Abstract
The essential (indispensable) amino acids (IAA) are neither synthesized nor stored in metazoans, yet they are the building blocks of protein. Survival depends on availability of these protein precursors, which must be obtained in the diet; it follows that food selection is critical for IAA homeostasis. If even one of the IAA is depleted, its tRNA becomes quickly deacylated and the levels of charged tRNA fall, leading to disruption of global protein synthesis. As they have priority in the diet, second only to energy, the missing IAA must be restored promptly or protein catabolism ensues. Animals detect and reject an IAA-deficient meal in 20 min, but how? Here, we review the molecular basis for sensing IAA depletion and repletion in the brain’s IAA chemosensor, the anterior piriform cortex (APC). As animals stop eating an IAA-deficient meal, they display foraging and altered choice behaviors, to improve their chances of encountering a better food. Within 2 h, sensory cues are associated with IAA depletion or repletion, leading to learned aversions and preferences that support better food selection. We show neural projections from the APC to appetitive and consummatory motor control centers, and to hedonic, motivational brain areas that reinforce these adaptive behaviors.
Keywords: Nutrient sensing, Anterior piriform cortex, Hypothalamus, Feeding circuits, Essential amino acids, Foraging, Learned aversion, Learned preference, GCN2
Introduction
Appropriate food selection is crucial for survival. Obtaining an adequate diet relies on awareness of the body’s present homeostatic state, or “nutrient sensing”, and appropriate responses. These responses should be quick because prolonged foraging in the wild can put an animal at risk of being the next meal for a predator. Nutrient levels are sensed by the brain, directly or indirectly; the brain also directs subsequent feeding-related responses, such as food choice and foraging. All of the macronutrients: proteins, fats and carbohydrates, contribute energy to the body. Also, proteins and fats provide essential molecules, which are indispensable in the diet because they cannot be synthesized in the body. Essential fatty acids have ample potential for storage, but because the indispensable amino acids (IAA) are not stored [1], they must be obtained from our food. After short-term energy needs are met, IAA are the nutrients with the highest priority. We review the homeostatic systems for maintaining IAA levels, detection of deficiency, and the neural circuits underlying behaviors that support diet selection and maintenance of IAA balance in the internal milieu.
Protein and IAA Deficiency
Given a choice, animals select (balanced) protein within a broad range of concentrations [2]; they avoid both very high (over 75 %) and very low-protein diets [3, 4]. It has long been known that rats will reject a low-protein diet, at 5–6 % or less [5]. Yet, such a low level might only be seen in the diets of fruitarians that avoid grains, seeds, and nuts. In contrast, White [6] showed that animals increase their food intake, becoming hyperphagic, if a diet, with a balanced IAA profile, is just marginally low in protein (8–10 % of the diet) [6–8], a level consistent with vegan-vegetarian diets. In White’s studies, the increases in feeding were associated with changes in orexigenic peptides in the hypothalamus [6]. At this level, the increased food eaten due to the generalized hyperphagia could provide enough protein for maintenance. Animals do not always select foods in a way that would show a “protein appetite” [9]; Morrison et al. [10] note that a mechanism for understanding the maintenance of protein homeostasis is lacking. Nevertheless, both animals [11] and humans [12] are reported to select a higher protein source after eating a low-protein meal; these choices typically are seen in a protein “need state” [13].
Protein quality is determined by the level of IAA precursors available for protein synthesis, specifically, the growth-limiting IAA in the food [5]. The most limiting IAA for protein synthesis is associated with the greatest accumulation of uncharged transfer RNA (tRNA). Because tRNA charging is competitive [14, 15], depletion can be delayed by high levels of circulating IAA, such as after a high protein meal [16]. Much of the work on IAA depletion has used the precisely defined basal, imbalanced and “corrected” IAA diets described by Harper and colleagues (see relative proportions in Fig. 1 of [5]). This model, also termed “aminoprivic”, provides appropriate nutritional controls. Note that the basal prefeeding protocol avoids unintended competition for the tRNA aminoacyl synthetases, allowing prompt sensing of IAA changes.
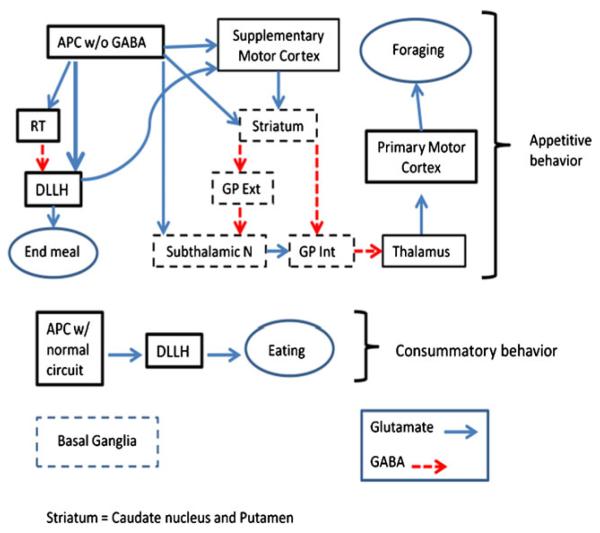
Fig. 1.
The projections from the APC to brain areas important to locomotor activity are shown for Appetitive behavior in foraging. Included are: the Supplementary Motor Cortex (AGm), the Basal Ganglia (BG) outlined in dashed rectangles, the dorsolateral perifornical hypothalamus (DLLH), which projects to the AGm, and the Primary Motor Cortex, which receives the processed output for locomotor activity from the BG, via the Thalamus. Included in this segment is the inhibitory RT, which projects GABAergic signals to the DLLH, to end the meal and allow foraging. In the Consummatory behavior segment, the APC, with its normal level of inhibitory function under IAA repletion, would not activate the RT’s inhibition of the DLLH, allowing feeding to proceed. The dashed arrows show GABAergic pathways and the solid arrows indicate glutamatergic pathways. Abbreviations are: APC, anterior piriform cortex; RT, reticular thalamus; DLLH, dorsolateral perifornical hypothalamus; GP ext, globus pallidus externa; GP Int, globus pallidus interna; N, nucleus
Sensing Limitation of Protein and IAA
The threshold for sensing the limiting IAA is quite low (rats detect differences as small as 0.009 % of the diet [17]). The biochemical signaling of IAA deficiency starts with accumulation of uncharged tRNA, which activates the general amino acid control non-derepressing kinase 2 (GCN2), in a pathway conserved from yeast [18, 19]. In metazoans, this occurs when any one of their IAA is deficient. Mice depleted of the GCN2 kinase are unable to detect (and do not reject) an IAA devoid meal, showing that GCN2 is required for detection of IAA deficiency [15]. Activated GCN2 phosphorylates eukaryotic initiation factor 2 alpha (eIF2α), which binds the tertiary initiation complex and halts initiation of translation in global protein synthesis [19]. Still, selected mRNAs are translated, such as those for: activating transcription factor ATF4, mitogen-activated protein kinase (MAPK), cJUN, and the sodium-coupled neutral amino acid transporter, SNAT2 [20, 21].
The Anatomy of Sensing IAA Deficiency
In rodents prefed a basal diet under the aminoprivic protocol, ingestion of an IAA-deficient meal results in rapid recognition of IAA depletion in the brain’s IAA chemosensor, the anterior piriform cortex (APC) [22]. This sensory role for the APC is based on the following: (a) ablations of the APC by brain lesions that show its essentiality for detection of IAA deficiency [23–25] (these lesions were replicated in the rat [26], and in the bird [27]), (b) repletion by injecting nanomole amounts of the limiting IAA into the APC to block the anorectic responses [28–30], and (c) electrophysiological measures that show neural activation of the APC in IAA depletion, both in vivo [31] and in vitro [32]. These findings have been reviewed in detail [25, 33–35]. The APC is a paleocortex, part of the most primitive mammalian cortex [36]; this is appropriate as a conserved sensor for such an important eukaryotic function. The neuronal circuit within the APC is well studied [37], as are its projections to the other elements of the olfactory cortex and beyond. The principal neurons are pyramidal cells having complex axons that excite each other in a recurrent excitatory (glutamatergic) pattern using n-methyl daspartate (NMDA) receptors [38]. This positive feedback loop is kept under control by inhibitory interneurons, many of which are gamma amino butyric acid (GABA)-ergic [39]. Along with the glutamatergic pyramidal cells, axons of other cell types (possibly including neuropeptide Y (NPY) [40]), provide the output of the APC [37], but glutamatergic axons predominate [41].
Using the isolated APC in an in vitro brain-slice preparation, we demonstrated that the APC is not only necessary but sufficient for sensing IAA depletion [32]. The precise location of the IAA-sensing neurons in the APC is shown by doubly labeled neurons for phosphorylated-mitogen-activated protein kinase (P-MAPK) and P-eIF2a [42], and by electrode placements determined in our electrophysiological recordings [32]. The responsive cells are found in a narrow band at 2.4–3.7 mm rostral of Bregma [32, 42]. This region corresponds to the “Area Tempestas” [43] described by Gale [43], which has a paucity of inhibitory elements. Limited inhibition explains the chemosensitivity of the APC [44], and is important for its sensitivity to the effects of IAA depletion.
The Mechanism
To describe the initial mechanism for sensing IAA deficiency in the APC [15], we reported that (a) the limiting IAA is decreased within the APC by 20 min after introduction of an IAA-deficient meal [45], (b) inhibition of tRNA charging, by injecting an IAA-alcohol into the APC, generates the same anorectic response as dietary IAA depletion, (c) GCN2 is required for the anorectic response and (d) its substrate, P-eIF2α, is activated in the APC [15, 46]. Major portions of these findings have been replicated by others [47, 48]. With initiation of protein synthesis inhibited in the APC [15], the already limited GABAergic control is lost [49], and the APC is disinhibited (activated) in minutes. When GABAergic function is so markedly decreased, other neurochemical systems are potentiated, especially glutamate.
