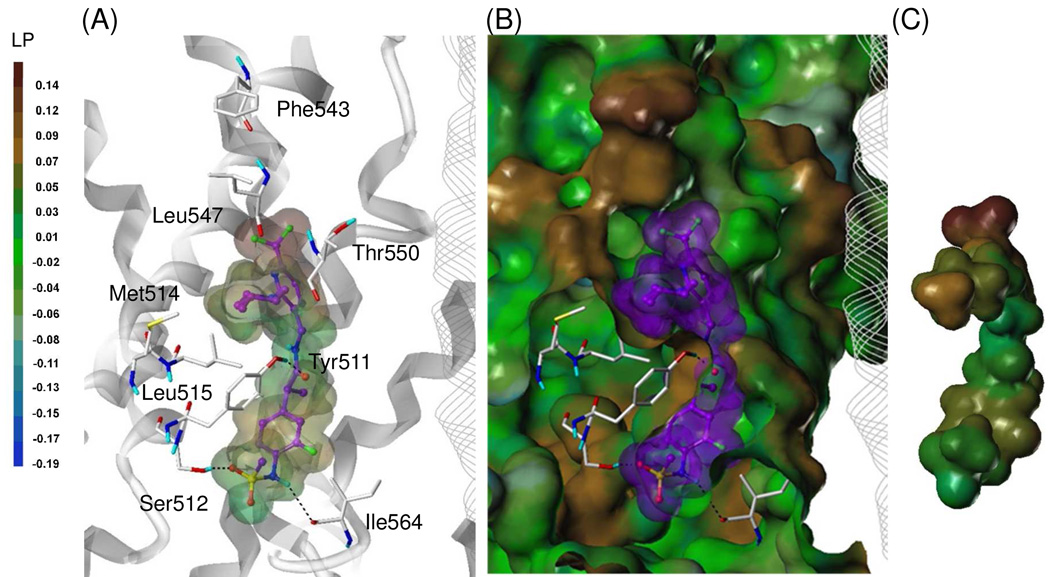
As we expected, compound 49S showed an excellent fit to the binding site and its 4-methylpiperidine ring in the C-region made an additional hydrophobic interaction with hTRPV1 (Figure 6). The sulfonylaminophenyl and amide moieties (A- and B-regions) made tight interactions with the binding site residues via the hydrophobic interaction with Tyr511 and hydrogen bonding with Tyr511, Ser512, and Ile564 as shown for 2. The 3-trifluromethyl group in the C-region, like the 4-t-butylbenzyl group of 2, formed a hydrophobic interaction with Leu547. Furthermore, the 4-methylpiperidinyl group in the C-region made an additional hydrophobic interaction with the hydrophobic region composed of Met514 and Leu515. That might explain why the activity of 49S was dramatically increased, compared with that of 2.
Figure 6.
Flexible docking of 49S in the hTRPV1 model.
(A) Binding mode of 49S. (B) Surface representations of the docked ligand and hTRPV1. (C) Van dar Waals surface of the ligand colored by its lipophilic potential property. The ligand is depicted as ball-and-stick with carbon atoms in purple; the details are the same as in Figure 5.
CONCLUSION
A variety of 2-amino substituted 6-trifluoromethyl-pyridin-3-yl-methylamines as the C-region were coupled with the well characterized A,B-region TRPV1 antagonistic template 2-(3-fluoro-4-methylsulfonylaminophenyl) propionic acid to provide a series of propanamides for evaluation as potent TRPV1 antagonists (12–99). The analysis of structure activity relationship indicated that a critical feature of the 2-amino substituents for activity was that they support a hydrophobic interaction with the receptor. Among the compounds of the series, compound 49S showed the best TRPV1 antagonism, blocking the activations by capsaicin, pH, heat and NADA. It was selective, and it demonstrated strong analgesic activity in a neuropathic pain model with almost no side effects. Consistent with its action in vivo being through TRPV1, compound 49S blocked capsacin-induced hypothermia but caused modest TRPV1-related hyperthermia in mice. The docking study with our homology model indicated that 49S showed an excellent fit to the binding site in which the sulfonylaminobenzyl and propanamide moieties interacted with Tyr511, Ser512, and Ile564 as found in 2 and the 4-methylpiperidinyl group in the C-region made an additional hydrophobic interaction with the hydrophobic region composed of Met514 and Leu515, resulting in its high potency.
EXPERIMENTAL SECTION
General
All chemical reagents were commercially available. Melting points were determined on a Büchi Melting Point B-540 apparatus and are uncorrected. Silica gel column chromatography was performed on silica gel 60, 230–400 mesh, Merck. Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were recorded on a JEOL JNM-LA 300 [300 MHz (1H), 75 MHz (13C)] and Bruker Avance 400 MHz FT-NMR [400 MHz (1H), 100 MHz(13C)] spectrometer. Chemical shifts are reported in ppm units with Me4Si as a reference standard. Infrared (IR) spectra were recorded on a JASCO FT/IR-4200 spectrometer. Mass spectra were recorded on a VG Trio-2 GC-MS and 6460 Triple Quad LC/MS. All final compounds were purified to >95% purity, as determined by high-performance liquid chromatography (HPLC). HPLC was performed on an Agilent 1120 Compact LC (G4288A) instrument using a Agilent Eclipse Plus C18 column (4.6 × 250 mm, 5 µm) and Daicel Chiralcel OD-H column (4.6 ×250 mm, 5 µm). Optical rotations were measured in a JASCO DIP-2000 digital polarimeter.
2-Hydroxy-6-(trifluoromethyl)nicotinonitrile (7)
To a ice cold solution of ethyl vinyl ether (9.58 mL, 0.1 mol), pyridine (8.1 mL, 0.1 mmol) in CHCl3 (anhydrous 100 mL) was added trifluroacetic anhydride (21 g, 0.1 mol) at 0 °C. The reaction mixture was gradually stirred at 0 °C to room temperature for 5 h. The mixture was concentrated in vacuo to obtain crude (E)-4-ethoxy-1,1,1-trifluorobut-3-en-2-one 6 (10.92 g, 65%) as a light yellow liquid, which was directly used for the next step without further purification.
A mixture of 6 (10 g, 59.5 mmol), 2-cyanoacetamide (5 g, 59.5 mmol) and K2CO3 (10.37 g, 75 mmol) in toluene (200 mL) was refluxed under a Dean-Stark trap for 9 h. The progress of the reaction was monitored by TLC (40% EtOAc/hexane, Rf~0.2). After the starting material disappeared, the reaction mixture was concentrated in vacuo to give the residue which was purified by flash column chromatography on silica gel using EtOAc:hexane (1:25) as eluant to yield compound 7 (8.6 g, 77%) as a yellow solid. mp 210–215 °C, 1H-NMR (300 MHz, CD3OD) δ 8.02 (d, J = 7.5 Hz, 1H), 6.97 (d, J = 7.68 Hz, 1H); 13C-NMR (100 MHz, CD3OD) δ 169.27, 151.01-149.98 (q, J = 34.3), 147.42, 127.00-118.83 (q, J = 272.4), 118.16, 109.05, 102.67; IR (KBr) 3408, 2825, 2704, 2231, 1827, 1680, 1590, 1562, 1484, 1437, 1348, 1312, 1290, 1218, 1199, 1180, 1153, 1116, 1095 cm−1; LC-MS (ESI) m/z 189 (MH+).
2-Chloro-6-(trifluoromethyl)nicotinonitrile (8)
A mixture of 7 (7.52 g, 40 mmol) and POCl3 (26 mL, 280 mmol) was refluxed for 3 h. The reaction mixture was cooled and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:20) as eluant to yield compound 8 (4.28 g, 52%) as a white solid. mp 37–40 °C 1H-NMR (300 MHz, CDCl3) δ 8.22 (d, J = 7.9 Hz, 1H), 7.77 (d, J = 7.9 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 153.19, 151.23-150.13 (q, J = 36.8), 144.42, 123.87-115.67 (q, J = 273.5), 119.11-119.04 (q, J = 2.7), 113.94, 113.47; IR (KBr) 3177, 3084, 3036, 2921, 2832, 2241, 1946, 1833, 1709, 1590, 1567, 1457, 1364, 1337, 1250, 1226, 1192, 1155, 1109, 1077 cm−1; LC-MS (ESI) m/z 207 (MH+).
General Procedure for Amination (9)
Method A (for compounds 13–16, 22, 27–31, 44)
A mixture of 2-chloro-6-trifluoromethyl-nicotinonitrile (1.0 mmol) was dissolved in amine (10.0 mmol). The mixture was stirred for 16 h at r.t. The reaction mixture was extracted with EtOAc (30 mL) twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:15) as eluant.
Method B (for compounds 17–21, 23, 32, 46)
A mixture of 2-chloro-6-trifluoromethyl nicotinonitrile (1.0 mmol), bis(triphenylphosphine)-palladium (II) dichloride (0.10 mmol) and copper iodide (0.20 mmol) were dissolved in 1-methyl-2-pyrrolidinone (10 mL). After 10 min stirring, alkyene and N,N-diisopropyl ethyl amine (2.0 mmol) were added to the mixture. The mixture was stirred for 12 h at 90–110 °C. The reaction was filtered over Celite and extracted with ether twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:15) as eluant.
Method C (for compounds 24–26, 33–43, 54, 57–61, 73–99)
A mixture of 6-tert-butyl-2-hydroxy-nicotinonitrile (1.0 mmol) and 1-bromo pentane (2.0 mmol), 18-crown-6-ether (cat.) and K2CO3 (4.0 mmol) were dissolved in CH3CN : DMF (1:2) solution. The mixture was refluxed for 12 h then cooled to room temperature. The mixture was extracted with EtOAc (30 mL). The organic phase was dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:1) as eluant.
Method D (for compounds 45, 47–53, 55–56, 62–72)
A mixture of 2-chloro-6-trifluoromethyl-nicotinonitrile (1.0 mmol), piperidine (2.0 mmol), and DBU (2.0 mmol) were dissolved in acetonitrile (10 mL). The mixture was stirred for 12 h at r.t. The reaction mixture was extracted with EtOAc (30 mL) twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:15) as eluant.
General Procedure for Reduction (10)
Method A (for compounds 17–21, 23–26, 32–35, 37–53, 57–81, 83, 85–86, 89–90, 93–99)
To a stirred solution of nitrile (2.00 mmol) in THF (anhydrous, 10 mL) was added a 2 M BH3·SMe2 in THF (3 ml, 3 equiv) at r.t. After being refluxed for 8 h, the mixture was cooled to r.t., 2 M HCl solution was added, and it was then refluxed for 30 min. After cooling to r.t., the mixture was neutralized by 2 M NaOH solution and extracted with EtOAc several times. The combined organic layers were washed with brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using CH2Cl2:MeOH (10:1) as eluant.
Method B (for compounds 13–16, 22, 27–31, 36, 56)
Nitrile (1.0 mmol) and NiCl2·6H2O (2.0 mmol) were dissolved in MeOH (8 mL). Sodium borohydride (4.0 mmol) was added slowly to the mixture. The mixture was refluxed for 12 h and then cooled to room temperature. The solution was filtered through Celite and dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using CH2Cl2:MeOH (10:1) as eluant.
Method C (for compounds 54–55, 82, 84, 87–88, 91, 92)
A suspension of nitrile compounds (5.0 mmol) and 10% Pd/C (500 mg) and c.HCl (3 mL) in MeOH (30 mL) was hydrogenated under a balloon of hydrogen for 6 h at room temperature and filtered through Celite. The filtrate was concentrated in vacuo and the residue was purified by flash column chromatography on silica gel using EtOAc as eluant.
General Procedure for Coupling (12–99)
A mixture of acid (10.0 mmol), amine (12.0 mmol) and 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (12.0 mmol) in DMF (20 mL) was stirred for 12 h at room temperature. The reaction mixture was extracted with EtOAc (50 mL). The aqueous phase was saturated with NaCl and extracted again with EtOAc (25 mL). The combined organic extracts were washed with 1 M HCl (25 mL) and brine (25 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexane (1:2) as eluant.
