Abstract
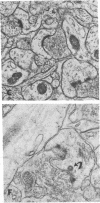
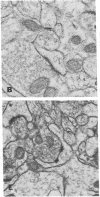
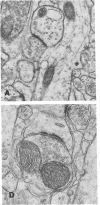
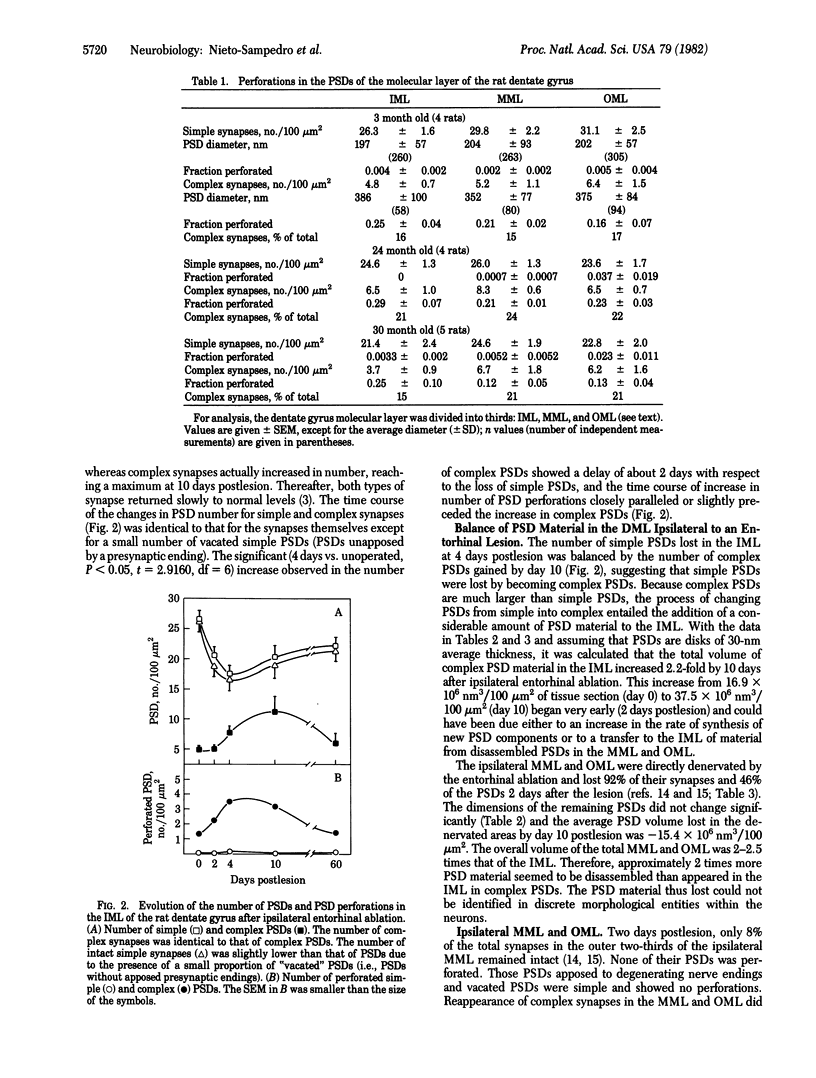
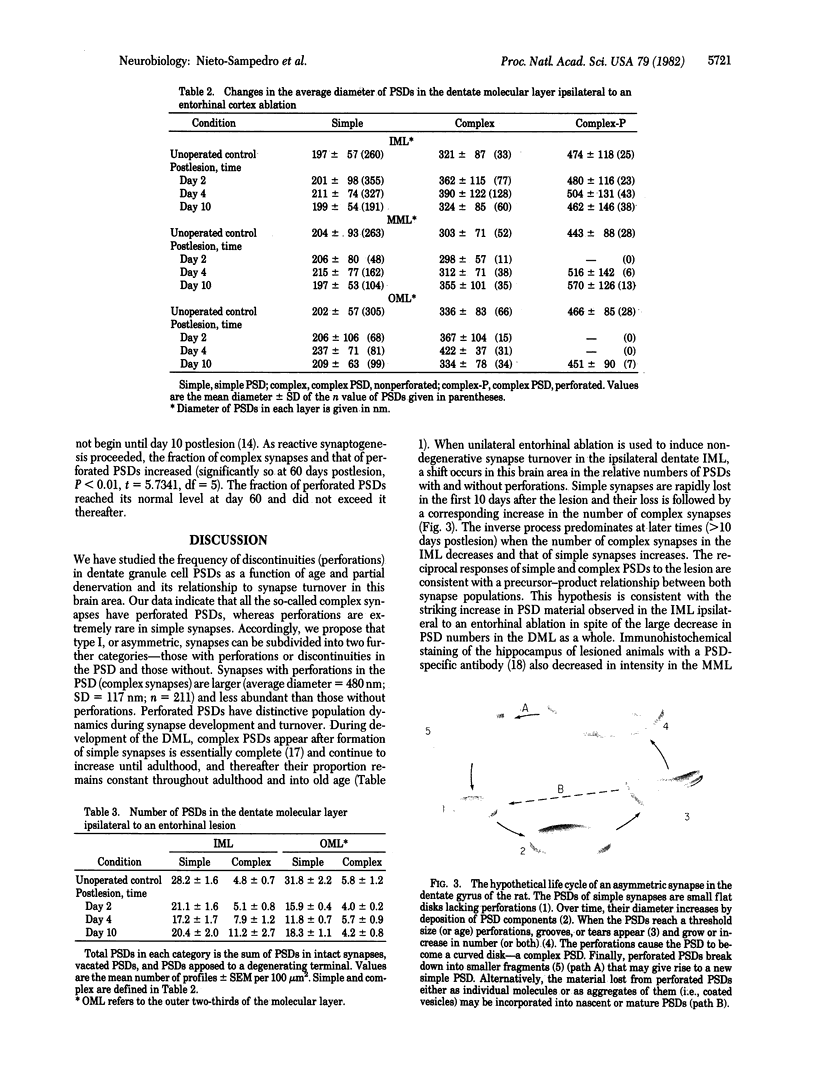
The molecular layer of the dentate gyrus of normal rats shows a large incidence of perforated postsynaptic densities (PSDs). The perforations or discontinuities occur almost exclusively in PSDs located in spines showing a U- or W-shaped junctional profile (complex PSDs). Perforated PSDs account for 16-25% of the total complex PSD profiles in young adult rats and 12-29% of those in aged animals. The frequency of perforations in the inner molecular layer of the dentate gyrus undergoes significant changes during a cycle of nondegenerative synapse turnover induced by ipsilateral ablation of the entorhinal cortex. During the first 2 days postlesion nonperforated PSDs (simple PSDs) decrease sharply, whereas perforated PSDs change little. However, at later times (4-10 days) there is a significant increase in the number of perforated PSDs that balances the number of simple PSDs lost. Beyond 10 days postlesion the proportion of both types of PSD is restored slowly to normal--i.e., nonperforated PSDs increase in number and perforated PSDs decrease, returning to the values in unoperated animals by 120 days postlesion. This inverse relationship between small nonperforated PSDs and large perforated PSDs suggests a precursor-product relationship between them. We propose that perforated PSDs are intermediates in an ongoing cycle of synapse turnover that is a part of the normal maintenance and adaptation of the nervous system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg F., Cohen R. S., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977 Jul;74(1):204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Siekevitz P. Form of the postsynaptic density. A serial section study. J Cell Biol. 1978 Jul;78(1):36–46. doi: 10.1083/jcb.78.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968 Jul;9(2):268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M. Brain function, synapse renewal, and plasticity. Annu Rev Psychol. 1982;33:371–401. doi: 10.1146/annurev.ps.33.020182.002103. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Dyson S. E., Jones D. G. Quantitation of terminal parameters and their inter-relationships in maturing central synapses: a perspective for experimental studies. Brain Res. 1980 Feb 3;183(1):43–59. doi: 10.1016/0006-8993(80)90118-3. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Greenough W. T., West R. W., DeVoogd T. J. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978 Dec 8;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Hatton J. D., Ellisman M. H. A restructuring off hypothalamic synapses is associated with motherhood. J Neurosci. 1982 Jun;2(6):704–707. doi: 10.1523/JNEUROSCI.02-06-00704.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff S. F., Scheff S. W., Benardo L. S., Cotman C. W. Lesion-induced synaptogenesis in the dentate gyrus of aged rats: I. Loss and reacquisition of normal synaptic density. J Comp Neurol. 1982 Mar 1;205(3):246–252. doi: 10.1002/cne.902050304. [DOI] [PubMed] [Google Scholar]