Other Potential Sensors for IAA Deficiency
It is important to note that olfaction, per se, is not necessary for the responses to IAA depletion [50], or for its role in learned behaviors [51]. To demonstrate activation of the APC without olfaction, Choi et al. [51] showed that neurons of the APC can be entrained to activate either aversive or appetitive behaviors, absent olfactory sensation. Similarly in IAA depletion, activation (disinhibition) of the APC is due to P-eIF2α blocking the initiation of protein translation, and the loss of inhibitory elements within the APC, rather than resulting from olfactory or other neural input [32].
Rogers and Leung [52] demonstrated that taste is not required for recognition of an IAA deficiency. They also showed that animals will eat a complete diet containing a bitter tastant in preference to a sweet-tasting IAA-imbalanced diet, so a bitter taste does not interfere with the choice of a better meal. Harper had earlier [5] argued that because animals can choose a corrected diet over an IAA-imbalanced diet, where each contain 6 % free amino acids, but differ by only 0.1 % of the limiting IAA, the sensing cannot be by taste or smell. Moreover, intravenous or intragastric infusions of imbalanced IAA, which bypass the sensory systems for taste and smell, cause food intake depression. Taken together, we can conclude that neither of these primary chemical senses is required for sensing IAA deficiency.
It is clear that the rejection of a food containing an IAA deficiency is not due to satiety, either. This was shown by the absence of the satiety sequence [53] and the observations that animals will eat almost anything else rather than the IAA-deficient food [54]; this shows that they are not satiated. Also the classical satiety center, the ventromedial hypothalamus (VMH), has no role in the sensing of an IAA-deficient diet [55], and the VMH does not receive projections from the relevant area of the APC [56].
Another putative amino acid sensor is the mammalian target of rapamycin (mTOR) [57]. To learn if mTOR would serve in the recognition of IAA deficiency in the APC, we injected rapamycin into the APC of intact rats. This well-described inhibitor of mTOR does inhibit the phosphorylation of S6K1 in APC tissue, as expected if the dose and drug are appropriate, but there is no effect on the behavioral responses to either IAA-deficient or control diets [58]. Injection of an mTOR agonist, isoleucine, into the APC also has no effect on intake of either isoleucine or threonine basal diets, or of a threonine-imbalanced diet [29]. These findings do not support a role for mTOR in sensing IAA depletion in the APC. Still, mTOR may have a role in recognizing increased levels of certain IAA, particularly the branched chain amino acids and methionine [59], see more about this below.
By definition, orexigenic peptides increase food intake. To test their effects on intake of a valine-deficient diet, peptides were administered both intraperitoneally (ip) and centrally [60]. Ghrelin injections into the third ventricle (intracerebroventricular, icv), but not ip, increase valine-deficient diet intake by about 1 g in 2 h, but this is below the level of control intake. Similar icv injections of NPY increase intake of the deficient diet fully to control levels in 2 h. When we [40] used smaller doses of NPY, injected into the APC, we found the reverse: significant dose-dependent decreases in intake of our threonine-deficient diet are seen with NPY after 3 h. The use of threonine vs valine-deficient diets should not explain the differences, so opposite results with NPY are more likely due to experimental differences such as injection sites and doses. Thus, it is not clear that peptides in the APC take part in the sensory response to IAA depletion, although they may be involved in “appetitive” responses, see below.
Many amino acid transporters are known to be upregulated in IAA deficiency [61]. In primary cultures of APC neurons, Blais et al. [62] studied the system A transporter (now known as SNAT1 or 2) as defined by its sodium dependency and its substrate, 2-methylamino-isobutyric acid (MeAIB) [63]. Uptake of labeled threonine or MeAIB is increased in threonine-deficient APC neurons within 10 min; this is inhibited by (a) the blockade of recruitment from the cytoplasm by a high dose of nocodazole, by (b) the mTOR inhibitor, rapamycin, by (c) the extracellular signal-related kinase (ERK) inhibitor, PD98059, and by (d) wortmannin, a phosphatidylinositol 3 (PI3)-kinase inhibitor. The results show that the increased transporter activity occurs too late for a sensory function, and is also secondary to PI3kinase, ERK or mTOR. Thus, the SNATs, while clearly involved in the response to IAA depletion, are unlikely to serve as the primary sensors. Additional findings by Goto and colleagues suggest that there may be amino acid sensing transporters in the tanycytes of the third ventricle in the midline of the hypothalamus [60], based on cFOS immunoreactivity in valine-deficient animals. How these cells contribute to signaling IAA deficiency requires further study.
Taken together, there is no consistent evidence, yet, of a role in sensing IAA depletion for: olfaction, taste, mTOR, orexigenic peptides, peptides requiring protein synthesis, or SNAT. We conclude that the uncharged tRNA/GCN2 system is the primary sensor of IAA deficiency in the APC, followed by loss of GABAergic inhibitory control in the APC circuit, and increased glutamatergic activity.
After Sensing, Signaling
To determine the neurotransmitters that provide signaling of IAA limitation in the APC, we used pharmacological agonists and antagonists microinjected into the APC. The effective receptor types for several neurotransmitters, included norepinephrine (NE), alpha 2, dopamine (DA), D2, GABAA, and the glutamate autoreceptor agonist, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA). Each inhibit the glutamatergic output cells of the APC, and maintain control of the circuitry there. When any of these receptors is blocked, IAA-deficient diet intake is increased (reviewed in [33, 64]); this shows the importance of inhibitory control in the APC’s response to IAA-deficient diets.
During the period leading up to rejection of an IAA-deficient diet, along with phosphorylation of eIF2α in the APC, one also sees increased phosphorylation of: the glutamate receptor (GluR1), MAPK (as ERK1/2), calcium calmodulin kinase II (CaMKII) [42, 65, 66], and increased expression of cJUN [46]. The phosphorylation of GluR1 is short-lived, consistent with brief activation of the glutamate output neurons, but both ERK1/2 and CaMKII remain phosphorylated longer. Secondary neurons, expressing P-CaMKII, are activated post-synaptically over a broader range of cells within the APC. These signaling molecules provide further evidence for projection sites from the APC.
Activation of the APC is followed within the first meal (20 min) by rejection of an IAA-deficient food [45] and increased locomotor activity. The activated APC then projects to other brain areas associated with the control of food intake, resulting in meal disruption [67] and termination [45]; appetitive behaviors including foraging ensue. Projections from the activated APC that lead to inhibition of eating and onset of foraging are sketched in Fig. 1. They extend to destinations that have been described in detail [68, 69]. Haberly and Price [68] injected horseradish peroxidase (HRP) into the APC to determine these destinations; most helpful to this review are results from injection sites that correspond to the locations identified by Sharp et al. [42] and Rudell [32] as responsive to IAA-deficient diets or media. Aja [69] confirmed and extended those findings by injecting biocytin into the precise locations in which an injection of the limiting IAA ameliorates the anorectic response to IAA-deficient diets [28–30]. What follows is a description of the rostral, medial, and caudal projections from the APC, as determined in the tract tracing studies, with notations about how they function in the integrated responses to IAA deficiency.
Rostral Projections from APC
Certain cortical sites that receive projections from the APC are relevant here, including the prefrontal cortex (PFC), reached either directly or via the mediodorsal thalamus [70]: the medial agranular cortex (AGm) is a rostral area of the PFC that serves as a supplementary premotor cortex [71], the orbitofrontal cortex (OFC), and the insular (taste cortex (IC). The AGm is activated just prior to the initiation of movement in a choice paradigm, as are other “value-related” neural areas such as the OFC and striatum (STR) [72]. The AGm shows significant P-MAPK expression (P<0.04) in response to a threonine devoid diet (unpublished data from a parallel study to reference [65]), and likely serves in the initiation of locomotor activity seen with IAA deficiency (see APC projecting to (→) supplementary motor cortex, AGm, in Fig. 1). The OFC integrates feeding-related information including taste, vision and touch (for “mouth feel” or the texture of food) [73, 74]. The IC, for taste, receives NTS projections via the parabrachial nucleus (PBN) and thalamus [75]. These projection sites may play a role in responses such as food choice and learned associations, discussed below.
Medial Projections from the APC
As can be seen in Fig. 2a, fibers from the APC cross from the globus pallidus (GP) through the internal capsule (single arrow at the top of the figure) on their way to the reticular thalamus (RT), identified by the three arrows. Many axons also follow the internal capsule to the dorsolateral perifornical hypothalamic area (DLLH), as seen in Fig. 2b (APC→DLLH in Fig. 1).
Fig. 2.
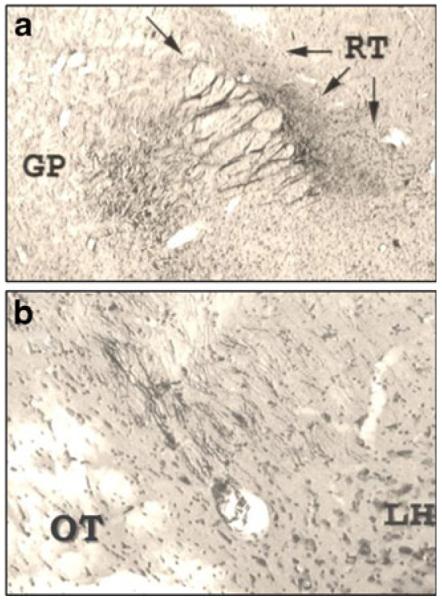
Photomicrographs of biocytin tract tracing from [69], showing: a Axons running from the globus pallidus (GP), left, crossing through the internal capsule (indicated by the single arrow at the top) to the reticular thalamus (RT) at the three arrows, on the right. b Monosynaptic axons from the highly chemosensitive region of the APC, which recognizes IAA deficiency using the GCN2 system, are shown following the internal capsule and ending in the dorsolateral perifornical LH (DLLH). Note that the internal capsule is in the same orientation in both frames. Abbreviations are OT, optic tract; LH, lateral hypothalamus
Several lines of evidence suggest that the DLLH is important to our understanding of the APC’s projection sites in IAA deficiency. Tract tracing studies using HRP show similar projections from the APC to this specific hypothalamic area [56]. Here are the terminations of the biocytin axons of Aja [69]. The hypocretin neurons of Karnani [76], in the DLLH, retrogradely label the IAA-sensitive region of the APC just deep to the lateral olfactory tract [56], where Beverly’s [29] IAA injections are effective. In line with these observations, injections of the limiting IAA, threonine, specifically into the dorsal, but not ventral, area of the LH increased threonine-imbalanced diet intake slightly, but significantly, at 6 h [77]. Note that when the limiting IAA is injected directly into the APC, the anti-anorectic effects are stronger and seen earlier [78] than when injected into the LH. Retrograde transport supports a monosynaptic connection between the two areas. Moreover, Monda [30] showed increased firing in LH neurons 30 min after injection of threonine into the APC of threonine-deficient rats; so, cells in the LH respond to IAA repletion in the APC of animals when they are deficient. Taken together with the work of Beverly [28, 29] and Torii’s group [79], these results strongly support the importance of the DLLH in the responses to IAA repletion.