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (12)
Yield 12%, white solid. mp 70 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.50 (s, 1H), 8.63 (br t, 1H), 8.56 (s, 1H), 7.82 (t, 2H), 7.32 (t, J = 8.28 Hz, 1H), 7.20 (d, J = 12.12 Hz, 1H), 7.12 (d, J = 8.34 Hz, 1H), 4.37 (m, 2H), 3.69 (q, J = 6.78 Hz, 1H), 3.00 (s, 3H), 1.36 (d, J = 6.78 Hz, 3H). MS (FAB) m/z 420 (MH+).
N-((2-(Butylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (13)
Yield 71%, white solid. mp 65–67 °C. 1H NMR (300 MHz, CDCl3) δ 7.50 (dd, J = 8.4 Hz, 1H), 7.22 (d, J = 7.3 Hz, 1H), 7.15 (dd, J = 11.2 Hz, 1H), 7.06 (d, 1H), 6.76 (d, J = 7.3 Hz, 1H), 6.51 (br s, 1H), 5.95 (br t, 1H), 5.71 (br t, 1H), 4.42–4.43 (m, 2H), 3.53-3.33 (m, 3H), 3.03 (s, 3H), 1.59-1.50 (m, 2H) 1.51 (d, J = 7.1 Hz, 3H), 1.45-1.33 (m, 2H), 0.95 (t, 3H). MS (FAB) m/z 491 (MH+).
N-((2-(Cyclohexylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (14)
Yield 93%, white solid. mp 82–84 °C. 1H NMR (300 MHz, CDCl3) δ 7.51 (t, 1H), 7.22 (d, J = 5.8 Hz, 1H), 7.18 (dd, J = 8.9, 1.5 Hz, 1H), 7.08 (d, J = 6.6 Hz, 1H), 6.74 (d, J = 5.8 Hz, 1H), 6.47 (br s, 1H), 5.84 (br d, 1H), 5.67 (br t, 1H), 4.32 (m, 2H), 3.91 (m, 1H), 3.48 (q, J = 5.7 Hz, 1H), 3.03 (s, 3H), 1.98-1.61 (m, 5H), 1.52 (d, J = 5.7 Hz, 3H), 1.42-1.07 (m, 5H). MS (FAB) m/z 517 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((4-methylcyclohexyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (15)
Yield 25%, yellowish solid. mp 82 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.50 (s, 1H), 8.57 (br t, 1H), 7.40 (dd, J = 33.18, 6.78 Hz, 1H), 7.31 (t, J = 8.28 Hz, 1H), 7.21 (d, J = 11.34 Hz, 1H), 7.12 (d, J = 8.28 Hz, 1H), 6.86 (dd, J = 18.12, 6.78 Hz, 1H), 6.30 (dd, J = 75.54, 6.78 Hz, 1H), 4.15 (m, 2H), 3.67 (m, 2H), 2.99 (s, 3H), 1.66 (m, 2H), 1.52 (m, 2H), 1.44 (m, 1H), 1.36 (d, J = 6.84 Hz, 3H), 1.17 (m, 2H), 0.98 (m, 2H), 0.88 (d, J = 6.06 Hz, 3H). MS (FAB) m/z 531 (MH+).
tert-Butyl 4-(((3-((2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)amino)methyl)piperidine-1-carboxylate (16)
Yield 55%, white solid. mp 83–85 °C. 1H NMR (300 MHz, CDCl3) δ 7.48 (dd, J = 8.2, 8.2 Hz, 1H), 7.25 (d, 1H), 7.16 (d, 1H), 7.06 (d, 1H), 6.77 (d, J = 7.3 Hz, 1H), 6.21 (br s, 1H), 5.93 (br s, 1H), 4.32 (m, 2H), 4.06 (m, 2H), 3.49 (q, J = 7.3 Hz, 1H), 3.32 (m, 2H), 2.66 (m, 2H), 1.76 (m, 2H), 1.51 (d, J = 7.0 Hz, 3H), 1.46 (s, 9H). MS (FAB) m/z 632 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(phenylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (17)
Yield 58%, white solid. mp 189–192 °C. 1H NMR (300 MHz, CD3OD) δ 7.67 (m, 2H), 7.59 (d, J = 8.2 Hz, 1H), 7.34 (dd, J = 8.2, 8.2 Hz, 1H), 7.19 (dd, J = 10.9, 1.9 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 7.06 (d, J = 7.7 Hz, 1H), 7.02-6.95 (m, 3H), 4.45 (m, 2H), 3.67 (q, J = 7.1 Hz, 1H), 2.89 (s, 3H), 1.47 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 511 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((4-fluorophenyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (18)
Yield 58%, white solid. mp 158 °C. 1H NMR (300 MHz, CD3OD) δ 7.90 (m, 2H), 7.59 (d, J = 7.5 Hz, 1H), 7.33 (dd, J = 8.3, 8.3 Hz, 1H), 7.24 (m, 1H), 7.21 (dd, J = 11.4, 1.8 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 7.06 (d, J = 7.5 Hz, 1H), 6.59 (m, 1H), 4.46 (m, 2H), 3.68 (q, J =7.1 Hz, 1H), 2.87 (s, 3H), 1.47 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 529 (MH+).
N-((2-((4-Chlorophenyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (19)
Yield 58%, white solid. mp 196 °C. 1H NMR (300 MHz, CD3OD) δ 7.10 (d, J = 9.0 Hz, 2H) 7.62 (d, J = 7.5 Hz, 1H), 7.34 (dd, J = 8.3, 8.3 Hz, 1H), 7.23 (d, J = 9.0 Hz, 2H), 7.19 (dd, J = 11.7, 2.0 Hz, 1H), 7.10 (d, J = 8.3 Hz, 1H), 7.09 (d, J = 7.5 Hz, 1H), 4.45 (m, 2H), 3.67 (q, J = 7.0 Hz, 1H), 2.88 (s, 3H), 1.47 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 545 (MH+).
N-((2-((3,4-Dimethylphenyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (20)
Yield 58%, white solid. mp 176 °C. 1H NMR (300 MHz, CDCl3) δ 7.56 (m, 2H), 7.43 (dd, J = 8.4, 8.4 Hz, 1H), 7.39 (d, J = 7.8 Hz, 1H), 7.14 (dd, J = 11.0, 2.2 Hz, 1H), 7.06 (d, J = 8.7 Hz, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.95 (d, J = 7.5 Hz, 1H), 6.41(br s, 1H), 5.85 (br t, 1H), 4.47 (d, J = 6.4 Hz, 2H), 3.52 (q, J =7.1 Hz, 1H), 2.96 (s, 3H), 2.27 (s, 3H), 2.23 (s, 3H), 1.52 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 539 (MH+).
N-((2-((5-Chloro-2-methylphenyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (21)
Yield 58%, pale yellow solid. mp 169 °C. 1H NMR (300 MHz, CDCl3) δ 7.68 (d, J = 2.2 Hz, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.42 (dd, J = 8.3, 8.3 Hz, 1H), 7.11 (d, J = 7.7 Hz, 1H), 7.08 (dd, J = 9.0, 2.2 Hz, 1H), 7.03-7.00 (m, 3H), 6.43 (br s, 1H), 5.87 (br t, 1H), 4.49 (m, 2H), 3.51 (q, J = 7.1 Hz, 1H), 3.02 (s, 3H), 2.25 (s, 3H), 1.48 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 559 (MH+).
N-((2-(Benzylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (22)
Yield 68%, white solid. mp 154–157 °C. 1H NMR (300 MHz, CD3OD) δ 7.42-7.14 (m, 8H), 6.82 (d, J = 7.2 Hz, 1H), 6.69 (br t, 1H), 4.68-4.44 (m, 2H), 4.25 (m, 2H), 3.62 (q, J = 7.1 Hz, 1H), 2.94 (s, 3H), 1.37 (d, J = 7.3 Hz, 3H). MS (FAB) m/z 525 (MH+).
N-((2-((4-Chlorobenzyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (23)
Yield 58%, white solid. mp 145 °C. 1H NMR (300 MHz, CDCl3) δ 7.48 (dd, J = 8.3, 8.3 Hz, 1H), 7.33 (d, J = 8.6 Hz, 2H), 7.25 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 7.5 Hz, 1H), 7.07 (dd, J = 11.2, 2.0 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.81 (d, J = 7.5 Hz, 1H), 6.71 (br t, 1H), 6.47 (br s, 1H), 5.72 (br s, 1H), 4.58 (m, 2H), 4.32 (m, 2H), 3.44 (q, J =7.1 Hz, 1H), 3.03 (s, 3H), 1.42 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 559 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((pyridin-2-ylmethyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (24)
Yield 62%, white solid. mp 155–165 °C. 1H NMR (300 MHz, CD3OD) δ 8.44 (m, 1H), 7.68 (td, J = 1.65, 7.68 Hz, 1H), 7.32–7.43 (m, 3H), 7.09–7.26 (m, 3H), 6.85 (d, J = 7.53 Hz, 1H), 4.72 (m, 2H), 4.33 (s, 2H), 3.63 (q, J = 7.14 Hz, 1H), 2.99 (s, 3H), 1.45 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 526 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((pyridin-3-ylmethyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (25)
Yield 65%, white solid. mp 80–100 °C. 1H NMR (300 MHz, CDCl3) δ 8.44–8.56 (m, 2H), 7.77 (m, 1H), 7.48 (m, 1H), 7.25 (m, 1H), 6.98–7.12 (m, 2H), 6.80–6.87 (m, 2H), 4.62 (m, 2H), 4.33 (m, 2H), 3.48 (m, 1H), 3.04 (s, 3H), 1.44 (d, J = 6.78 Hz, 3H). MS (FAB) m/z 526 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((pyridin-4-ylmethyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (26)
Yield 68%, white solid. mp 80–100 °C. 1H NMR (300 MHz, CDCl3) δ 8.46–8.49 (m, 2H), 7.47 (t, J = 8.24 Hz, 1H), 7.24–7.31 (m, 3H), 7.02–7.09 (m, 2H), 6.84 (m, 1H), 5.92 (m, 1H), 4.64 (m, 2H), 4.38 (m, 2H), 3.46 (m, 1H), 3.03 (s, 3H), 1.45 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 526 (MH+).
N-((2-(Dimethylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (27)
Yield 75%, white solid. mp 65–67 °C. 1H NMR (300 MHz, CDCl3) δ 7.46-7.40 (m, 2H), 7.20-7.00 (m, 3H), 6.60 (br t, 1H), 4.50 (br d, 2H), 3.60 (m, 1H), 3.00 (s, 3H), 2.80 (s, 6H), 1.49 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 463 (MH+).
N-((2-(Diethylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (28)
Yield 74%, white solid. mp 58–60 °C. 1H NMR (300 MHz, CDCl3) δ 7.50 (dd, J = 8.0, 8.0 Hz, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.20-7.00 (m, 3H), 6.50 (br s, 1H), 6.30 (br s, 1H), 4.50 (m, 2H), 3.50 (q, J = 7.0 Hz, 1H), 3.20 (m, 2H), 3.00 (s, 3H), 1.50 (d, J = 7.0 Hz, 3H), 0.99 (t, J = 7.2 Hz, 6H). MS (FAB) m/z 491 (MH+).
N-((2-(Dipropylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (29)
Yield 58%, white solid. mp 96–98 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.44 (m, 2H), 7.18-7.07 (m, 3H), 6.63 (br s, 1H), 6.20 (br t, 1H), 4.44 (m, 2H), 3.55 (q, J = 7.3 Hz, 1H), 3.12-3.07 (m, 4H), 3.02 (s, 3H), 1.54-1.40 (m, 4H), 0.83 (t, J = 7.3 Hz, 6H). MS (FAB) m/z 519 (MH+).