- Hoff S. F., Scheff S. W., Kwan A. Y., Cotman C. W. A new type of lesion-induced synaptogenesis: I. Synaptic turnover in non-denervated zones of the dentate gyrus in young adult rats. Brain Res. 1981 Oct 5;222(1):1–13. doi: 10.1016/0006-8993(81)90936-7. [DOI] [PubMed] [Google Scholar]
- Hoff S. F., Scheff S. W., Kwan A. Y., Cotman C. W. A new type of lesion-induced synaptogenesis: II. The effect of aging on synaptic turnover in non-denervated zones. Brain Res. 1981 Oct 5;222(1):15–27. doi: 10.1016/0006-8993(81)90937-9. [DOI] [PubMed] [Google Scholar]
- Jones D. G., Devon R. M. An ultrastructural study into the effects of pentobarbitone on synaptic organization. Brain Res. 1978 May 19;147(1):47–63. doi: 10.1016/0006-8993(78)90771-0. [DOI] [PubMed] [Google Scholar]
- Laatsch R. H., Cowan W. M. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J Comp Neurol. 1966 Nov;128(3):359–395. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Cotman C., Lynch G. An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. I. Magnitude and time course of degeneration. Brain Res. 1976 Oct 8;115(1):1–21. doi: 10.1016/0006-8993(76)90819-2. [DOI] [PubMed] [Google Scholar]
- Medosch C. M., Diamond M. C. Rat occipital cortical synapses after ovariectomy. Exp Neurol. 1982 Jan;75(1):120–133. doi: 10.1016/0014-4886(82)90012-7. [DOI] [PubMed] [Google Scholar]
- Müller L., Pattiselanno A., Vrensen G. The postnatal development of the presynaptic grid in the visual cortex of rabbits and the effect of dark-rearing. Brain Res. 1981 Jan 26;205(1):39–48. doi: 10.1016/0006-8993(81)90718-6. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Bussineau C. M., Cotman C. W. Isolation, morphology, and protein and glycoprotein composition of synaptic junctional fractions from the brain of lower vertebrates: antigen PSD-95 as a junctional marker. J Neurosci. 1982 Jun;2(6):722–734. doi: 10.1523/JNEUROSCI.02-06-00722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969 Sep 22;100(4):487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Sampedro M. N., Bussineau C. M., Cotman C. W. Postsynaptic density antigens: preparation and characterization of an antiserum against postsynaptic densities. J Cell Biol. 1981 Sep;90(3):675–686. doi: 10.1083/jcb.90.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrensen G., Cardozo J. N. Changes in size and shape of synaptic connections after visual training: an ultrastructural approach of synaptic plasticity. Brain Res. 1981 Aug 10;218(1-2):79–97. doi: 10.1016/0006-8993(81)90990-2. [DOI] [PubMed] [Google Scholar]