The DLLH also projects to the AGm in the rat [72] for initiation of locomotion. In line with this, stimulation of the DLLH causes locomotor activity in the limbs of anesthetized rats [80], and the LH projects caudally to the midbrain locomotor center as well [81]. Thus, these projections are useful in foraging.
While not directly sensing deficiency of an IAA, other hypothalamic areas may be related to food seeking. Both the PVN and the DMH have shown increased labeling for P-MAPK and cFOS with IAA deficiency [65, 82, 83], and fibers around the DMH, although not cells within the DMH, have been shown to be involved in the responses to IAA depletion, as well [84, 85]. So, these hypothalamic areas may play roles in secondary integrative responses, in addition to the importance of the DLLH in foraging.
The APC projects biocytin label [69] to several motor areas also associated with initiation of locomotor activity, including the STR (the combined caudate and putamen), and other sites in the basal ganglia (BG; APC→STR and subthalamic nucleus, Figs. 1 and 3). Cortical areas project to the BG providing initiation and guidance for locomotor activities through projections back to the motor cortex via the thalamus. (A simplified sketch of the BG is included in Fig. 1, the relevant BG segments are surrounded by dashed lines in the figures). The STR is also associated with the DA feeding-reward and motivation system [86], which will become relevant later.
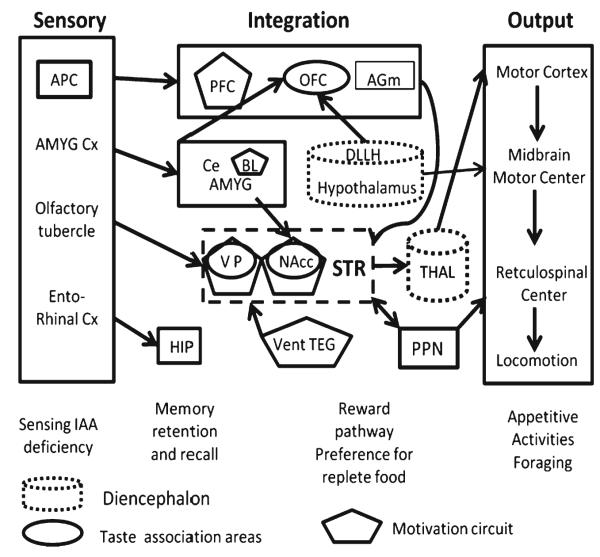
Fig. 3.
The brain areas associated with the sensory, integrative, and motor output functions associated with the sensing of and responses to IAA-deficient diets. Under Sensory, the regions belonging to the olfactory cortex, with their projections, are shown on the left of the figure. Under Integration, the top box represents cortical areas, prefrontal (PFC), orbitofrontal (OFC), and supplementary motor (AGm). As subcortical structures, central and basolateral amygdala (Ce, BL AMYG) and DLLH hypothalamus are as in Fig. 1. In the dashed box, ventral pallidum (VP) and nucleus accumbens (NAcc) are members of the basal ganglia in the striatum (STR). The ventral tegmentum (vent TEG) projects to the STR in the motivation circuit (Reward); at this level the pedunculopontine nucleus (PPN) also receives input from the STR and projects to reticulospinal centers. Members of the motivation circuit are in five-sided boxes, taste association areas are in ovals. The diencephalon includes the hypothalamus (and DLLH) and thalamus (THAL), indicated in dotted cylinders, for comparison with the lamprey circuit. Under Output are the motor centers for appetitive activities such as foraging. The hippocampus (HIP) is important for spatial memory
Several regions of the STR that receive glutamatergic projections from the APC, and are associated with the initiation of locomotor activity, also contain hedonic centers (for reward), important in food choice. The ventral pallidum (VP) and nucleus accumbens (NAcc) also belong to the STR and are components of the reward system, in which DA plays a major role [87]. The NAcc sends GABAergic axons to the VP. The studies of Yamamoto and Ueji [88] led to the idea that after development of an aversion, the conditioned stimulus causes increased GABA release in the VP via AMYG projections to the NAcc. The “Motivation circuit” (in Fig. 3) represents these areas, and is important in food selection, to be discussed later. This anatomy is consistent with our findings that dopamine, via the D1 receptor (tested with a systemic D1 antagonist) is involved in aminoprivic feeding [89]. Also, interstitial homovanillic acid, a DA metabolite, was decreased in APC by 20 min after IAA repletion, suggesting that when the needed IAA is replenished, DA activity in the APC is decreased [90]. Moreover, DA inhibits glutamatergic activity in the APC via the D2 receptor [64]. The interactions among glutamate, GABA and DA in the BG are complex and beyond the scope of this review, but clearly, the APC is positioned to initiate locomotor activity via its glutamate projections to the BG, and DA’s activity in hedonic centers is important in this system. Both the subsequent locomotor activity and the hedonic responses fit the classical roles of these areas.
Caudal Projections
The APC is the most anterior region of the olfactory cortex, and its caudal projections reach the rest of the olfactory cortical areas, defined as those areas receiving primary input from the main olfactory bulb. Taken together, the olfactory cortex (Fig. 3, left side) includes the anterior olfactory nucleus, the APC and posterior piriform cortex with the deeper endopiriform area, the olfactory tubercle, the transitional entorhinal cortex, and the amygdalar cortex (AMYG Cx) [37, 56]. Further caudally, biocytin-labeled neuron terminals were also seen in the subthalamic nucleus (STN) of the BG (seen earlier in Fig. 1) and the ventral zona incerta (ZI) [69]. Haberly and Price [68] reported ipsilateral projections to the ventral tegmental area (vent TEG) (the A10 dopaminergic area) showing that the mesolimbic DA system (also associated with the BG, and motivation [91]) receives projections from the APC (Fig. 3). Also at the level of the TEG, the pedunculopontine nucleus (PPN) affects locomotor activity via connections with the BG and reticulospinal center (Fig. 3).
Olfaction and Locomotor Activity, a Primitive Model for the Anatomical Circuit
The APC’s importance in locomotor activity (for foraging), once IAA deficiency has been detected, is also a conserved function. How does activation of an olfactory cortex lead to changes in motor behavior? The most primitive olfactory–locomotor circuit, described in detail so far, has well-defined neuronal links between the olfactory tuberculum and the command neurons for locomotion [92]. The sea lamprey, a jawless fish, was used in a reduced preparation; olfactory stimuli were applied to the preparation. The responding circuit included olfactory inputs conveyed to the tuberculum, then to the diencephalic locomotor center and from there via the midbrain locomotor region to brainstem reticulospinal neurons that activate swimming [92]. Clearly, the lamprey is a very primitive, parasitic animal, but it does orient toward molecules, including amino acids, that might lead to host fish. We suggest that this simple neural circuit may provide a model for work in higher animals.
In the rat analogous structures to those in the lamprey exist: (a) the olfactory tubercle is part of the olfactory cortex, (b) the thalamus and hypothalamus, including the DLLH, make up the diencephalon (see the cylinders outlined by dots in Fig. 3), and (c) the midbrain locomotor region is important in the initiation of motor activity [81]. Similarly in the rat, the LH projects to the midbrain locomotor center, as described above, which projects to the reticulospinal center for initiation of “appetitive” locomotion [81] (again, see Fig. 3).
Behavioral Responses to IAA Deficiencies and Incomplete Protein
Checking Out a New Food and Rejection of an IAA-Deficient Meal
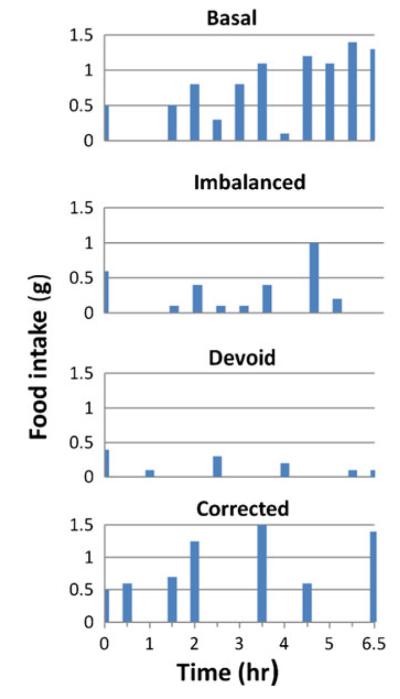
Rats have a strategy for investigating novel foods, termed neophobia [74]; it requires the central nucleus of the amygdala (CeAMYG) and the IC (reviewed in [93]). This is adaptive as clearly animals have no way to know the biological value of a new food in advance. In demonstrating neophobia, rats sample the food and then wait a bit (lengthening the first intermeal interval), before they gradually take smaller samples, and finally accept a meal that has not yielded adverse effects. Computer recordings made in the 1970s from rats eating their first isoleucine (an IAA)-deficient or corrected meal show these patterns. In Fig. 4 (replotted from Figs. 1 and 2 in reference [24]), all four diets, basal, imbalanced, devoid, and corrected, were eaten similarly for the first 0.5 h. With the basal-prefeeding diet and both deficient diets, one sees a delay of 1–1.5 h before feeding resumes, consistent with neophobia. After the delay, it is also clear in the figure that, with the imbalanced and devoid diets, rats continue taking small samples. No full meals are seen for the 6.5-h period shown; and, even less is eaten of the more deficient, devoid diet than of the imbalanced diet. In contrast, if there are no deleterious effects, the meal continues, as seen in the isoleucine basal and “corrected” (control diet) recordings (Fig. 4). In the responses to the imbalanced and devoid diets, the APC is activated, eating is disrupted (shown in full detail using microstructural analysis [67]), and the meal ends prematurely. These behaviors have been termed anorexic, so the mechanism for meal termination could be inhibition of the “feeding center”, the LH [94].