N-((2-(Dibutylamino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (30)
Yield 70%, white solid. mp 102–104 °C. 1H NMR (300 MHz, CDCl3) δ 7.51-7.43 (m, 2H), 7.19-7.07 (m, 3H), 6.96 (br s, 1H), 6.40 (br t, 1H), 4.50 (m, 2H), 3.56 (q, J = 7.1 Hz, 1H), 3.13 (m, 4H), 3.02 (s, 3H), 1.52 (d, J = 7.1 Hz, 3H) 1.50 (m, 4H), 1.31-1.10 (m, 4H), 0.87 (t, J = 7.1 Hz, 6H). MS (FAB) m/z 547 (MH+).
N-((2-(Butyl(methyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (31)
Yield 60%, white solid. mp 66 °C. 1H NMR (300 MHz, CDCl3) δ 7.53 (dd, J = 8.3, 8.3 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H), 7.05–7.19 (m, 3H), 6.52 (br s, 1H), 6.13 (br t, 1H), 4.46 (d, J = 5.9 Hz, 2H), 3.56 (q, J = 7.1 Hz, 1H), 3.05–3.12 (m, 2H), 3.04 (s, 3H), 2.80 (s, 3H), 1.42–1.58 (m, 5H), 1.20–1.38 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). MS (FAB) m/z 505 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(methyl(phenyl)amino)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (32)
Yield 83%, white solid. mp 76–84 °C. 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 7.9 Hz, 1H), 7.53 (dd, J = 8.2, 8.2 Hz, 1H) 7.29-7.23 (m, 3H), 7.10-7.01 (m, 3H), 6.83 (m, 2H), 6.48(br s, 1H), 5.42 (br t, 1H), 3.88 (d, J = 6.0 Hz, 2H), 3.38 (s, 3H), 3.37 (q, J = 7.1 Hz, 1H), 3.04 (s, 3H), 1.43 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 525 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(pyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (33)
Yield 80%, white solid. mp 130–135 °C. 1H NMR (300 MHz, CDCl3) δ 7.51 (dd, J = 8.1, 8.1 Hz, 1H), 7.38 (d, J = 7.5 Hz, 2H), 7.13 (dd, J = 11.1, 2.0 Hz, 1H), 7.07 (dd, J = 7.8, 1.8 Hz, 1H), 6.94 (d, J = 7.5 Hz, 1H), 5.72 (br t, 1H), 4.47 (d, J = 5.3 Hz, 2H), 3.52 (q, J = 6.9 Hz, 1H), 3.42–3.46 (m, 4H), 3.02 (s, 3H), 1.82–1.89 (m, 4H), 1.50 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 489 (MH+).
(2S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(2-methylpyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (34)
Yield 75%, white solid. mp 80–85 °C. [α]D25 - 12.740 (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.53-7.35 (m, 2H), 7.17-6.98 (m, 3H), 6.52 (br s, 1H), 5.85 (br s, 1H), 4.57 (m, 1H), 4.28 (m, 2H), 3.53 (m, 3H), 3.14 (m, 1H), 3.02 (d, J = 3.66 Hz, 3H), 2.14 (m, 1H), 1.90 (m, 2H), 1.59 (m, 1H), 1.50 (d, J = 1.65 Hz, 3H), 1.10 (d, J = 6.03 Hz, 3H). MS (FAB) m/z 503 (MH+).
(S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((S)-2-(hydroxymethyl)pyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (35)
Yield 42%, white solid. mp 123–129 °C. [α]D25 - 45.620 (c 0.1, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.44 (m, 2H), 7.13-7.00 (m, 3H), 6.68 (br s, 1H), 6.17 (br s, 1H), 4.59 (m, 2H), 4.35 (m, 1H), 3.78 (m, 1H), 3.66-3.45 (m, 4H), 3.19 (m, 2H), 3.10 (s, 3H), 2.04-1.72 (m, 4H), 1.50 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 519 (MH+).
(S)-1-(3-(((S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)-N,N-dimethylpyrrolidine-2-carboxamide (36)
Yield 55%, white solid. mp 90–100 °C. [α]D25 - 170.019 (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3) δ 8.06 (m, 1H), 7.46 (d, J = 7.32 Hz, 1H), 7.40 (t, J = 8.25 Hz, 1H), 7.26 (m, 1H), 7.18 (m, 1H), 6.99 (d, J = 7.50 Hz, 1H), 5.40 (t, J = 7.35 Hz, 1H), 4.52 (dd, J = 15.03, 6.42 Hz, 1H), 4.35 (d, J = 14.82 Hz, 1H), 3.75 (m, 2H), 3.23 (s, 3H), 2.98 (s, 3H), 2.95 (s, 3H), 2.35 (m, 1H), 2.15 (m, 1H), 2.04 (m, 1H), 1.89 (m, 1H), 1.50 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 560 (MH+).
(S)-tert-butyl 1-(3-(((S)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)pyrrolidine-2-carboxylate (37)
Yield 62%, white solid. mp 80–95 °C. [α]D25 - 33.2 (c 0.15, MeOH). 1H NMR (300 MHz, CDCl3) δ 6.83–7.56 (m, 5H), 6.58 (s, 1H), 4.69 (s, 1H), 4.43 (m, 2H), 3.91 (m, 1H), 3.58 (m, 4H), 3.00 (s, 3H), 1.85 (m, 2H), 1.50 (d, J = 7.1 Hz, 3H), 1.44 (s, 9H). MS (FAB) m/z 589 (MH+).
(S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((R)-3-hydroxypyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (38)
Yield 62%, white solid. mp 88–90 °C. [α]D25 - 1.6 (c 0.05, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.49 (m, 2H), 7.13-7.05 (m, 2H), 6.99 (d, J = 7.5 Hz, 1H), 5.97 (br s, 1H), 4.56-4.37 (m, 2H), 3.75-3.36 (m, 6H), 3.03 (s, 3H), 2.04-1.97 (m, 1H), 1.83-1.74 (m, 2H), 1.50 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 505 (MH+).
(S)-N-((2-((R)-3-(Benzyloxy)pyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (39)
Yield 62%, white solid. mp 109–112 °C. [α]D25 - 31.108 (c 1.0, MeOH). 1H NMR (300 MHz, CDCl3) δ 7.40 (dd, J = 8.1 Hz, 2H), 7.33-7,28 (m, 5H), 7.10 (dd, J = 11 Hz, 1H), 6.97 (dd, J = 7.8 Hz, 2H), 6.72 (s, 1H), 5.98 (br s, 1H), 4.54 (d, 2H), 4.20 (br s, 1H), 3.75-3.39 (m, 5H), 2.97 (s, 1H), 2.16-2.11 (m, 1H), 2.05-1.94 (m, 1H), 1.76 (s, 1H), 1.45 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 595 (MH+).
tert-Butyl ((R)-1-(3-(((S)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)pyrrolidin-3-yl)carbamate (40)
Yield 65%, white solid. mp 78–79 °C. [α]D25 10.8925 (c 0.8, MeOH). 1H NMR (300 MHz, CD3OD) δ 7.48-7.41 (dd, J = 8.1 Hz, 2H), 7.22-7.14 (dd, J = 8.1 Hz, 2H), 6.97 (d, J = 7.68Hz, 1H), 4.42 (q, 2H), 4.07 (q, 1H), 3.70 (m, 2H), 3.52 (m, 2H), 3.02 (s, 3H), 2.06 (m, 1H), 1.82 (m, 1H), 1.44 (m, 12H). MS (FAB) m/z 604 (MH+).
tert-Butyl ((S)-1-(3-(((S)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)pyrrolidin-3-yl)carbamate (41)
Yield 58%, white solid. mp 78–80 °C. [α]D25 - 5.62 (c 0.1, MeOH). 1H NMR (300 MHz, CD3OD) δ 7.43 (d, J = 8.22 Hz, 2H), 7.41 (s, 1H), 7.22-7.13 (dd, J = 8.1 Hz, 2H), 6.97 (d, J = 7.5 Hz, 1H), 4.60 (q, 2H), 4.50-4.32 (m, 2H), 3.72 (m, 2H), 3.57-3.48 (m, 1H), 3.02 (s, 3H), 2.15-2.06 (m, 1H), 1.89-1.82 (m, 1H), 1.44 (m, 12H). MS (FAB) m/z 604 (MH+).
(S)-N-((2-((R)-3-(Dimethylamino)pyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (42)
Yield 67%, white solid. mp 75–77 °C. [α]D25 16.294 (c 1.0, MeOH). 1H NMR (300 MHz, CDCl3) δ 7.47 (dd, J = 8.1 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 7.16 (dd, J = 10.5 Hz, 1H), 7.06 (d, J = 9.5 Hz, 1H), 6.97 (d, J = 6.9 Hz, 1H), 5.98 (br s, 1H), 4.79 (s, 3H), 4.55 (dd, J = 6.9 Hz, 1H), 4.40 (dd, J = 6.9 Hz, 1H), 3.64-3.48 (m, 4H), 3.36 (t, 1H), 3.02 (s, 3H), 2.71-2.66 (m, 4H), 2.29 (s, 6H), 2.11-2.05 (m, 1H), 1.83-1.76 (m, 2H), 1.49 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 532 (MH+).
(S)-N-((2-((S)-3-(Dimethylamino)pyrrolidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (43)
Yield 65%, white solid. mp 78–79 °C. [α]D25 - 28.355 (c 0.6, MeOH). 1H NMR (300 MHz, CDCl3) δ 7.50 (dd, J = 8.1 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 7.13 (dd, J = 10.5 Hz, 1H), 7.08 (d, J = 9.5 Hz, 1H), 6.98 (d, J = 6.9 Hz, 1H), 5.98 (br s, 1H), 4.55 (dd, J = 6.9 Hz, 1H), 4.42(dd, J = 6.9 Hz, 1H), 3.66-3.45 (m, 4H), 3.36 (t, 1H), 3.02, (s, 3H), 2.69 (m, 1H), 2.28 (s, 6H), 2.11-2.05 (m, 1H), 1.83-1.74(m, 2H), 1.49 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 532 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(isoindolin-2-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (44)
Yield 55%, white solid. mp 175–177 °C. 1H NMR (300 MHz, CDCl3) δ 7.46 (d, J = 7. 5 Hz, 1H), 7.25–7.37 (m, 5H), 7.01–7.10 (m, 3H), 6.24 (br s, 1H), 5.75 (br t, 1H), 4. 84 (s, 4H), 4.59 (d, J = 5.7 Hz, 2H), 3.52 (q, J = 7.2 Hz, 1H), 2.94 (s, 3H), 1.49 (d, J = 7.2 Hz, 3H). MS (FAB) m/z 537 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (45)
Yield 88%, white solid. mp 75–79 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.55 (m, 2H), 7.07–7.22 (m, 3H), 6.33 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 6.9 Hz, 1H), 3.00–3.05 (m, 7H), 1.61 (m, 6H), 1.52 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 503 (MH+).
(S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (45S)
Yield 65%, white solid. mp 75–79 °C. [α]D25 - 1.39 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.47–7.55 (m, 2H), 7.07–7.22 (m, 3H), 6.33 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 6.9 Hz, 1H), 3.00–3.05(m, 7H), 1.52 (d, J = 6.9 Hz, 3H), 1.61 (m, 6H). MS (FAB) m/z 503 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((6'-(trifluoromethyl)-3,6-dihydro-2H-[1,2'-bipyridin]-3'-yl)methyl)propanamide (46)
Yield 60%, white solid. mp 82–85 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.52 (m, 2H), 7.06–7.22 (m, 4H), 6.68 (br s, 1H), 6.40 (br t, 1H), 5.79–5.83 (m, 2H), 4.49 (d, J = 5.7 Hz, 2H), 3.69 (m, 2H), 3.56 (q, J = 7.2 Hz, 1H), 3.21 (m, 2H), 3.02 (s, 3H), 2.27 (m, 2H), 1.52 (d, J = 7.2 Hz, 3H). MS (FAB) m/z 501 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(2-methylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (47)
Yield 60%, white solid. mp 78–80 °C. 1H NMR (300 MHz, CDCl3) δ 7.62-7.48 (m, 2H), 7.30 (m, 1H), 7.18-7.07 (m, 2H), 6.71 (br t, 1H), 6.58 (br s, 1H), 4.67-4.57 (m, 1H), 4.35 (m, 1H), 3.56-3.46 (m, 2H), 3.02 & 3.01 (s, 3H), 3.01-2.95 (m, 1H), 2.79 (m, 1H), 1.80-1.50 (m, 9H), 0.90 & 0.85 (d, 3H). MS (FAB) m/z 517 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(3-methylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (48)
Yield 82%, white solid. mp 67–69 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.47 (m, 2H), 7.21 (d, J = 7.7 Hz, 1H), 7.15-7.07 (m, 2H), 6.64 (br s, 1H), 6.34 (br t, 1H), 4.48 (d, J = 5.9 Hz, 2H), 3.56 (q, J = 7.0 Hz, 1H), 3.32-3.17 (m, 2H), 3.03 (s, 3H), 2.74 (m, 1H), 2.46 (m, 1H), 1.82-1.61 (m, 4H), 1.53 (d, J = 7.1 Hz, 3H), 1.13-1.01 (m, 1H), 0.91 (m, 3H). MS (FAB) m/z 517 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-methylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (49)
Yield 75%, white solid. mp 85 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.55 (m, 2H), 7.07–7.22 (m, 3H), 6.29 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 6.9 Hz, 1H), 3.30 (m, 2H), 3.03 (s, 3H), 2.82 (m, 2H), 1.71 (m, 2H), 1.52 (d, J = 6.9 Hz, 3H), 1.24 (m, 3H), 0.97 (d, J = 6.6 Hz, 3H). MS (FAB) m/z 517 (MH+).
(S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-methylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (49S)
Yield 86%, white solid. mp 75–77 °C. [α]D20 - 2.73 (c 1.00, CHCl3). 1H-NMR (300 MHz, CDCl3) δ 7.54 (d, J = 8.25 Hz, 1H), 7.48 (d, J = 8.43 Hz, 1H), 7.20 (d, J = 7.89 Hz, 1H), 7.15-7.07 (m, 2H), 6.44 (br s, 1H), 6.27 (br t, 1H), 4.47 (d, J = 5.49 Hz, 2H), 3.55 (q, J = 6.93 Hz, 1H), 3.30 (t, 2H), 3.03 (s, 3H), 2.82 (tt, 2H), 1.73 (m, 2H), 1.53 (d, J = 7.14 Hz, 3H), 1.23 (m, 3H), 0.97 (d, J = 6.39 Hz); 13C-NMR (100 MHz, CDCl3) δ 173.48, 161.14, 155.55-153.10 (d, J = 244.6), 145.47-144.44 (q, J = 34.1), 140.25-140.18 (d, J = 6.6), 137.64, 128.03, 124.16, 124.11-124.08 (d, J = 3.4), 123.68-123.56 (d, J = 12.7), 125.51-117.34 (q, J = 272.1), 114.91-114.71 (d, J = 20), 114.05-114.02 (d, J = 2.8), 50.49-50.42 (d, J = 6.3), 46.39, 39.84, 39.82, 34.34-34.30 (d, J = 3.8), 30.60, 21.81, 18.53; MS (FAB) m/z 517 (MH+); HRMS calcd for C23H29F4N4O3S (M+H), 517.1897, found 517.1904
(R)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-methylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (49R)
Yield 50%, white solid. mp 78–80 °C. [α]D20 - 2.30 (c 1.00, CHCl3). 1H-NMR (300 MHz, CDCl3) δ 7.54 (d, J = 8.25 Hz, 1H), 7.48 (d, J = 8.43 Hz, 1H), 7.20 (d, J = 7.89 Hz, 1H), 7.15-7.07 (m, 2H), 6.44 (br s, 1H), 6.27 (br t, 1H), 4.47 (d, J = 5.49 Hz, 2H), 3.55 (q, J = 6.93 Hz, 1H), 3.30 (t, 2H), 3.03 (s, 3H), 2.82 (tt, 2H), 1.73 (m, 2H), 1.53 (d, J = 7.14 Hz, 3H), 1.23 (m, 3H), 0.97 (d, J = 6.39 Hz); 13C-NMR (100 MHz, CDCl3) δ 173.48, 161.14, 155.55-153.10(d, J = 244.6), 145.47-144.44(q, J = 34.1), 140.25-140.18 (d, J = 6.6), 137.64, 128.03, 124.16, 124.11-124.08 (d, J = 3.4), 123.68-123.56 (d, J = 12.7), 125.51-117.34 (q, J = 272.1), 114.91-114.71 (d, J = 20), 114.05-114.02 (d, J = 2.8), 50.49-50.42 (d, J = 6.3), 46.39, 39.84, 39.82, 34.34-34.30 (d, J = 3.8), 30.60, 21.81, 18.53. MS (FAB) m/z 517 (MH+).
N-((2-(4-Ethylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (50)
Yield 61%, white solid. mp 78–80 °C. 1H NMR (300 MHz, CD3OD) δ 7.47–7.52 (m, 2H), 7.19 (d, J = 7.8 Hz, 1H), 7.06–7.14 (m, 2H), 6.69 (br s, 1H), 6.37 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.56 (q, J = 6.9 Hz, 1H), 3.33 (m, 2H), 3.02 (s, 3H), 2.80 (m, 2H), 1.76 (m, 2H), 1.52 (d, J = 6.9 Hz, 3H), 1.21–1.32 (m, 5H), 0.91 (t, J = 7.2 Hz, 3H). MS (FAB) m/z 531 (MH+).
N-((2-(4,4-Dimethylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (51)
Yield 80%, white solid. mp 128 °C. 1H NMR (300 MHz, CDCl3) δ 7.53 (dd, J = 8.4, 8.4 Hz, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 7.14 (dd, J = 11.4, 1.9 Hz, 1H), 7.09 (d, J = 8.8 Hz, 1H), 6.47 (br s, 1H), 6.26 (br t, 1H), 4.47 (d, J = 5.0 Hz, 2H), 3.56 (q, J = 7.1Hz, 1H), 3.08-3.04 (m, 4H), 3.03 (s, 3H), 1.53 (d, J = 7.1 Hz, 3H), 1.48-1.43 (m, 4H), 0.99 (s, 6H). MS (FAB) m/z 531 (MH+).
N-((2-(3,5-Dimethylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (52)
Yield 68%, white solid. mp 175 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.54 (m, 2H), 7.21 (d, J = 7.8 Hz, 1H), 7.13 (dd, J = 8.1, 1.8 Hz, 1H), 7.07 (d, J = 8.1 Hz, 1H), 6.48 (br s, 1H), 6.28 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 6.9 Hz, 1H), 3.23 (m, 2H), 3.03 (s, 3H), 2.35 (m, 2H), 1.54–1.76 (m, 2H), 1.52 (d, J = 6.9 Hz, 3H), 0.90 (d, J = 5.7 Hz, 3H), 0.88 (d, J = 5.7 Hz, 3H); MS (FAB) m/z 531 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-phenylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (53)
Yield 75%, white solid. mp 138–141 °C. 1H NMR (300 MHz, CDCl3) δ 7.48–7.51 (m, 2H), 7.08–7.36 (m, 8H), 6.52 (s, 1H), 6.23 (br s, 1H), 4.53 (d, J = 5.1 Hz, 2H), 3.56 (q, J = 7.2 Hz, 1H), 3.46 (m, 2H), 2.95–3.00 (m, 5H), 2.03 (m, 2H), 1.82 (m, 2H), 1.54 (d, J = 7.2 Hz, 3H). MS (FAB) m/z 579 (MH+).
(S)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-phenylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (53S)
Yield 85%, white solid, mp = 135 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.58 (br t, 1H), 7.55 (d, J = 7.56 Hz, 1H), 7.37 (d, J = 7.56 Hz, 1H), 7.29 (m, 5H), 7.21 (m, 2H), 7.15 (d, J = 7.56 Hz, 1H), 4.32 (m, 2H), 3.72 (q, J = 7.56 Hz, 1H), 3.51 (m, 2H), 2.99 (s, 3H), 2.90 (q, 2H), 2.71 (m, 1H), 1.81 (m, 4H), 1.38 (d, J = 7.56 Hz, 3H). MS (FAB) m/z 579 (MH+).
(R)-2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-phenylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (53R)
Yield 82%, white solid. mp 142 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.58 (br t, 1H), 7.55 (d, J = 7.56 Hz, 1H), 7.37 (d, J = 7.56 Hz, 1H), 7.29 (m, 5H), 7.21 (m, 2H), 7.15 (d, J = 7.56 Hz, 1H), 4.32 (m, 2H), 3.72 (q, J = 7.56 Hz, 1H), 3.51 (m, 2H), 2.99 (s, 3H), 2.90 (q, 2H), 2.71 (m, 1H), 1.81 (m, 4H), 1.38 (d, J = 7.56 Hz, 3H). MS (FAB) m/z 579 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((4-phenyl-6'-(trifluoromethyl)-3,6-dihydro-2H-[1,2'-bipyridin]-3'-yl)methyl)propanamide (54)
Yield 65%, white solid. mp 71–73 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.02 (m, 10H), 6.64 (br s, 1H), 6.34 (m, 1H), 6.18 (br s, 1H), 4.52 (m, 2H), 3.88 (d, J = 2.73 Hz, 2H), 3.59-3.36 (m, 4H), 2.99 (m, 5H), 2.67 (m, 2H), 1.51 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 577 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(4-fluorophenyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (55)
Yield 66%, white solid. mp 117–119 °C. 1H NMR (300 MHz, CDCl3) δ 7.46–7.51 (m, 2H), 6.97–7.25 (m, 7H), 6.72 (br s, 1H), 6.24 (br t, 1H), 4.50 (d, J = 5.7 Hz, 2H), 3.59 (q, J = 6.9 Hz, 1H), 3.45 (m, 2H), 3.00 (s, 3H), 2.93 (m, 2H), 1.92 (m, 2H), 1.76 (m, 3H), 1.51 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 597 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((4-(4-fluorophenyl)-6'-(trifluoromethyl)-3,6-dihydro-2H-[1,2'-bipyridin]-3'-yl)methyl)propanamide (56)
Yield 55%, white solid. mp = 83–85 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.36 (m, 4H), 7.23 (d, J = 7.71 Hz, 1H), 7.13-7.02 (m, 3H), 6.21 (m, 1H), 6.12 (m, 1H), 4.53 (d, J = 5.49 Hz, 2H), 3.87 (m, 2H), 3.55 (q, J = 6.96 Hz, 1H), 3.37 (t, J = 5.67 Hz, 2H), 3.00 (s, 3H), 2.65 (m, 2H), 1.51 (d, J = 7.14 Hz, 3H). MS (FAB) m/z 595 (MH+).
N-((2-(4-Benzylpiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (57)
Yield 78%, white solid. mp 81–83 °C. 1H NMR (300 MHz, CDCl3) δ 7.53 (dd, J = 8.2, 8.2 Hz, 1 H), 7.48 (d, J = 7.9 Hz, 1H), 7.29-7.14 (m, 7H), 7.07 (d, J = 8.1 Hz, 1H), 6.49 (br s, 1H), 6.23 (br t, 1H), 4.46 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 7.0 Hz, 1H), 3.31 (m, 2H), 3.02 (s, 3H), 2.78 (m, 2H), 2.59 (d, J = 6.6 Hz, 2H), 1.78-1.71 (m, 3H), 1.52 (d, J = 7.1 Hz, 3H), 1.30 (m, 2H). MS (FAB) m/z 593 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(4-methylbenzyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (58)
Yield 64%, white solid. mp 110–130 °C. 1H NMR (300 MHz, CDCl3) δ 7.45–7.53 (m, 2H), 7.18 (m, 1H), 6.98–7.12 (m, 6H), 6.41 (s, 1H), 6.19 (t, J = 3.87 Hz, 1H), 4.44 (d, J = 4.11 Hz, 2H), 3.51 (q, J = 5.28 Hz, 1H), 3.28 (m, 2H), 3.00 (s, 3H), 2.76 (t, J = 9.21 Hz, 2H), 2.51 (d, J = 5.01 Hz, 2H), 2.31 (s, 3H), 1.66 (m, 3H), 1.50 (m, 3H), 1.23 (m, 2H). MS (FAB) m/z 607 (MH+).