Fig. 4.
Food intake data showing feeding responses to IAA basal, imbalanced, devoid or corrected diets, are replotted, with permission from [24]. The data are taken from computerized food intake records of two individual rats eating the diets listed at the top of each panel. The top two panels represent one rat’s feeding patterns; the bottom two panels are from the second rat. Food intake is given in grams per half-hour bins. The time is plotted on the X-axis, for the first 6.5 h of each animal’s first exposure to imbalanced, devoid or corrected diets, compared with the basal control (prefeeding) diet
There are at least two straightforward routes for the disinhibited (activated) APC to inhibit the LH feeding center, based on anatomical evidence: first, projections from the APC may be inhibitory, so that an inhibitory neurotransmitter from the output neurons could reach the LH. There are GABAergic neurons in the APC, which are inhibitory, but they tend to be interneurons. Moreover, GABA in the APC affects feeding in general, and is not selective for IAA status [49]. Because GABA function is decreased in the APC in IAA deficiency, the immediate APC output should be glutamatergic. Using both NMDA and AMPA receptor antagonists, we saw that glutamate in the APC is associated with increased food intake [95]. Yet, feeding is disrupted rather than increased, so this first route may not provide the mechanism.
A second route involving glutamatergic projections from the APC seems more likely: axons migrate through the globus pallidus (GP) in the BG, terminating in the reticular thalamus (RT), as clearly seen in Fig. 2a. The RT contains GABAergic cells that are known to project to, and inhibit the activity of, the LH [96]. So, in this route, glutamatergic output from the APC stimulates the RT [69], resulting in GABAergic inhibition of the LH and the meal ends. Repletion of the limiting IAA would reverse this sequence, leading to consummatory behavior (eating the better meal), consistent with the evidence for repletion, below. This is seen with the corrected diet in Fig. 4, as feeding resumes within 30 min of the first corrected meal. Repletion will be discussed later.
Appetitive Behavior: Foraging
Along with ending the meal, the animals dig into their food cups, increasing food spillage [97], and they become more active [54]; these behaviors are indicators of foraging. Because eating and foraging are mutually exclusive, at least in quadrupeds, the animal must leave the food cup, effectively ending the meal, in order to begin foraging. A precedent for this is seen in the “NPY paradox” in which “appetitive” ingestive behavior (searching for food) is distinguished from “consummatory” ingestive behavior (chewing and swallowing) [98]. Consistent with our results with NPY given above, this orexigenic peptide is reported to have its main effect on appetitive behavior, rather than eating [98]. Again, the DLLH and the hypothalamic projection sites of Aja [69] and Price et al., [56] provide the anatomical substrates. An intriguing finding is that the same perifornical region of the DLLH contains orexin/hypocretin neurons projecting to motor areas that control foraging [76]. These neurons (a) respond to increases in non-essential amino acids, which may be increased in IAA deficiency, (b) are required for foraging in fasted mice, and (c) are thought to be important in activating feeding circuits based on the macronutrient balance of the animal [76]. Clearly, foraging and food choice strategies are preferred over anorexia and catabolism. Therefore, rather than the APC inhibiting the LH feeding center, the stimulatory effects may result in foraging and altered food choice.
After the Initial Signaling
By 2 h after the first meal, tRNA charging in the APC has returned to normal or higher levels [99]; this shows that the supply of IAA at the cellular level is restored, likely by catabolism [100]. The GCN2 system in the APC is no longer activated; the synthesis of inhibitory neural elements is in progress and GABAergic control over the glutamatergic output resumes. In feeding records, if rats have no other choice, they will take another, smaller, meal, but only after a prolonged intermeal interval. This can be seen in the imbalanced diet feeding pattern (in Fig. 4, at 4 h), or by using microstructural analysis [67]. Electrophysiology in vivo shows that the activation of the APC is diminished by a few hours after the first IAA-deficient meal [31]. At this point, the APC’s role as the initial IAA deficiency sensor is finished, and alternative strategies are needed.
Learned Responses, Aversion and Preference
Animals learn to associate IAA-deficient diets with taste, olfactory, auditory, visual, and spatial cues [79, 101, 102]. Then they continue to forage for additional food choices, avoiding the deficient food and any cues with which there is an association (see for example [102–105]). This is known as the “Garcia effect”, or conditioned aversion [106]. Associative learning takes time, as it requires protein synthesis, which takes longer than the 20-min period seen before rejection of the first IAA-deficient meal [45], but the Garcia effect is a long-lasting form of single-trial learning. In the work of Simson and Booth [107], at some point between one and four hours after gavage feeding of a preparation deficient of histidine (a conditional IAA), paired with exposure to an odor cue, rats acquire an aversion to the odor associated with the deficiency. This timing helps to distinguish sensing of IAA deficiency (which we have covered in detail above) from the development of aversion to an IAA-deficient food.
The development of such an aversion also may be associated with omnivory. For example, the cat, as a representative carnivore, can recognize a methionine-deficient food at its first day of introduction, selecting the higher level of the limiting IAA, yet they do not appear to recognize a threonine deficient diet [108]. In contrast, in an omnivorous rat, any IAA-deficient diet will be rejected immediately the next day, demonstrating its aversion to the food. If the cat has failed to develop the ability to learn an aversion to an IAA-deficient food other than methionine, which is high in meat, it may be that during the evolution of the carnivore, their meals rarely, if ever, contained a deficient IAA profile. So again, sensing IAA depletion can be separated from aversion to an IAA-deficient food.
The APC also serves as an association cortex, and can drive differing responses based on learning [51]. However, rats with effective lesions of the APC cannot sense the IAA-deficient diet, but they still show an aversion in a lithium chloride test [109]; they do not reject the IAA-deficient diet, but they still can develop an aversion to a cue paired with a toxin. Thus, several aspects of the mechanisms serving the sensing and initial rejection of an IAA-deficient food differ from those active in the development of aversion [25, 74, 82, 83, 93, 110]; additional brain regions must be involved.
The Anatomy Associated with Conditioned Food Aversions
The neural circuitry for conditioned taste aversion (CTA) has been described [88, 111, 112]; a large literature on the topic exists [113], and other sensors, providing associative cues, such as taste, smell, etc., are involved. Note that the sensory input from the taste pathway interacts with many of the same brain areas that serve the earlier responses to IAA deficiency (see Fig. 5). Included in the neural circuit for olfactory-assisted CTA are: the AMYG [69, 114], both basolateral (BL) [111] and central (Ce) [82] for emotional learning, the IC for taste [74], the PBN, a secondary taste relay shown to be important in IAA aversion by Fromentin et al. [115]. Flavors are associated with aversion to an IAA-deficient diet in a typical CTA [52], which also requires the AMYG [109]. In addition, MRI data show involvement of the NAcc and the VP, both hedonic centers [112]. Implicated in taste aversion are the area postrema (AP), a portion of the blood brain barrier that is leaky and can allow toxins along with nutritional molecules entry into the brain, and the ventromedial globus pallidus (GP) of the BG, for the hedonic responses. The hippocampus (HIP), for spatial learning [111], receives input from the APC via the entorhinal cortex [116]. Leung and Rogers [117] showed that HIP lesions do not disrupt the initial rejection of an IAA-imbalanced diet, but do facilitate adaptation to the diet, suggesting disruption of the learned responses [117]. It is now thought that the HIP receives input from the AMYG for “contextual fear formation” [88], which should aid the learning process.
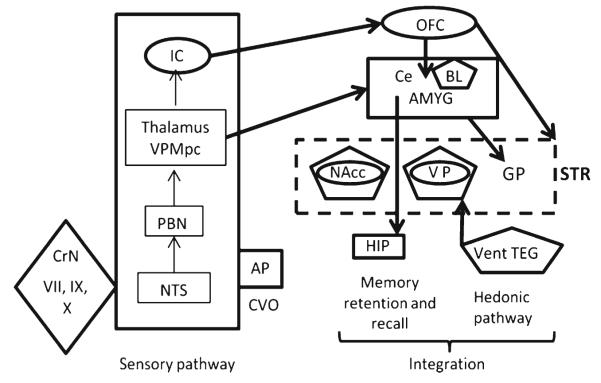
Fig. 5.
Anatomical features of the taste-aversion or preference pathways. The Sensory pathway for taste is at the left of the figure, with cranial nerves for taste (CrN VII, IX, and X) coming in to the brainstem at the level of the nucleus tractus solitarius (NTS). The NTS projects to the parabrachial nucleus (PBN) and then to the taste relay in the thalamus (VPMpc) and finally to the insular cortex (IC). The area postrema (AP) is a circumventricular organ (CVO) allowing molecules in the blood to enter the brainstem at the level of the NTS. Other elements of the figure, for Integration with the learned aspects of the responses, are the same as in Fig. 3
After Successful Foraging, Recognition of Repletion
After initiation of foraging, if a food source providing IAA repletion is found (as with the corrected diet in Fig. 4), or external injections have supplied the limiting IAA [28, 29], a different sequence ensues (a) protein synthesis resumes, : (b) GABAergic function is restored in the APC, (c) appetitive foraging can subside, and (d) normal consummatory feeding behavior resumes within 30 min. Intact protein synthesis in the APC is required for recognition of repletion [118]. Note also, in Fig. 4, that a second 0.5 g of the corrected diet was eaten at 30 min. The APC shows no activation in electrophysiological recordings made from formerly IAA-deficient animals who receive the corrected diet for the first time [64]. The early acceptance of the corrected diet is consistent with the work of Monda [30] and many elegant studies, using lysine-deficient animals, by Torii’s group [79, 119], showing that neurons in the LH increase firing with repletion of the limiting IAA.