N-((2-(4-(3,4-Difluorobenzyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (59)
Yield 59%, white solid. mp 100–120 °C. 1H NMR (300 MHz, CDCl3) δ 7.43–7.48 (m, 2H), 6.92–7.28 (m, 6H), 6.86 (m, 1H), 6.51 (m, 1H), 4.45 (d, J = 5.49Hz, 2H), 3.60 (q, J = 6.96 Hz, 1H), 3.33 (t, J = 11.60 Hz, 2H), 3.01 (s, 3H), 2.78 (t, J = 11.72 Hz, 2H), 2.53 (d, J = 6.60 Hz, 2H), 1.63–1.72 (m, 3H), 1.50 (d, J = 7.23 Hz, 3H), 1.28 (m, 2H). MS (FAB) m/z 629 (MH+).
N-((2-(4-(3,5-Difluorobenzyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (60)
Yield 61%, white solid. mp 70 °C. 1H NMR (300 MHz, CDCl3) δ 7.46–7.53 (m, 2H), 7.07–7.27 (m, 3H), 6.08 (s, 1H), 6.61–6.69 (m, 3H), 6.28 (t, J = 5.60 Hz, 1H), 4.46 (d, J = 5.49 Hz, 2H), 3.58 (q, J = 7.14 Hz, 1H), 3.31 (m, 2H), 3.05 (s, 3H), 2.80 (m, 2H), 2.56 (d, J = 6.78 Hz, 2H), 1.70–1.74 (m, 3H), 1.53 (d, J = 7.14 Hz, 3H), 1.32 (m, 2H). MS (FAB) m/z 629 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(4-fluorobenzoyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (61)
Yield 65%, white solid. mp 100–115 °C. 1H NMR (300 MHz, CDCl3) δ 7.52 (m, 2H), 7.23-7.03 (m, 7H), 6.54 (br s, 1H), 6.22 (m, 1H), 4.45 (m, 2H), 3.54 (q, J = 6.96 Hz, 1H), 3.37 (m, 2H), 3.04 (s, 3H), 2.80 (m, 2H), 2.01 (m, 2H), 1.75 (m, 1H), 1.51 (d, J = 6.96 Hz, 3H), 1.42 (m, 3H). MS (FAB) m/z 625 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-fluoropiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (62)
Yield 55%, white solid. mp 207–208 °C. 1H NMR (300 MHz, CD3OD) δ 7.50 (d, J = 8.1 Hz, 1H), 7.43 (t, J = 8.1Hz, 1H), 7.14–7.26 (m, 3H), 4.75 (d, J = 5.0 Hz, 1H), 4.38 (d, J = 5.7 Hz, 2H) 3.71 (q, J = 7.2 Hz, 1H), 3.30 (m, 2H), 3.03 (m, 2H), 2.96 (s, 3H), 1.88 (m, 4H), 1.46 (d, J = 7.2 Hz, 3H). MS (FAB) m/z 521 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((6-(trifluoromethyl)-2-(4-(trifluoromethyl)piperidin-1-yl)pyridin-3-yl)methyl)propanamide (63)
Yield 75%, white solid. mp 82–84 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.51 (m, 2H), 7.25 (d, J = 7.8 Hz, 1H), 7.08–7.15 (m, 2H), 6.34 (br s, 1H), 6.04 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.61 (q, J = 6.9 Hz, 1H), 3.43 (m, 2H), 3.01 (s, 3H), 2.84 (t, J = 11.1 Hz, 2H), 1.95 (m, 2H), 1.66 (m, 1H), 1.53 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 571 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-oxopiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (64)
Yield 44%, white solid. mp 86–88 °C. 1H NMR (300 MHz, CDCl3) δ 7.55-7.49 (m, 2H), 7.29 (d, J = 7.9 Hz, 1H), 7.17 (dd, J = 11.2, 2.0 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 6.70 (br s, 1H), 6.04 (br t, 1H), 4.54 (d, J = 5.7 Hz, 2H), 3.61 (q, J = 7.0 Hz, 1H), 3.49 (t, J = 6.0 Hz, 4H), 3.04 (s, 3H), 2.55 (t, J = 6.1 Hz, 4H), 1.55 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 517 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-hydroxypiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (65)
Yield 87%, white solid. mp 81–83 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.49 (m, 2H), 7.23 (d, J = 7.7 Hz, 1H), 7.16-7.09 (m, 2H), 6.69 (br s, 1H), 6.25 (br t, 1H), 4.48 (m, 2H), 3.84 (m, 1H), 3.58 (q, J = 7.3 Hz, 1H), 3.38-3.26 (m, 2H), 3.04 (s, 3H), 2.97-2.88 (m, 2H), 2.02-1.92 (m, 2H), 1.75 (s, 1H), 1.53 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 519 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-methoxypiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (66)
Yield 50%, white solid. mp 65–67 °C. 1H NMR (300 MHz, CDCl3) δ 7.53-7.47 (m, 2H), 7.22 (d, J = 7.7 Hz, 1H), 7.15-7.07 (m, 2H), 6.77 (br s, 1H), 6.32 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.58 (q, J = 7.1Hz, 1H), 3.40-3.25 (m, 3H), 3.37 (s, 3H), 3.03 (s, 3H), 2.95-2.86 (m, 2H), 2.04-1.95 (m, 2H), 1.63-1.50 (m, 2H), 1.53 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 533 (MH+).
N-((2-(4-Ethoxypiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (67)
Yield 59%, white solid. mp 69–71 °C. 1H NMR (300 MHz, CDCl3) δ 7.55-7.48 (m, 2H), 7.23 (d, J = 7.7 Hz, 1H), 7.15-7.08 (m, 2H), 6.54 (br s, 1H), 6.23 (br t, 1H), 4.48 (d, 2H), 3.58-3.23 (m, 6H), 3.04 (s, 3H), 2.94-2.86 (m, 2H), 2.05-1.95 (m, 2H), 1.63-1.50 (m, 2H), 1.53 (d, J = 7.1 Hz, 3H), 1.24 (t, J = 7.0 Hz, 3H). MS (FAB) m/z 547 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(methoxymethyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (68)
Yield 60%, white solid. mp 117–119 °C. 1H NMR (300 MHz, CDCl3) δ 7.46–7.52 (m, 2H), 7.20 (d, J = 7.8 Hz, 1H), 7.07–7.15 (m, 2H), 6.82 (br s, 1H), 6.37 (br t, 1H), 4.46 (d, J = 5.7 Hz, 2H), 3.58 (q, J = 6.9 Hz, 1H), 3.26–3.38 (m, 5H), 3.02 (s, 3H), 2.82 (m, 2H), 1.79 (m, 3H), 1.51 (d, J = 6.9 Hz, 3H), 1.25–1.30 (m, 4H). MS (FAB) m/z 547 (MH+).
N-((2-(4-Butoxypiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (69)
Yield 74%, white solid. mp 70–72 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.48 (m, 2H), 7.21 (d, J = 7.5 Hz, 1H), 7.14-7.07 (m, 2H), 6.64 (br s, 1H), 6.26 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.57 (q, J = 7.1 Hz, 1H), 3.50-3.26 (m, 5H), 3.03 (s, 3H), 2.94-2.86 (m, 2H), 2.02-1.95 (m, 2H), 1.62-1.50 (m, 7H), 1.45-1.33 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). MS (FAB) m/z 575 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-isopropoxypiperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (70)
Yield 44%, white solid. mp 77–79 °C. 1H NMR (300 MHz, CDCl3) δ 7.55-7.48 (m, 2H), 7.22 (d, J = 7.7 Hz, 1H), 7.15-7.08 (m, 2H), 6.56 (br s, 1H), 6.23 (br t, 1H), 4.47 (d, J = 5.9 Hz, 2H), 3.74 (m, 1H), 3.60-3.45 (m, 2H), 3.37-3.33 (m, 2H), 3.04 (s, 3H), 2.94-2.85 (m, 2H), 1.98-1.90 (m, 2H), 1.62-1.50 (m, 2H), 1.53 (d, J = 7.0 Hz, 3H), 1.18 (d, J = 6.1 Hz, 6H). MS (FAB) m/z 561 (MH+).
1-(3-((2-(3-Fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)piperidin-4-yl acetate (71)
Yield 55%, white solid. mp 115–117 °C. 1H NMR (300 MHz, CDCl3) δ 7.57-7.48 (m, 2H), 7.24 (d, J = 8.1 Hz, 1H), 7.17-7.09 (m, 2H), 6.47 (br s, 1H), 6.05 (br t, 1H), 4.93 (m, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.57 (q, J = 7.0 Hz, 1H), 3.35-3.25 (m, 2H), 3.07-2.97 (m, 2H), 3.04 (s, 3H), 2.08 (s, 3H), 2.02-1.92 (m, 2H), 1.80-1.70 (m, 2H), 1.54 (d, J = 7.3 Hz, 3H). MS (FAB) m/z 561 (MH+).
1-(3-((2-(3-Fluoro-4-(methylsulfonamido)phenyl)propanamido)methyl)-6-(trifluoromethyl)pyridin-2-yl)piperidin-4-yl pivalate (72)
Yield 52%, white solid. mp 86–88 °C. 1H NMR (300 MHz, CDCl3) δ 7.54-7.47 (m, 2H), 7.22 (d, J = 7.7 Hz, 1H), 7.15 (dd, J = 11.0, 1.8 Hz, 1H), 7.10 (m, 1H), 6.49 (br s, 1H), 6.01 (br t, 1H), 4.94 (m, 1H), 4.47 (d, J = 6.0 Hz, 2H), 3.58 (q, J = 7.0 Hz, 1H), 3.32-3.22 (m, 2H), 3.13-3.03 (m, 2H), 3.04 (s, 3H), 2.00-1.90 (m, 2H), 1.82-1.70 (m, 2H), 1.55 (d, J = 7.1 Hz, 3H), 1.21 (s, 9H). MS (FAB) m/z 603 (MH+).
N-((2-(4-((Dimethylamino)methyl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (73)
Yield 60%, white solid. mp 85–130 °C. 1H NMR (300 MHz, CDCl3) δ 7.44–7.49 (m, 2H), 7.05–7.24 (m, 3H), 6.22 (br t, 1H), 4.42 (d, J = 5.7 Hz, 2H), 3.55 (q, J = 7.2 Hz, 1H), 3.28 (m, 2H), 3.00 (s, 3H), 2.90 (m, 2H), 2.63 (d, J = 4.2 Hz, 2H), 2.58 (s, 6H), 1.89–2.13 (m, 4H), 1.49 (d, J = 6.9 Hz, 3H), 1.36 (m, 2H). MS (FAB) m/z 560 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(phenylamino)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (74)
Yield 48%, white solid. mp 67–69 °C. 1H NMR (300 MHz, CDCl3) δ 7.49–7.54 (m, 2H), 7.08–7.25 (m, 6H), 6.72 (t, J = 7.2 Hz, 1H), 6.63 (d, J = 8.1 Hz, 2H), 6.21 (br t, 1H), 4.48 (d, J = 5.7 Hz, 2H), 3.57 (q, J = 6.9 Hz, 1H), 3.35–3.46 (m, 3H), 3.01–3.04 (m, 5H), 2.60 (m, 2H), 2.17 (m, 2H), 1.52 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 594 (MH+).