Other Potential Sensors for IAA Repletion
Although taste is not involved in early sensing of IAA limitation, or immediate repletion [120], it does reinforce odor-based learning and flavors can be used as an associative cue in secondary learned food choices, aversions and preferences. Individual amino acids, obtained either from the food directly or after protein digestion, are sensed by the taste receptors of the T1R (G protein-coupled) category for sweet or savory stimuli [121]. Along with vagal afferents, amino acid-related signals project to the NTS via the cranial nerves for taste [122]. Afferents from taste cells synapse with the facial (VII), glossopharyngeal (IX), and vagal (X) cranial nerves terminating in the rostral NTS [123], which has secondary projections to more rostral brain sites [124] (Fig. 5). The neurochemistry and neurophysiology of taste are beyond the scope of this review (as an example of many reviews, see [125–128]).
An increase in the most abundant IAA, leucine, which may signal protein and IAA sufficiency, activates the mTOR system [57]. As leucine is relatively selective among the amino acids as an agonist for mTOR and the selective inhibitor, rapamycin, inhibits feeding when injected into the hypothalamus, Cota and colleagues [129] concluded that leucine-activated mTOR controls feeding by acting in the hypothalamus. If leucine is the signal carrier for rich diets, the decrease in food intake that they saw would support this idea. However, mTOR is involved in cellular metabolism, energy homeostasis and cell growth as well [130], and mTOR also responds to energy and fatty acids, but importantly, not to all IAAs. For example in adipocytes, the branched chain amino acids, and methionine, were the only amino acids activating mTOR [59]. Thus a role for mTOR, either as a general amino acid sensor, or in the recognition of an appropriate balance of IAA, seems unlikely.
Other sensors for increases of amino acids have been reported; amino acids can be recognized by specialized transporters that may provide sensing [131, 132]. Because amino acid transporter proteins are found in the cell membrane, and yeast have a modified amino acid transporter that does sense amino acids in the medium [133], these transporters may sense amino acids in the extracellular space [134]. Amino acids are also recognized by calcium-sensing receptors (CaSR) [135]. In line with this, L-phenylalanine (an IAA) is sensed by the CaSR in gut cells [136]. The CaSR belong to the G-protein coupled receptor family along with the T1Rs, and some of the glutamate and GABA receptors [137], yet, the CaSR respond to increases in amino acids generally, and are not selective for IAA.
Sodium-dependent amino acid transporters, such as the SNAT family [63], could also serve as amino acid signals in neurons if they are rheogenic [138], where the entry of sodium, linked to the amino acid being transported, depolarizes the neuron and initiates a signal. However, this was ruled out in hippocampal neurons, because neural activation was due to excitation by glutamate’s action as a neurotransmitter, rather than to SNAT causing depolarization of the postsynaptic cell [139].
Given the importance of the hypothalamus to food intake in general, we measured neurotransmitters and amino acids in the VMH, along with the APC, at 2 h after introduction of IAA-imbalanced meals. NE is decreased in APC, along with the limiting IAA, but increased in the VMH, while the limiting IAA is unchanged there [55]. In addition, the NE alpha 2 receptor is decreased in APC and trends in the same direction in the VMH [140]. Perhaps NE, in the VMH, is involved in the satiety following a replete meal.
In summary, for sensing repletion, taste can be a cue in the CTA/preference response, but does not serve the immediate (30 min) sensing of repletion. The other possibilities considered: mTOR, SNAT, and CaSR, do not seem selective for an individual missing IAA, although they may play a role in responding to increased protein intake, about which we have more to learn [10].
Finally, in the rapid recognition of IAA repletion with a corrected diet, or injections of the IAA into the APC, there is good evidence for reversal of the APC-RT-DLLH circuit (which interrupts the first IAA-deficient meal and initiates foraging as was described above and sketched in Fig. 1). In this model, the APC is under control and the RT no longer inhibits the DLLH. In IAA repletion, (as long as protein synthesis is intact [118]), we suggest the following sequence: (a) activity subsides in the APC, itself [31], along with several locomotor activity centers, and (b) together: foraging stops, the better food is chosen, and eating resumes.
Repletion of the Missing IAA and Preference
When an IAA-deficient animal is offered the limiting IAA in a repletion (corrected) diet, or the IAA balance is restored by injecting the limiting IAA into the APC [28–30], feeding resumes quickly and foods (or flavors, colors, places, etc.) associated with foods containing the limiting IAA [11, 101, 103, 141, 142] are preferred. Hedonic centers in the BG, including the NAcc, will be stimulated likely via the LH. Cells in the LH increase firing rates after the animal has learned to associate a cue-sound with availability of the repleting IAA in its drinking water [79], which it licks avidly, demonstrating consummatory ingestive behavior. The resumption of feeding when the “corrected” diet is presented occurs within 30 min (Fig. 4 and reference [34]), but association of cues with repletion does require more training trials than aversions [93], which can be learned in a single exposure. Also, IAA-deficient rats learn to associate the positive effects of gastric infusions that provide repletion of the limiting IAA, with the place where these infusions were given [102]; this shows that spatial learning, a HIP function, can be involved in conditioned preferences. We [105, 142] used the Sclafani [143] method for determining preferences for flavored water paired with test foods. Although the appropriate associations are made, aversions are achieved more quickly than preferences. Later, only rats that are IAA-deficient prefer solutions previously paired with the needed IAA; if they are replete, they no longer prefer that flavor. This shows an IAA-preference based on their IAA “need state”.
Dietary Choice
Sanahuja and Harper, in 1962 [141], were the first to show dietary choice in which rats avoid IAA-imbalanced diets, selecting a protein-free diet instead. This is considered an adverse choice, because the mild imbalance used in their tests support life, while the protein-free diet does not. That study provided graphic evidence for the power of aversion to an IAA-imbalanced diet. Harper and colleagues provided a very detailed review [5] (section III.B.9, pp. 519–522), which covers a series of choice experiments. Since those first experiments, many choice studies have been done; they show that animals will eat just about anything else, rather than an IAA-deficient or imbalanced diet, reviewed in [35, 54, 142]. Hrupka [144] showed that rats adapted to a lysine-supplemented wheat gluten diet (which is deficient in the IAA, lysine) selected a higher level of supplemental lysine (at a difference of 0.01 %) without reducing their food intake. Thus, food choice is a more sensitive indicator of IAA repletion than intake.
Many species, including birds [145], pigs [146], and fish [147], have shown recognition of IAA limitation. (For a comparative review see [35], and for comparable projections from olfactory structures to other brain areas see [148]). Recent work with pigs [149] shows rejection of a diet deficient of the IAA, valine, within 1 h. Comparative neuroanatomical data suggest that the integrative centers required for the neural circuits to serve the food seeking and selection tasks are also conserved across vertebrate species. The puzzle presented by the failure of consistent appropriate protein selection seen by some [9] may be addressed by considering the experimental problems around palatability and aversion (which can be difficult to extinguish), or the matter of chance associated with foraging. The ability to compose a diet containing a complete IAA profile, albeit imperfectly, if given appropriate choices is reviewed by Koehnle [54].
In the Absence of a Choice, Adaptation Over Several Days
Rats do not adapt to an IAA devoid diet, which is lethal over time, as survival is similar to that seen with a protein free diet [5]. However, over a period of days, rodents can adapt fully to an IAA-imbalanced diet, which contains at least a small amount of the limiting IAA [5]. The responses to protein and IAA levels are graded; more severe limitation or imbalance causes the most pronounced response (compare feeding on the milder imbalanced diet with the devoid diet in Fig. 4). Such adaptation includes both increasing the activity of amino acid metabolizing enzymes in the liver for those amino acids that are in relative excess [150, 151], and altered feeding patterns [52]. Two recent reports addressing the responses to mild imbalances show adaptation to imbalanced IAA diets.
Using a soy protein diet that is deficient, but not devoid of, sulfur-containing amino acids (SCAA), Sikaladis and Stipanuk [152] showed that rats adapted by altering their feeding patterns, similar to the patterns seen by Leung and colleagues [16], in the imbalanced diet model described by Harper et al. [5]. These soy-fed, mildly SCAA-deficient rats had increased liver levels of P-eIF2α, showing that complete depletion of an IAA is not necessary for the GCN2 response [152], at least in the liver. Methionine (another SCAA) restriction has also been studied recently, over long feeding trials (months). The diets used [153] again closely follow the pattern of Harper’s [5] classical amino acid-imbalanced diets, to which rats adapt within a week (see the controls in ref [117]). Of interest is the reported increase in nighttime energy expenditure in the methionine restricted rats [153]. Rats are nocturnal animals and on an IAA-imbalanced diet they exhibit increased locomotor activity during the dark period, which may be beneficial to the amino acid profile [154]. Increased activity has been shown for water, food and other deprivations, as well [155]. However, in the methionine-restricted animals, interestingly, there was no evidence reported that could link locomotor activity to the increased energy expenditure during the dark period [153]. These authors also report beneficial effects of SCAA restriction, including increased longevity and metabolic changes. Because the effects of this methionine-imbalanced diet can be reversed by the SCAA, cysteine [156], which spares methionine nutritionally, the effects may be due to the limiting of SCAA, or sulfur, in these diets.
Taken together, we have reviewed findings to show that, with time after sensing IAA limitation, animals can make IAA-relevant choices and associations, aversions and preferences for nutrient selection in the maintenance of IAA, and thus protein homeostasis, and survival. We have also seen how the neuroanatomy serving these behaviors is integrated with sensory, motivational and locomotor centers.
Summary and Conclusions
Although the APC is a major component of the olfactory cortex, olfactory sensation is not required for recognition of an IAA-deficient meal [50]. The sequence can be summarized as follows: IAA deficiency in the APC blocks the initiation of protein synthesis via the GCN2 system; loss of the GABAergic elements [49] that rapidly turn over, results in disinhibition (activation) of the glutamate output from the APC. Glutamatergic axons reach many brain areas associated with the behavioral responses.
The key link may be the relay at the RT, between the APC and the DLLH. When the APC is activated, not only do the locomotor activities commence because of the Sherringtonian sum of stimuli to various locomotor activity circuits, but the RT is stimulated, sending GABAergic inhibition to the LH. When the limiting IAA is restored in the APC so that the GABA elements can be expressed there, this source of inhibition, from the RT to the LH, is reduced and feeding resumes.
Based on the evidence reviewed here, we may trace the anatomy of a homeostatic response from (a) sensing deficiency of the limiting IAA [GCN2 and P-eIF2α causing loss of GABAergic control, followed by neural potentiation of the APC] to (b) activation of the glutamate output neurons, to (c) stimulation of brain areas for locomotor activity including foraging [supplementary premotor cortex (AGm), DLLH and BG], plus activation of the RT for GABAergic inhibition of the DLLH, to (d) recognition of repletion after successful foraging, food choices and increased intake of the replete meal [DLLH] and to (e) preference for cues associated with IAA repletion [hedonic centers, NAcc, TEG]. We review several behavioral strategies for dealing with challenges to IAA homeostasis as well, if foraging fails; many of these behaviors require the same brain areas.
In prioritizing food selection, once energy is sufficient for the short term, maintenance of IAA homeostasis is the crucial challenge. Metazoans cannot synthesize or store their IAA, and catabolism of functional protein is not an effective strategy over time. Therefore, dietary selection for appropriate IAA balance has been essential for omnivores across the evolutionary spectrum [35]. Here, we have reviewed several mechanisms in the brain’s IAA chemosensor, and how activation of the APC may result in distributed signaling to neural circuits for increases in foraging and food selection. We suggest that these circuits support foraging and choice, rather than anorexia in response to IAA depletion, following the appetitive vs. consummatory model [98]. Given the conserved status of the APC as a paleocortex, we also suggest that investigation of olfactory cortex-locomotion links may be fruitful in learning just how animals initiate the complex maneuvers required to find and ingest beneficial foods.
Acknowledgements
The tract-tracing work of Dr. Aja was supported by National Institutes of Health (NIH) grant DK 09271. DWG had support from NIH grants DK42274, NS 043210, and NS 33347, and from Ajinomoto Co., Inc., Tokyo. We extend particular thanks to Dr. Kunio Torii for his kind advice and collaboration. The authors are grateful to the many students and postdoctoral fellows and technicians in the Food Intake Laboratory at the University of California, Davis, who provided assistance with the animal and biochemical studies (to all those who weighed spill papers, special thanks). The authors extend our profound apologies to those whose volumes of work could not be included due to space limitations.
Abbreviations
- AGm
Medial agranular (supplementary motor) cortex
- AMYG
Amygdala
- AP
Area postrema
- APC
Anterior piriform cortex
- ATF
Activating transcription factor
- BG
Basal ganglia
- BL
Basolateral
- CaSR
Calcium sensing receptor
- CaMKII
Calcium calmodulin kinase II
- Ce
Central
- CTA
Conditioned taste aversion
- CVO
Circumventricular organ
- Cx
Cortex
- DA
Dopamine
- D1or 2
Dopamine receptor categories 1or 2
- DLLH
Dorsolateral perifornical lateral hypothalamus
- DMH
Dorsomedial hypothalamus
- eIF2
Eukaryotic initiation factor 2
- ERK
Extracellular signal-related kinase
- GCN2
General amino acid control non-derepressing kinase 2
- GP
Globus pallidus
- GluR1
Glutamate receptor 1
- HIP
Hippocampus
- HRP
Horseradish peroxidase
- IAA
Indispensable (essential in the diet) amino acid
- IC
Insular (taste) cortex
- icv
Intracerebroventricular
- LH
Lateral hypothalamic area
- MAPK
Mitogen-activated protein kinase
- MeAIB
2-Methylamino isobutyric acid
- mTOR
Mammalian target of rapamycin
- NAcc
Nucleus accumbens
- NE
Norepinephrine
- NTS
Nucleus of the tractus solitarius
- OFC
Orbitofrontal cortex
- PBN
Parabrachial nucleus
- PVN
Paraventricular nucleus of the hypothalamus
- PI3kinase
Phosphatidylinositol 3 kinase
- PFC
Prefrontal cortex
- RT
Reticular thalamus
- SCAA
Sulfur-containing amino acid
- SNAT
Sodium-coupled neutral amino acid transporter
- STR
Striatum (caudate + putamen)
- tRNA
Transfer ribonucleic acid
- vent TEG
Ventral tegmentum
- VMH
Ventromedial hypothalamus
- VP
Ventral pallidum
- ZI
Zona incerta
Contributor Information
Dorothy W. Gietzen, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, University of California, Davis, One Shields Ave, Davis, CA 95616, USA
Susan M. Aja, Department of Neuroscience, Johns Hopkins University School of Medicine, 855 North Wolfe Street, Rangos 472, Baltimore, MD 21205, USA
References
- 1.Geiger E. Experiments with delayed supplementation of incomplete amino acid mixtures. J Nutr. 1947;34(1):97–111. doi: 10.1093/jn/34.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Peters JC, Harper AE. Influence of dietary protein level on protein self-selection and plasma and brain amino acid concentrations. Physiol Behav. 1984;33(5):783–790. doi: 10.1016/0031-9384(84)90048-9. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008;16(3):566–571. doi: 10.1038/oby.2007.58. doi:10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- 4.Tome D. Protein, amino acids and the control of food intake. Br J Nutr. 2004;92(Suppl 1):S27–S30. doi: 10.1079/bjn20041138. [DOI] [PubMed] [Google Scholar]
- 5.Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970;50(3):428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- 6.White BD, He B, Dean RG, Martin RJ. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J Nutr. 1994;124(8):1152–1160. doi: 10.1093/jn/124.8.1152. [DOI] [PubMed] [Google Scholar]
- 7.Riggs AJ, White BD, Gropper SS. Changes in energy expenditure associated with ingestion of high protein, high fat versus high protein, low fat meals among underweight, normal weight, and overweight females. Nutr J. 2007;6:40. doi: 10.1186/1475-2891-6-40. doi:10.1186/1475-2891-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr. 2000;130(3):514–521. doi: 10.1093/jn/130.3.514. [DOI] [PubMed] [Google Scholar]
- 9.Galef BG. Is there a specific appetite for protein? In: Berthoud HR, Seeley RJ, editors. Neural and metabolic control of macronutrient intake. CRC Press; Boca Raton: 2000. pp. 19–28. [Google Scholar]
- 10.Morrison CD, Reed SD, Henagan TM. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302(8):R917–R928. doi: 10.1152/ajpregu.00609.2011. doi:10.1152/ajpregu.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiBattista D, Mercier S. Role of learning in the selection of dietary protein in the golden hamster (Mesocricetus auratus) Behav Neurosci. 1999;113(3):574–586. doi: 10.1037//0735-7044.113.3.574. [DOI] [PubMed] [Google Scholar]
- 12.Gibson EL, Wainwright CJ, Booth DA. Disguised protein in lunch after low-protein breakfast conditions food-flavor preferences dependent on recent lack of protein intake. Physiol Behav. 1995;58(2):363–371. doi: 10.1016/0031-9384(95)00068-t. [DOI] [PubMed] [Google Scholar]
- 13.Gibson EL, Booth DA. Acquired protein appetite in rats: dependence on a protein-specific need state. Experientia. 1986;42(9):1003–1004. doi: 10.1007/BF01940706. [DOI] [PubMed] [Google Scholar]
- 14.Hansen BS, Vaughan MH, Wang L. Reversible inhibition by histidinol of protein synthesis in human cells at the activation of histidine. J Biol Chem. 1972;247(12):3854–3857. [PubMed] [Google Scholar]
- 15.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307(5716):1776–1778. doi: 10.1126/science.1104882. doi:10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 16.Leung PM, Rogers QR, Harper AE. Effect of amino acid imbalance in rats fed ad libitum, interval-fed or force-fed. J Nutr. 1968;95(3):474–482. doi: 10.1093/jn/95.3.474. [DOI] [PubMed] [Google Scholar]
- 17.Hrupka BJ, Lin YM, Gietzen DW, Rogers QR. Small changes in essential amino acid concentrations alter diet selection in amino acid-deficient rats. J Nutr. 1997;127(5):777–784. doi: 10.1093/jn/127.5.777. [DOI] [PubMed] [Google Scholar]