N-((2-([1,4'-Bipiperidin]-1'-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (75)
Yield 30%, white solid. mp 103 °C. 1H NMR (300 MHz, CD3OD) δ 7.51 (d, J = 7.7 Hz, 1H), 7.42 (dd, J = 8.2, 8.2 Hz, 1H), 7.53 (d, J = 7.7 Hz, 1H), 7.13–7.21 (m, 2H), 4.30–4.47 (m, 2H), 3.71 (q, J = 7.0 Hz, 1H), 3.48–3.52 (m, 2H), 2.97 (s, 3H), 2.80–2.84 (m, 2H), 2.55–2.75 (m, 5H), 1.88–2.00 (m, 2H), 1.60–1.75 (m, 6H), 1.50–1.55 (m, 2H), 1.46 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 586 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(pyrrolidin-1-yl)piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (76)
Yield 37%, white solid. mp 144 °C. 1H NMR (300 MHz, CD3OD) δ 7.50 (d, J = 7.7 Hz, 1H), 7.42 (dd, J = 8.3, 8.3 Hz, 1H), 7.25 (d, J = 7.7 Hz, 1H), 7.10–7.22 (m, 2H), 4.29–4.45 (m, 2H), 3.72 (q, J = 7.1 Hz, 1H), 3.40–3.50 (m, 2H), 2.70–2.92 (m, 6H), 2.40 (m, 1H), 1.95–2.10 (m, 2H), 1.81–2.10 (m, 4H), 1.57–1.74 (m, 2H), 1.46 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 572 (MH+).
N-((2-(3-(tert-Butyl)-1-oxa-2,8-diazaspiro[4.5]dec-2-en-8-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (77)
Yield 20%, beige-colored solid. mp 124 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.56 (br t, 1H), 7.55 (d, J = 7.56 Hz, 1H), 7.37 (d, J = 7.56 Hz, 1H), 7.32 (t, J = 8.34 Hz, 1H), 7.22 (d, J = 9.84 Hz, 1H), 7.14 (d, J = 8.34 Hz, 1H), 4.28 (m, 2H), 3.70 (q, J = 6.78 Hz, 1H), 3.19 (m, 2H), 3.08 (m, 2H), 3.00 (s, 3H), 2.83 (s, 2H), 1.76 (m, 4H), 1.37 (d, J = 6.78 Hz, 3H), 1.13 (s, 9H). MS (FAB) m/z 615 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(3-phenyl-1-oxa-2,8-diazaspiro[4.5]dec-2-en-8-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (78)
Yield 20%, yellowish solid. mp 131 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.58 (br t, 1H), 7.68 (m, 2H), 7.56 (d, J = 8.34 Hz, 1H), 7.44 (m, 3H), 7.39 (d, J = 7.56 Hz, 1H), 7.33 (t, J = 8.34 Hz, 1H), 7.22 (d, J = 10.56 Hz, 1H), 7.15 (d, J = 7.56 Hz, 1H), 4.32 (m, 2H), 3.71 (q, J = 6.84 Hz, 1H), 3.28 (m, 2H), 3.14 (m, 2H), 3.00 (s, 3H), 1.90 (m, 4H), 1.37 (d, J = 6.84 Hz, 3H). MS (FAB) m/z 634 (MH+).
N-((2-(Azepan-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (79)
Yield 78%, white solid. mp 126–130 °C. 1H NMR (300 MHz, CDCl3) δ 7.52 (dd, J = 8.1, 8.1 Hz, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.14 (dd, J = 8.1, 1.8 Hz, 1H), 7.08 (d, J = 8.1 Hz, 1H), 7.03 (d, J = 7.5 Hz, 1H), 5.86 (br t, 1H), 4.43 (d, J = 5.7 Hz, 2H), 3.54 (q, J = 6.9 Hz, 1H), 3.38 (m, 4H), 3.03 (s, 3H), 1.75 (m, 4H), 1.57 (m, 4H), 1.52 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 531 (MH+).
N-((2-(Azocan-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (80)
Yield 68%, white solid. mp 138–141 °C. 1H NMR (300 MHz, CDCl3) δ 7.48 (t, J = 8.1 Hz, 1H), 7.36 (d, J = 7.8 Hz, 1H), 7.14 (dd, J = 2.1, 11.1 Hz, 1H), 7.07 (d, J = 8.1 Hz, 1H), 6.96 (d, J = 7.8 Hz, 1H), 6.94 (br s, 1H), 5.97 (br s, 1H), 4.39 (d, J = 5.1 Hz, 2H), 3.59 (q, J = 7.2 Hz, 1H), 3.46 (m, 4H), 3.01 (s, 3H), 1.68 (m, 4H), 1.51 (m, 6H). MS (FAB) m/z 531 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (81)
Yield 42%, white solid. mp 44–48 °C. 1H NMR (300 MHz, CD3OD) δ 7.62 (d, J = 7.5 Hz, 1H), 7.37–7.45 (m, 2H), 7.14–7.21 (m, 2H), 4.60 (s, 2H), 4.42 (q, J = 15.6 Hz, 2H) 3.73 (q, J = 6.9 Hz, 1H), 3.25 (m, 4H) 3.06 (q, J = 15.6 Hz, 2H), 2.99 (s, 3H), 1.46 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 504 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-methylpiperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (82)
Yield 68%, pale yellow solid. mp 97–99 °C. 1H NMR (300 MHz, CDCl3) δ 7.54 (dd, J = 8.4, 8.4 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.23 (d, J = 7.7 Hz, 1H), 7.14 (dd, J = 11.2, 1.9 Hz, 1H), 7.09 (d, J = 8.2 Hz, 1H), 6.21 (br t, 1H), 4.47 (m, 2H), 3.57 (q, J = 7.1 Hz, 1H), 3.19–3.15 (m, 4H), 3.04 (s, 3H), 2.53–2.49 (m, 4H), 2.34 (s, 3H), 1.54 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 518 (MH+).
N-((2-(4-Cyclohexylpiperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (83)
Yield 72%, white solid. mp 112–114 °C. 1H NMR (300 MHz, CDCl3) δ 7.47–7.53 (m, 2H), 7.23 (d, J = 7.8 Hz, 1H), 7.07–7.15 (m, 2H), 6.26 (br t, 1H), 4.44 (d, J = 5.7 Hz, 2H), 3.58 (q, J = 6.9 Hz, 1H), 3.27 (m, 4H), 3.03 (s, 3H), 2.84 (m, 4H), 2.50 (m, 1H), 1.94 (m, 2H), 1.85 (m, 2H), 1.51 (d, J = 6.9 Hz, 3H), 1.25–1.30 (m, 6H). MS (FAB) m/z 586 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-phenylpiperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (84)
Yield 58%, white solid. mp 87–92 °C. 1H NMR (300 MHz, CDCl3) δ 7.54 (d, J = 7.7Hz, 1H), 7.48 (dd, J = 8.2, 8.2 Hz, 1H), 7.31 (m, 3H), 7.13 (dd, J = 11.0, 1.8 Hz, 1H), 7.08 (d, J = 8.8 Hz, 1H), 6.96-6.89 (m, 3H), 6.33 (br s, 1H), 6.20 (br t, 1H), 4.54 (d, J = 6.0 Hz, 2H), 3.57 (q, J = 7.0 Hz, 1H), 3.32-3.29 (m, 8H), 2.99 (s, 3H), 1.53 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 580 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(m-tolyl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (85)
Yield 60%, white solid. mp 61–63 °C. 1H NMR (300 MHz, CDCl3) δ 7.45–7.54 (m, 2H), 7.06–7.28 (m, 4H), 6.73–6.76 (m, 3H), 6.28(br s, 1H), 6.20 (br t, 1H), 4.52 (d, J = 5.7 Hz, 2H), 3.56 (q, J = 6.9 Hz, 1H), 3.24 (m, 8H), 2.98 (s, 3H), 2.35 (s, 3H), 1.52 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 594 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(p-tolyl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (86)
Yield 67%, white solid. mp 83–85 °C. 1H NMR (300 MHz, CDCl3) δ 7.55 (m, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.24–7.32 (m, 2H), 6.96–7.12 (m, 4H), 6. 78–6.81 (m, 3H), 4.40 (d, J = 5.7 Hz, 2H), 3.56 (q, J = 6.9 Hz, 1H), 3.12 (m, 8H), 2.86 (s, 3H), 2.18 (s, 3H), 1.40 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 594(MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(2-fluorophenyl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (87)
Yield 67%, white solid. mp 178–180 °C. 1H NMR (300 MHz, CDCl3) δ 7.53 (d, J = 8.1 Hz, 1H), 7.52 (dd, J = 8.3, 8.3 Hz, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.11 (m, 4H), 6.98 (m, 2H), 6.40 (br s, 1H), 6.16 (br t, 1H), 4.53 (d, J = 4.6 Hz, 2H), 3.58 (q, J = 7.3 Hz, 1H), 3.32-3.28 (m, 4H), 3.18-3.15 (m, 4H), 3.01 (s, 3H), 1.54 (d, J = 7.0 Hz, 3H). MS (FAB) m/z 598 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(4-fluorophenyl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (88)
Yield 80%, white solid. mp 87–92 °C. 1H NMR (300 MHz, CDCl3) δ 7.53 (d, J = 7.5Hz, 1H), 7.49 (dd, J = 7.9, 7.9 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.14 (d, J = 11.0 Hz, 1H), 7.08 (d, J = 8.4 Hz, 1H), 6.99 (m, 2H), 6.90 (m, 2H), 6.58 (br s, 1H), 6.17 (br t, 1H), 4.52 (d, J = 5.7 Hz, 2H), 3.58 (q, J = 6.8 Hz, 1H), 3.29-3.25 (m, 4H), 3.22-3.18 (m, 4H), 3.01 (s, 3H), 1.53 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 598 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((6-(trifluoromethyl)-2-(4-(4-(trifluoromethyl)phenyl)piperazin-1-yl)pyridin-3-yl)methyl)propanamide (89)
Yield 52%, white solid. mp 164–166 °C. 1H NMR (300 MHz, CD3OD) δ 7.39–7.52 (m, 5H), 7.27 (d, J = 7.8 Hz, 1H), 7.11–7.20 (m, 2H), 4.46 (d, J = 5.7 Hz, 2H), 3.67 (q, J = 6.9 Hz, 1H), 3.33–3.38 (m, 8H), 3.00 (s, 3H), 1.53 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 648 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(4-methoxyphenyl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (90)
Yield 73%, white solid. mp 86–88 °C. 1H NMR (300 MHz, CDCl3) δ 7.45–7.54 (m, 2H), 7.26 (d, J = 7.5 Hz, 1H), 7.06–7.14 (m, 2H), 6.85–6.93 (m, 4H), 6.41 (br s, 1H), 6.23 (br t, 1H), 4.53 (d, J = 5.7 Hz, 2H), 3.79 (s, 3H), 3.56 (q, J = 6.9 Hz, 1H), 3.79 (m, 4H), 3,12 (m, 4H), 2.99 (s, 3H), 1.51 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 610 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(pyridin-2-yl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (91)
Yield 24%, yellowish solid, mp = 133 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.51 (s, 1H), 8.60 (br t, 1H), 8.14 (d, J = 4.5 Hz, 1H), 7.57 (m, 2H), 7.41 (d, J = 8.28 Hz, 1H), 7.33 (t, J = 8.34 Hz, 1H), 7.22 (d, J = 11.34 Hz, 1H), 7.15 (d, J = 8.28 Hz, 1H), 6.86 (d, J = 8.28 Hz, 1H), 6.67 (t, J = 6.84 Hz, 1H), 4.34 (m, 2H), 3.72 (q, J = 6.78 Hz, 1H), 3.61 (s, 4H), 3.20 (s, 4H), 3.00 (s, 3H), 1.38 (d, J = 6.78 Hz, 3H). MS (FAB) m/z 582 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(4-(pyridin-4-yl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (92)
Yield 58%, white solid. mp 132–134 °C. 1H NMR (300 MHz, CDCl3) δ 8.28 (d, J = 6.4 Hz, 2H), 7.55 (d, J = 7.9 Hz, 1H), 7.50 (dd, J = 8.2, 8.2 Hz, 1H), 7.29 (d, J = 7.9 Hz, 1H), 7.14 (dd, J = 11.4, 2.0 Hz, 1H), 7.09 (d, J = 8.3 Hz, 1H), 6.69 (d, J = 6.6 Hz, 2H), 6.26 (br t, 1H), 4.52 (d, J = 5.7 Hz, 2H), 3.60 (q, J = 7.0 Hz, 1H), 3.43-3.38 (m, 4H), 3.29-3.25 (m, 4H), 3.02 (s, 3H), 1.54 (d, J = 7.1 Hz, 3H). MS (FAB) m/z 581 (MH+).