- 18.Hinnebusch AG, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell. 2002;1(1):22–32. doi: 10.1128/EC.01.1.22-32.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wek RC, Jiang HY, Anthony TG. Coping with stress: EIF2 kinases and translational control. Biochem Soc Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. doi:10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 20.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20(9):436–443. doi: 10.1016/j.tem.2009.05.008. doi:10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilberg MS, Balasubramanian M, Fu L, Shan J. The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr. 2012;3(3):295–306. doi: 10.3945/an.112.001891. doi:10.3945/an.112.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koehnle TJ, Russell MC, Morin AS, Erecius LF, Gietzen DW. Diets deficient in indispensable amino acids rapidly decrease the concentration of the limiting amino acid in the anterior piriform cortex of rats. J Nutr. 2004;134(9):2365–2371. doi: 10.1093/jn/134.9.2365. [DOI] [PubMed] [Google Scholar]
- 23.Leung PM, Rogers QR. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol. 1971;221(3):929–935. doi: 10.1152/ajplegacy.1971.221.3.929. [DOI] [PubMed] [Google Scholar]
- 24.Rogers QR, Leung PM. The influence of amino acids on the neuroregulation of food intake. Fed Proc. 1973;32(6):1709–1719. [PubMed] [Google Scholar]
- 25.Gietzen DW. Neural mechanisms in the responses to amino acid deficiency. J Nutr. 1993;123(4):610–625. doi: 10.1093/jn/123.4.610. [DOI] [PubMed] [Google Scholar]
- 26.Noda K, Chikamori K. Effect of ammonia via prepyriform cortex on regulation of food intake in the rat. Am J Physiol. 1976;231(4):1263–1266. doi: 10.1152/ajplegacy.1976.231.4.1263. [DOI] [PubMed] [Google Scholar]
- 27.Firman JD, Kuenzel WJ. Neuroanatomical regions of the chick brain involved in monitoring amino acid deficient diets. Brain Res Bull. 1988;21(4):637–642. doi: 10.1016/0361-9230(88)90203-1. [DOI] [PubMed] [Google Scholar]
- 28.Beverly JL, Gietzen DW, Rogers QR. Effect of dietary limiting amino acid in prepyriform cortex on meal patterns. Am J Physiol. 1990;259(4 Pt 2):R716–R723. doi: 10.1152/ajpregu.1990.259.4.R716. [DOI] [PubMed] [Google Scholar]
- 29.Beverly JL, Gietzen DW, Rogers QR. Effect of dietary limiting amino acid in prepyriform cortex on food intake. Am J Physiol. 1990;259(4 Pt 2):R709–R715. doi: 10.1152/ajpregu.1990.259.4.R709. [DOI] [PubMed] [Google Scholar]
- 30.Monda M, Sullo A, De Luca V, Pellicano MP, Viggiano A. L-threonine injection into PPC modifies food intake, lateral hypothalamic activity, and sympathetic discharge. Am J Physiol. 1997;273(2 Pt 2):R554–R559. doi: 10.1152/ajpregu.1997.273.2.R554. [DOI] [PubMed] [Google Scholar]
- 31.Hasan Z, Woolley DE, Gietzen DW. Responses to indispensable amino acid deficiency and replenishment recorded in the anerior piriform cortex of the behaving rat. Nutr Neurosci. 1998;1:373–381. doi: 10.1080/1028415X.1998.11747247. [DOI] [PubMed] [Google Scholar]
- 32.Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci. 2011;31(5):1583–1590. doi: 10.1523/JNEUROSCI.4934-10.2011. doi:10.1523/JNEUROSCI.4934-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gietzen DW. Amino acid recognition in the central nervous system. In: Berthoud HR, Seeley RJ, editors. Neural and metabolic control of macronutrient intake. CRC Press; Boca Raton: 2000. pp. 339–357. [Google Scholar]
- 34.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr. 2007;27:63–78. doi: 10.1146/annurev.nutr.27.061406.093726. doi:10.1146/annurev.nutr.27.061406.093726. [DOI] [PubMed] [Google Scholar]
- 35.Gietzen DW, Rogers QR. Nutritional homeostasis and indispensable amino acid sensing: a new solution to an old puzzle. Trends Neurosci. 2006;29(2):91–99. doi: 10.1016/j.tins.2005.12.007. doi:10.1016/j.tins.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Rowe TB, Macrini TE, Luo ZX. Fossil evidence on origin of the mammalian brain. Science. 2011;332(6032):955–957. doi: 10.1126/science.1203117. doi:10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd G. The synaptic organization of the brain. 2nd edn Oxford University Press; New York: 1979. Olfactory cortex; pp. 289–307. [Google Scholar]
- 38.Kanter ED, Haberly LB. NMDA-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res. 1990;525(1):175–179. doi: 10.1016/0006-8993(90)91337-g. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518(10):1670–1687. doi: 10.1002/cne.22295. doi:10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- 40.Cummings SL, Truong BG, Gietzen DW. Neuropeptide Y and somatostatin in the anterior piriform cortex alter intake of amino acid-deficient diets. Peptides. 1998;19(3):527–535. doi: 10.1016/s0196-9781(97)00468-3. [DOI] [PubMed] [Google Scholar]
- 41.Jung MW, Larson J, Lynch G. Role of NMDA and non-NMDA receptors in synaptic transmission in rat piriform cortex. Exp Brain Res. 1990;82(2):451–455. doi: 10.1007/BF00231264. [DOI] [PubMed] [Google Scholar]
- 42.Sharp JW, Ross-Inta CM, Hao S, Rudell JB, Gietzen DW. Co-localization of phosphorylated extracellular signal-regulated protein kinases 1/2 (ERK1/2) and phosphorylated eukaryotic initiation factor 2alpha (eIF2alpha) in response to a threonine-devoid diet. J Comp Neurol. 2006;494(3):485–494. doi: 10.1002/cne.20817. doi:10.1002/cne.20817. [DOI] [PubMed] [Google Scholar]
- 43.Gale K, Zhong P, Miller LP, Murray TF. Amino acid neurotransmitter interactions in ‘area tempestas’: an epileptogenic trigger zone in the deep prepiriform cortex. Epilepsy Res Suppl. 1992;8:229–234. doi: 10.1016/b978-0-444-89710-7.50034-3. [DOI] [PubMed] [Google Scholar]
- 44.Ekstrand JJ, Domroese ME, Johnson DM, Feig SL, Knodel SM, Behan M, Haberly LB. A new subdivision of anterior piriform cortex and associated deep nucleus with novel features of interest for olfaction and epilepsy. J Comp Neurol. 2001;434(3):289–307. doi: 10.1002/cne.1178. [DOI] [PubMed] [Google Scholar]
- 45.Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr. 2003;133(7):2331–2335. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- 46.Gietzen DW, Ross CM, Hao S, Sharp JW. Phosphorylation of eIF2alpha is involved in the signaling of indispensable amino acid deficiency in the anterior piriform cortex of the brain in rats. J Nutr. 2004;134(4):717–723. doi: 10.1093/jn/134.4.717. [DOI] [PubMed] [Google Scholar]
- 47.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1(4):273–277. doi: 10.1016/j.cmet.2005.03.004. doi:10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Mitsuda T, Hayakawa Y, Itoh M, Ohta K, Nakagawa T. ATF4 regulates gamma-secretase activity during amino acid imbalance. Biochem Biophys Res Commun. 2007;352(3):722–727. doi: 10.1016/j.bbrc.2006.11.075. doi:10.1016/j.bbrc.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 49.Truong BG, Magrum LJ, Gietzen DW. GABA(A) and GABA(B) receptors in the anterior piriform cortex modulate feeding in rats. Brain Res. 2002;924(1):1–9. doi: 10.1016/s0006-8993(01)03213-9. [DOI] [PubMed] [Google Scholar]
- 50.Leung PM, Larson DM, Rogers QR. Food intake and preference of olfactory bulbectomized rats fed amino acid imbalanced or deficient diets. Physiol Behav. 1972;9(4):553–557. doi: 10.1016/0031-9384(72)90011-x. [DOI] [PubMed] [Google Scholar]
- 51.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146(6):1004–1015. doi: 10.1016/j.cell.2011.07.041. doi:10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers QR, Leung PMB. The control of food intake: when and how are amino acids involved? In: Kare MR, Maller O, editors. The chemical senses and nutrition. Academic Press. Inc.; New York: 1977. pp. 213–249. [Google Scholar]
- 53.Feurte S, Tome D, Gietzen DW, Even PC, Nicolaidis S, Fromentin G. Feeding patterns and meal microstructure during development of a taste aversion to a threonine devoid diet. Nutr Neurosci. 2002;5(4):269–278. doi: 10.1080/10284150290032003. [DOI] [PubMed] [Google Scholar]
- 54.Koehnle TJ, Gietzen DW. Modulation of feeding behavior by amino acid-deficient diets: present findings and future directions. In: Lieberman HR, Kanarek RB, Prasad C, editors. Nutritional neuroscience. Taylor and Francis Group/CRC Press; Boca Raton: 2005. pp. 147–161. [Google Scholar]
- 55.Gietzen DW, Leung PM, Rogers QR. Dietary amino acid imbalance and neurochemical changes in three hypothalamic areas. Physiol Behav. 1989;46(3):503–511. doi: 10.1016/0031-9384(89)90028-0. [DOI] [PubMed] [Google Scholar]
- 56.Price JL, Slotnick BM, Revial MF. Olfactory projections to the hypothalamus. J Comp Neurol. 1991;306(3):447–461. doi: 10.1002/cne.903060309. doi:10.1002/cne.903060309. [DOI] [PubMed] [Google Scholar]
- 57.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296(4):E592–E602. doi: 10.1152/ajpendo.90645.2008. doi:10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao S, Ross-Inta CM, Gietzen DW. The sensing of essential amino acid deficiency in the anterior piriform cortex, that requires the uncharged tRNA/GCN2 pathway, is sensitive to wortmannin but not rapamycin. Pharmacol Biochem Behav. 2010;94(3):333–340. doi: 10.1016/j.pbb.2009.09.014. doi:10.1016/j.pbb.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr. 2001;131(3):861S–865S. doi: 10.1093/jn/131.3.861S. [DOI] [PubMed] [Google Scholar]
- 60.Goto S, Nagao K, Bannai M, Takahashi M, Nakahara K, Kangawa K, Murakami N. Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience. 2010;166(1):333–340. doi: 10.1016/j.neuroscience.2009.12.013. doi:10.1016/j.neuroscience.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78(4):969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 62.Blais A, Huneau JF, Magrum LJ, Koehnle TJ, Sharp JW, Tome D, Gietzen DW. Threonine deprivation rapidly activates the system A amino acid transporter in primary cultures of rat neurons from the essential amino acid sensor in the anterior piriform cortex. J Nutr. 2003;133(7):2156–2164. doi: 10.1093/jn/133.7.2156. [DOI] [PubMed] [Google Scholar]