N-((2-(4-(3-Chloropyridin-2-yl)piperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (93)
Yield 48%, white solid, mp 135 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.50 (s, 1H), 8.60 (br t, 1H), 8.24 (d, J = 4.5 Hz, 1H), 7.82 (d, J = 7.56 Hz, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.41 (d, J = 7.5 Hz, 1H), 7.32(t, J = 8.34 Hz, 1H), 7.22 (d, J = 12.06 Hz, 1H), 7.14 (d, J = 8.28 Hz, 1H), 7.02 (m, 1H), 4.33 (m, 2H), 3.71 (q, J = 6.84 Hz, 1H), 3.40 (s, 4H), 3.25 (s, 4H), 2.99 (s, 3H), 1.37 (d, J = 6.78 Hz, 3H). MS (FAB) m/z 616 (MH+).
N-((2-(4-(3-Chloropyridin-2-yl)-2-methylpiperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (94)
Yield 23 %, beige-colored solid. mp 141 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.50 (s, 1H), 8.57 (d, J = 6.06 Hz, 1H), 8.24 (d, J = 4.56 Hz, 1H), 7.81 (d, J = 7.56 Hz, 1H), 7.61 (dd, J = 18.84, 7.5 Hz, 1H), 7.45 (t, J = 6.84 Hz, 1H), 7.33 (t, J = 8.28 Hz, 1H), 7.22 (d, J = 11.34 Hz, 1H), 7.15 (d, J = 8.34 Hz, 1H), 7.02 (t, 1H), 4.34 (m, 2H), 3.72 (q, J = 6.78 Hz, 1H), 3.48 (m, 2H), 3.38 (m, 4H), 3.20 (m, 1H), 3.00 (s, 3H), 1.37 (d, J = 6.78 Hz, 3H), 1.02 (d, 3H). MS (FAB) m/z 629 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((6-(trifluoromethyl)-2-(4-(3-(trifluoromethyl)pyridin-2-yl)piperazin-1-yl)pyridin-3-yl)methyl)propanamide (95)
Yield 69%, white solid. mp 77–79 °C. 1H NMR (300 MHz, CDCl3) δ 8.50 (d, J = 3.6 Hz, 1H), 7.91 (dd, J = 7.8, 1.8 Hz, 1H), 7.54 (d, J = 6.9 Hz, 1H), 7.49 (dd, J = 8.1, 8.1 Hz, 1H), 7.05–7.17 (m, 4H), 6.40 (br t, 1H), 4.52 (d, J = 5.7 Hz, 2H), 3.61 (q, J = 6. 9 Hz, 1H), 3.35 (m, 8H), 3.02 (s, 3H), 1.53 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 649 (MH+).
N-((2-(4-Benzylpiperazin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (96)
Yield 58%, white solid. mp 105–109 °C. 1H NMR (300 MHz, CDCl3) δ 7.48–7.54 (m, 2H), 7.21–7.34 (m, 7H), 7.11 (dd, J = 8.1, 2.1 Hz, 1H), 7.07 (d, J = 8.1 Hz, 1H), 6.24 (br t, 1H), 4.46 (d, J = 5.7 Hz, 2H), 3.51–3.58 (m, 3H), 3.14 (m, 4H) 3.02 (s, 3H), 2.54 (m, 4H), 1.52 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 594 (MH+).
2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-morpholino-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (97)
Yield 60%, white solid. mp 82–84 °C. 1H NMR (300 MHz, CDCl3) δ 7.49–7.56 (m, 2H), 7.26 (d, J = 7.5 Hz, 1H), 7.08–7.17 (m, 2H,), 6.53 (br s, 1H), 6.06 (br t, 1H), 4.48 (d, J = 5.7 Hz, 2H), 3.76 (m, 4H), 3.57 (q, J = 6.9 Hz, 1H), 3.13 (m, 4H), 3.04 (s, 3H), 1.55 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 505 (MH+).
N-((2-((2S,6R)-2,6-Dimethylmorpholino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (98)
Yield 80%, white solid. mp 66–68 °C. [α]D25 - 1.33 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.48–7.52 (m, 2H), 7.10–7.25 (m, 3H), 6.52 (br s, 1H), 6.06 (br t, 1H), 4.47 (d, J = 5.7 Hz, 2H), 3.70 (m, 2H), 3.58 (q, J = 6.9 Hz, 1H), 3.16 (m, 2H), 3.04 (s, 3H), 2.64 (m, 2H), 1.55 (d, J = 6.9 Hz, 3H), 1.19 (d, J = 6.3 Hz, 6H). MS (FAB) m/z 533 (MH+).
N-((2-(1,1-Dioxidothiomorpholino)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (99)
Yield 70%, white solid. mp 77–79 °C. 1H NMR (300 MHz, CDCl3) δ 7.50–7.56 (m, 2H), 7.33 (d, J = 7.5 Hz, 1H), 7.09–7.17 (m, 2H), 5.94 (br t, 1H), 4.46 (d, J = 5.7 Hz, 2H), 3.72 (m, 4H), 3.60 (q, J = 6.9 Hz, 1H), 3.16 (m, 4H), 3.02 (s, 3H), 1.54 (d, J = 6.9 Hz, 3H). MS (FAB) m/z 553 (MH+).
Biological study
Functional Investigations on the Vanilloid Receptor 1 (TRPV1)
For determination of agonistic or antagonistic compound activity, the FLIPR-3 instrument (Molecular Devices Corp.) was used for the capsaicin, NADA, and pH assays.
Capsaicin Assay
CHO-K1 cells stably expressing human, rat or mouse TRPV1 are plated on poly-D-lysine-coated black 96-well plates with a clear bottom (BD Biosciences) at a density of 20,000 cells/well in a volume of 100µl Ham´s F12 medium with L-glutamine, 10% v/v fetal calf serum (Gibco Invitrogen) and 20 µg/ml L-proline (Sigma). The cells are then incubated overnight at 37 °C, 5% CO2 and approximately 98% relative humidity. The following day, the cells are incubated with Fluo-4 (Molecular Probes) and 0.01 Vol% Pluronic F127 (Molecular Probes) in Hank’s buffered saline solution (HBSS, Gibco Invitrogen) for 30 minutes at 37 °C. The plates are then washed 3 times with HBSS buffer and, after a further incubation for 15 minutes at room temperature, used in the FLIPR assay for the Ca2+ measurement (wavelength λex=488 nm, λem=540 nm). The quantification is carried out by measuring the highest fluorescence intensity (FC, Fluorescence Counts) over time.
The FLIPR protocol consists of 2 additions of substances. To test for agonistic activity, the test compounds (3× concentrated in 150 µl total volume, final screening concentration 10 µM, or dose-response curves from 0.01 µM to 25 µM) are added to the cells and the Ca2+ inflow is compared to the control (capsaicin 10 µM). To test for antagonistic activity, 100 nM capsaicin are added 6 min after test compound addition by the FL IPR pipettor, and the inflow of Ca2+ is determined again. Desensitising agonists and antagonists lead to a suppression of the Ca2+ inflow after addition of capsaicin. The inhibition [%] compared to the maximum achievable inhibition with the reference antagonist is calculated.
To test for non-specific inhibition of the fluorescence assay by the test compounds, plates containing wild-type CHO-K1 cells are activated with ATP (50µl/well, 10µM final concentration). Each assay plate contains a reference standard (e.g. BCTC) as well as vehicle controls (HBSS-DMSO) and positive controls (10 µM capsaicin). A capsaicin dose response curve is generated on a separate plate, to determine the EC50 concentration.
For data analysis, FLIPR raw data (fluorescence units) are transferred to Excel, to determine %-stimulation (as compared to the response elicited by 10 µM capsaicin) in the first part of the experiment or %-inhibition (of the signal elicited by 100 nM capsaicin) in the second part of the experiment for the drugs under investigation. EC50/IC50 values are calculated using GraphPad Prism (GraphPad Software, San Diego, USA). Functional (f)Ki-values are calculated according to the modified Cheng-Prusoff equation.21
pH Assay
In the pH-assay, human TRPV1-transfected cells are activated with HBSS containing 60mM MES (Sigma; instead of capsaicin) resulting in a final pH of 6.0 to 6.3 in the assay medium. Data are recorded and processed as described above.
Temperature Assay
In the temperature assay, CHO-K1 cells expressing human TRPV1 are stimulated by a final medium temperature of 45 °C. For the indirect measurement of intracellular calcium using Fluo-4 dye, CHO-K1/TRPV1 cells are seeded in 100 µl of complete HAM`s F12 Nutrient Mixture medium in clear bottom black 96 MTPs (Corning Cellbind Surface Assay Plates) at 3.000 c/w 96 h before analysis. The day of the experiment, medium is replaced and cells are loaded for 90 min at 37°C with 50 µl buffer B. After removal of buffer B, the plate is then incubated in 50 µl buffer A for 15 min at room temperature and protected from light. 25 µl of reference antagonist or test compound (3 fold concentrated in buffer A giving the final concentration in the wells) are added to start the experiment, followed by an incubation step at room temperature for 5 min. During this incubation period, the baseline fluorescence prior to stimulation is measured at λex=488 nm, λem= 540 nm using the FLX800 Fluorescence Reader (Biotek). After 5 min, the plate is transferred into the PCR cycler PTC200, MJ Research (flat bottom insert) and the temperature cycling program is started (using heated lid, 2 min 57°C, 1 min 20°). Directly after this temperature stimulus resulting in a final temperature 45°C (approx. EC80 of temperature stimulation response) in the wells, the fluorescence is measured again. The difference of fluorescence units before and after temperature stimulation are calculated and used for the IC50 determination of the antagonists. Each experiment is also conducted on CHO-K1 wildtype cells to detect unspecific drug interactions in this assay system.; Buffer A: HBSS + CaCl2 + MgCl (Gibco) containing 2.5 mM probenecid and 10 mM HEPES, pH 7.4, Buffer B: HBSS + CaCl2 + MgCl (Gibco) containing 2.17 µM fluo-4 AM (Molecular Probes), 2.5 mM probenecid, 10 mM HEPES, pH 7.4.
NADA Assay
The effect of test compounds to inhibit a N-arachidonoyl-dopamine stimulus acting on TRPV1 or to perform as agonist on calcium release in TRPV1 transfected eukaryotic cells is analysed. NADA (N-arachidonoyl-dopamine, Tocris) is dissolved at in ethanol to 10 mM stock concentration and stored at −20°C. Working solutions are freshly prepared by dilution in buffer A. The test compounds are freshly prepared as 10 mM solutions in DMSO (Fluka) and then diluted in buffer B. To keep the DMSO concentration below 0.1%, the highest concentration of compounds used in the test is 10 µM. For indirect measurement of intracellular calcium by FLIPR3 using Fluo-4 dye, CHO-K1/TRPV1 cells are seeded in 100 µl of complete HAM`s F12 Nutrient Mixture medium in clear bottom black 96 MTP’s (Corning Cellbind Surface Assay Plates) at 20.000 c/w 24 h before analysis. The day of experiment, medium is replaced and cells are loaded with 50 µl buffer C for 40 min at 37°C. After washing twice with buffer D, 100 µl buffer D is added to the cells followed by 15 min incubation at room temperature in the dark. Then, in the FLIPR instrument, 50 µl of known antagonist or test compound (3 fold concentrated in buffer B, giving 0.1% BSA final concentration in the wells) are added to the cells followed by the addition of 50 µl of NADA as agonist (approx. EC90; 4 fold concentrated in buffer A, final BSA concentration 0.1%, final volume 200 µl). Fluorescence measurements are performed for about 9 min before and for about 5 min after agonist injection. (f)Ki values are calculated according to Cheng-Prusoff.25; Buffer A: (HBSS +CaCl2 + MgCl (Gibco No. 14025-050) containing 2.5 mM probenecid, 0.1% BSA (PAA No. K05-013, 30% stock solution), 10 mM HEPES, pH 7.4), Buffer B: (HBSS +CaCl2 + MgCl (Gibco No. 14025-050) containing 2.5 mM probenecid, 0.3% BSA (PAA No. K05-013, 30% stock solution), 10 mM HEPES, pH 7.4, Buffer C: HBSS + CaCl2 + MgCl (Gibco No. 14025-050) containing 2.17 µM fluo-4 AM (Molecular Probes No. F14201, 50 µg), 2.5 mM probenecid, 10 mM HEPES, pH 7.4, Buffer D: HBSS +CaCl2 + MgCl (Gibco No. 14025-050) containing 2.5 mM probenecid, 10 mM HEPES, pH 7.4.
Animal pain model assay
Adult male Sprague Dawley rats (170–310 g, obtained from Janvier, Le Genest Saint Isle, France) were used in the study. Animals were kept under standard laboratory conditions with free access to standard laboratory food and tap water. The experiments were performed in accordance with ECC guidelines (86/609/EEC) for the use of laboratory animals. All efforts were made to minimize animal suffering and to reduce the number of animals used. Animals were tested in randomized groups of 10 for each dose and vehicle controls. Although the operators performing behavioral tests were not formally ‘blinded’ with respect to the treatment, they were not aware of the nature of the differences between the drugs and the study hypothesis. Rats underwent surgery involving four loose ligations of the sciatic nerve according to the Bennett model. Under pentobarbital anesthesia (Narcoren®, 60 mg/kg i.p.), the right common sciatic nerve was exposed by blunt dissection at the level of midthigh and four loose ligatures (softcat® chrom USP 4/0, metric2; Braun Melsungen, Germany) were placed around the nerve, taking care not to interrupt the epineural circulation. After operation, animals were allowed to recover for one week. Cold allodynia was stable for several weeks and was tested on a metal plate cooled by a water bath to a constant temperature of 4° C by means of counting the number of brisk paw withdrawals during 2 min. Animals were observed for periods of 2 min before and 15, 30, 45 and 60 min after administration of test compounds or vehicle control (compound 2: 10% DMSO, 10% Glucose, in 0.9% NaCl; compound 49S: 10% DMSO, 50% PEG400, in H2O). % MPE of each time point was calculated according to the formula: [(T0–T1) / T0] × 100, where T0 and T1 were numbers of paw withdrawal reactions before and after drug administration, respectively. Data were analysed by means of two-factor analysis of variance (ANOVA) with repeated measures. In case of a significant treatment effect, pair-wise comparison was performed at the every test time point on raw data by post hoc analysis with Bonferroni adjustment. Results were considered statistically significant if p < 0.05.
Care and handling of mice
Animals were housed under a 12:12 h light-dark cycle (lights on at 06:00 a.m.), and with room temperature 20–24° C, relative air humidity 35–70 %, 15 air changes per hour, and air movement < 0.2 m/s. The animals had free access to standard laboratory food (Ssniff R/M-Haltung, Ssniff Spezialdiäten GmbH, Soest, Germany) and tap water. All animals were used only once in all pain models. There were at least five days between delivery of the animals and the start of experiment Animal testing was performed in accordance with the recommendations and policies of the International Association for the Study of Pain26 and the German Animal Welfare Law. All study protocols were approved by the local government committee for animal research, which is also an ethics committee. Animals were assigned randomly to treatment groups. Different doses and vehicle were tested in a randomised fashion. Although the operators performing the behavioural tests were not formally 'blinded' with respect to the treatment, they were not aware of the study hypothesis or the nature of differences between drugs.
Capsaicin induced hypothermia in mice
The administration of the TRPV1 agonist capsaicin is known to produce a dramatic fall in body temperature.20–21 The studies were conducted with male NMRI mice (30–35 g) supplied by a commercial breeder (Charles River, Sulzfeld, Germany). Mice were habituated to the experimental room (23–24 °C) for up to 1 ½ hours prior to treatment. Intraperitoneal injection of capsaicin 3 mg/kg was used to analyze TRPV1 agonist-induced hypothermia. The rectal temperature was measured twice before (baseline) and 15, 30, 60, 90 and 120 min after capsaicin with a thermocouple probe (connected to a digital thermometer; Thermalert TH-5, physitemp, Clifton NJ). Compound 49S at the dose of 0.3 mg/kg or vehicle (10% DMSO, 50% PEG in H2O) was administered alone to investigate a possible intrinsic effect on body temperature and 15 min before intraperitoneal capsaicin injection.
Analysis of body temperature in wild type and TRPV1 knock-out mice
For the experiments on the effect of the substance on body temperature, male TRPV1 knockout mice (B6.129S4-Trpv1tm1Jul/J, Jax mice, USA; 20–30 g) were used on congenic background and compared to male C57BL/6J wild type mice (Charles River, Sulzfeld, Germany; 20–30 g). The rectal temperature was measured as described above, twice before (baseline) and 15, 30, 60 and 90 min after substance. Compound 49S dissolved in 10% DMSO, 50% PEG in H2O was administered at the dose of 1 mg/kg 15 min before intraperitoneal capsaicin (3 mg/kg) injection.
Molecular modeling
The human TRPV1 (hTRPV1) tetramer homology model was constructed by the Build Mutants protocol in Discovery Studio v.3.1 (Accelrys Inc., San Diego, CA, USA), using the capsaicin-docked form of our rat TRPV1 tetramer homology model20 as a template. The 27 residues different between rat and human TRPV1 sequences were computationally mutated as Phe439Leu, Ala450Met, Glu458Aap, Tyr463Phe, Leu465Met, Lys466Glu, Asn467Lys, Ser483Leu, Leu503Met, Ser505Thr, Ile514Met, Val518Leu, Val525Ala, Ser526Thr, Gln533His, Arg534Leu, Met547Leu, Leu585Ile, Asn605Asp, Met609Ser, Pro613Ser, Lys615Arg, Cys616Trp, Ser619Pro, Lys622Arg, Gly624Pro, and Asn625Asp. The side chains and backbone within 4.5 Å of the mutated residues were optimized by the MODELER 9v8 program through a combination of conjugate gradient minimization and molecular dynamics with simulated annealing. Among the resulting five models, the model with the lowest probability density function (PDF) total energy was selected. Then, it was energy minimized with the backbone atoms fixed, using a CHARMm force field until the rms of the conjugate gradient was lower than 0.05 kcal mol−1A−1. The 3D structures of the ligands were generated with Concord and energy minimized using an MMFF94s force field and an MMFF94 charge until the rms of the Powell gradient was 0.05 kcal mol−1A−1 in SYBYL-X 1.2 (Tripos Int., St. Louis, MO, USA). The flexible docking study on our hTRPV1 model was performed by GOLD v.5.0.1 (Cambridge Crystallographic Data Centre, Cambridge, UK), which uses a genetic algorithm (GA) and allows for full ligand flexibility and partial protein flexibility. The binding site was defined as 8 Å around the capsaicin, complexed in the hTRPV1 model. The side chains of the nine residues (i.e., Tyr511, Ser512, Met514, Leu515, Leu518, Phe543, Leu547, Thr550, and Asn551) which are thought to be important for ligand binding were set to be flexible with ‘crystal mode’ in GOLD. The ligands were docked using the GoldScore scoring function with 30 GA runs, and other parameters were set as default. All computation calculations were undertaken on an Intel® Xeon™ Quad-core 2.5 GHz workstation with Linux Cent OS release 5.5.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by Research Grants from Grunenthal, Grants from the National Research Foundation of Korea (NRF) (R11-2007-107-02001-0), Grants from the NLRL program (2011-0028885), the 21st Century Frontier Research Program (2011-K000289) and NCRC program (2012-0000952), the Ewha Global Top 5 Grant 2011 and in part by the Intramural Research Program of NIH, Center for Cancer Research, NCI (Project Z1A BC 005270).
ABBREVIATIONS USED
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- SAR
Structure Activity Relationship
Footnotes
ASSOCIATED CONTENT
Supporting Information
The HPLC purities of all final compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 2.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walpole CSJ, Wrigglesworth R. Capsaicin in the Study of Pain. San Diego, CA: Academic Press; 1993. pp. 63–82. [Google Scholar]
- 7.Appendino G, Szallasi A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- 8.Szallasi A, Cruz F, Geppetti P. TRPV1: a therapeutic target for novel analgesic drugs? Trends in Mol. Med. 2006;12:545–554. doi: 10.1016/j.molmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Voight EA, Kort ME. Transient receptor potential vanilloid-1 antagonists: a survey of recent patent literature. Exp. Opin. Ther. Pat. 2010;20:1–16. doi: 10.1517/13543776.2010.497756. [DOI] [PubMed] [Google Scholar]
- 10.Lazar J, Gharat L, Khairathkar-Joshi N, Blumberg PM, Szallasi A. Screening TRPV1 antagonists for the treatment of pain: lessons learned over a decade. Exp. Opin. Drug Discov. 2009;4:159–180. doi: 10.1517/17460440802681300. [DOI] [PubMed] [Google Scholar]
- 11.Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Disc. Today. 2009;14:56–67. doi: 10.1016/j.drudis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Kym PR, Kort ME, Hutchins CW. Analgesic potential of TRPV1 antagonists. Biochem. Pharmacol. 2009;78:211–216. doi: 10.1016/j.bcp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Kil M-J, Kang S-U, Ryu H, Kim MS, Cho Y, Bhondwe RS, Thorat SA, Sun W, Liu K, Lee JH, Choi S, Pearce LV, Pavlyukovets VA, Morgan MA, Tran R, Lazar J, Blumberg PM, Lee J. N-4-t-Butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamide TRPV1 antagonists: Structure–activity relationships in the A-region. Bioorg. Med. Chem. 2012;20:215–224. doi: 10.1016/j.bmc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min KH, Suh Y-G, Park M-K, Park H-G, Park Y-H, Kim H-D, Oh U, Blumberg PM, Lee J. High affinity antagonists of the vanilloid receptor. Mol. Pharm. 2002;62:947–956. doi: 10.1124/mol.62.4.947. [published erratum appears in Mol. Pharmacol.. 2003, 63, 958] [DOI] [PubMed] [Google Scholar]
- 16.Sun W, Liu K, Ryu H, Kang DW, Kim YS, Kim MS, Cho Y, Bhondwe RS, Thorat SA, Kim HS, Pearce LV, Pavlyukovets VA, Tran R, Morgan MA, Lazar J, Ryder CB, Toth A, Blumberg PM, Lee J. 2-(4-Methylsulfonylaminophenyl) propanamide TRPV1 antagonists: structure activity relationships in the B and C-regions. Bioorg. Med. Chem. 2012;20:1310–1318. doi: 10.1016/j.bmc.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty EM, Fotsch C, Bo Y, Chakrabarti PP, Chen N, Gavva N, Han N, Kelly MG, Kincaid J, Klionsky L, Liu Q, Ognyanov VI, Tamir R, Wang X, Zhu J, Norman MH, Treanor JJS. Discovery of potent, orally available vanilloid receptor-1 antagonists. Structure-activity relationship of N-Aryl cinnamides. J. Med. Chem. 2005;48:71–90. doi: 10.1021/jm049485i. [DOI] [PubMed] [Google Scholar]
- 18.Lang RW, Wenk PF. Synthesis of selectively trifluoromethylated pyridine derivatives as potential antihypertensives. Hel. Chim. Acta. 1988;71:596–601. [Google Scholar]
- 19.Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 20.Szikszay M, Obál F, Jr, Obál F. Dose-response relationships in the thermoregulatory effects of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:97–100. doi: 10.1007/BF00506307. [DOI] [PubMed] [Google Scholar]
- 21.De Vries DJ, Blumberg PM. Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci. 1989;44:711–715. doi: 10.1016/0024-3205(89)90382-2. [DOI] [PubMed] [Google Scholar]
- 22.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 23.Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J. Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S. Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J. Comput. Aided Mol. Des. 2011;25:317–327. doi: 10.1007/s10822-011-9421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.