- 63.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447(5):784–795. doi: 10.1007/s00424-003-1117-9. doi:10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 64.Gietzen DW, Magrum LJ. Molecular mechanisms in the brain involved in the anorexia of branched-chain amino acid deficiency. J Nutr. 2001;131(3):851S–855S. doi: 10.1093/jn/131.3.851S. [DOI] [PubMed] [Google Scholar]
- 65.Sharp JW, Magrum LJ, Gietzen DW. Role of MAP kinase in signaling indispensable amino acid deficiency in the brain. Brain Res Mol Brain Res. 2002;105(1–2):11–18. doi: 10.1016/s0169-328x(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 66.Sharp JW, Ross CM, Koehnle TJ, Gietzen DW. Phosphorylation of Ca2+/calmodulin-dependent protein kinase type II and the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor in response to a threonine-devoid diet. Neuroscience. 2004;126(4):1053–1062. doi: 10.1016/j.neuroscience.2004.03.066. doi:10.1016/j.neuroscience.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 67.Koehnle TJ, Stephens AL, Gietzen DW. Threonine-imbalanced diet alters first-meal microstructure in rats. Physiol Behav. 2004;81(1):15–21. doi: 10.1016/j.physbeh.2003.11.009. doi:10.1016/j.physbeh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178(4):711–740. doi: 10.1002/cne.901780408. doi:10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- 69.Aja SM. Dissertation. University of California; Davis: 1999. Neurotransmitters and neural circuitry supporting aminoprivic feeding. [Google Scholar]
- 70.Price JL, Carmichael T, Haberly LB. Olfactory input to the prefrontal cortex. In: Davis JL, Eichenbaum H, editors. Olfaction a model system for computational neuroscience. MIT Press; London: 1991. pp. 101–120. [Google Scholar]
- 71.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396(1):77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 72.Sul JH, Jo S, Lee D, Jung MW. Role of rodent secondary motor cortex in value-based action selection. Nat Neurosci. 2011;14(9):1202–1208. doi: 10.1038/nn.2881. doi:10.1038/nn.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolls ET. The neural control of feeding in primates. In: Booth DA, editor. Neurophysiology of ingestion. Pergamon Press; Oxford: 1993. pp. 137–169. [Google Scholar]
- 74.Rolls ET. Chemosensory learning in the cortex. Front Syst Neurosci. 2011;5:78. doi: 10.3389/fnsys.2011.00078. doi:10.3389/fnsys.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172(4):687–722. doi: 10.1002/cne.901720408. doi:10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 76.Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, Burdakov D. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72(4):616–629. doi: 10.1016/j.neuron.2011.08.027. doi:10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 77.Blevins JE, Dixon KD, Hernandez EJ, Barrett JA, Gietzen DW. Effects of threonine injections in the lateral hypothalamus on intake of amino acid imbalanced diets in rats. Brain Res. 2000;879(1–2):65–72. doi: 10.1016/s0006-8993(00)02734-7. [DOI] [PubMed] [Google Scholar]
- 78.Russell MC, Koehnle TJ, Barrett JA, Blevins JE, Gietzen DW. The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutr Neurosci. 2003;6(4):247–251. doi: 10.1080/1028415031000151567. [DOI] [PubMed] [Google Scholar]
- 79.Tabuchi E, Ono T, Nishijo H, Torii K. Amino acid and NaCl appetite, and LHA neuron responses of lysine-deficient rat. Physiol Behav. 1991;49(5):951–964. doi: 10.1016/0031-9384(91)90208-6. [DOI] [PubMed] [Google Scholar]
- 80.Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotion in the anesthetized rat. Prog Neurobiol. 1993;41(3):323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- 81.Jordan LM. Initiation of locomotion in mammals. Ann N Y Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Cummings SL, Gietzen DW. Temporal-spatial pattern of c-Fos expression in the rat brain in response to indispensable amino acid deficiency. II. The learned taste aversion. Brain Res Mol Brain Res. 1996;40(1):35–41. doi: 10.1016/0169-328x(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Cummings SL, Gietzen DW. Temporal-spatial pattern of c-Fos expression in the rat brain in response to indispensable amino acid deficiency. I. The initial recognition phase. Brain Res Mol Brain Res. 1996;40(1):27–34. doi: 10.1016/0169-328x(96)00032-0. [DOI] [PubMed] [Google Scholar]
- 84.Bellinger LL, Evans JF, Gietzen DW. Dorsomedial hypothalamic lesions alter intake of an imbalanced amino acid diet in rats. J Nutr. 1998;128(7):1213–1217. doi: 10.1093/jn/128.7.1213. [DOI] [PubMed] [Google Scholar]
- 85.Bellinger LL, Evans JF, Tillberg CM, Gietzen DW. Effects of dorsomedial hypothalamic nuclei lesions on intake of an imbalanced amino acid diet. Am J Physiol. 1999;277(1 Pt 2):R250–R262. doi: 10.1152/ajpregu.1999.277.1.R250. [DOI] [PubMed] [Google Scholar]
- 86.Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44(4–5):599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- 87.Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551(1–2):308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto T, Ueji K. Brain mechanisms of flavor learning. Front Syst Neurosci. 2011;5:76. doi: 10.3389/fnsys.2011.00076. doi:10.3389/fnsys.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aja SM, Chan P, Barrett JA, Gietzen DW. DA1 receptor activity opposes anorectic responses to amino acid-imbalanced diets. Pharmacol Biochem Behav. 1999;62(3):493–498. doi: 10.1016/s0091-3057(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 90.Wang CX, Erecius LF, Beverly JL, 3rd, Gietzen DW. Essential amino acids affect interstitial dopamine metabolites in the anterior piriform cortex of rats. J Nutr. 1999;129(9):1742–1745. doi: 10.1093/jn/129.9.1742. [DOI] [PubMed] [Google Scholar]
- 91.Hoebel BG. Brain reward and aversion systems in the control of feeding and sexual behavior. Nebr Symp Motiv. 1975;22:49–112. [PubMed] [Google Scholar]
- 92.Derjean D, Moussaddy A, Atallah E, St-Pierre M, Auclair F, Chang S, Ren X, Zielinski B, Dubuc R. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010;8(12):e1000567. doi: 10.1371/journal.pbio.1000567. doi:10.1371/journal.pbio.1000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott TR. Learning through the taste system. Front Syst Neurosci. 2011;5:87. doi: 10.3389/fnsys.2011.00087. doi:10.3389/fnsys.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stellar E. The physiology of motivation. Psychol Rev. 1954;61(1):5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 95.Blevins JE, Truong BG, Gietzen DW. NMDA receptor function within the anterior piriform cortex and lateral hypothalamus in rats on the control of intake of amino acid-deficient diets. Brain Res. 2004;1019(1–2):124–133. doi: 10.1016/j.brainres.2004.05.089. doi:10.1016/j.brainres.2004.05.089. [DOI] [PubMed] [Google Scholar]
- 96.Barone FC, Cheng JT, Wayner MJ. GABA inhibition of lateral hypothalamic neurons: role of reticular thalamic afferents. Brain Res Bull. 1994;33(6):699–708. doi: 10.1016/0361-9230(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 97.Rozin P. Specific aversions as a component of specific hungers. J Comp Physiol Psychol. 1967;64(2):237–242. doi: 10.1037/h0088047. [DOI] [PubMed] [Google Scholar]
- 98.Sodersten P, Nergardh R, Bergh C, Zandian M, Scheurink A. Behavioral neuroendocrinology and treatment of anorexia nervosa. Front Neuroendocrinol. 2008;29(4):445–462. doi: 10.1016/j.yfrne.2008.06.001. doi:10.1016/j.yfrne.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Magrum LJ, Teh PS, Kreiter MR, Hickman MA, Gietzen DW. Transfer ribonucleic acid charging in rat brain after consumption of amino acid-imbalanced diets. Nutr Neurosci. 2002;5(2):125–130. doi: 10.1080/10284150290018982. [DOI] [PubMed] [Google Scholar]
- 100.Kadowaki M, Kanazawa T. Amino acids as regulators of proteolysis. J Nutr. 2003;133(6 Suppl 1):2052S–2056S. doi: 10.1093/jn/133.6.2052S. [DOI] [PubMed] [Google Scholar]
- 101.Simson PC, Booth DA. Olfactory conditioning by association with histidine-free or balanced amino acid loads in rats. Q J Exp Psychol. 1973;25(3):354–359. doi: 10.1080/14640747308400356. doi:10.1080/14640747308400356. [DOI] [PubMed] [Google Scholar]
- 102.Fromentin G, Feurte S, Nicolaidis S. Spatial cues are relevant for learned preference/aversion shifts due to amino-acid deficiencies. Appetite. 1998;30(2):223–234. doi: 10.1006/appe.1997.0132. doi:10.1006/appe.1997.0132. [DOI] [PubMed] [Google Scholar]
- 103.Booth DA, Simson PC. Food preferences acquired by association with variations in amino acid nutrition. Q J Exp Psychol. 1971;23(1):135–145. doi: 10.1080/00335557143000149. doi:10.1080/00335557143000149. [DOI] [PubMed] [Google Scholar]
- 104.Fromentin G, Gietzen DW, Nicolaidis S. Aversion-preference patterns in amino acid- or protein-deficient rats: a comparison with previously reported responses to thiamin-deficient diets. Br J Nutr. 1997;77(2):299–314. doi: 10.1079/bjn19970031. [DOI] [PubMed] [Google Scholar]
- 105.Gietzen DW, McArthur LH, Theisen JC, Rogers QR. Learned preference for the limiting amino acid in rats fed a threonine-deficient diet. Physiol Behav. 1992;51(5):909–914. doi: 10.1016/0031-9384(92)90069-e. [DOI] [PubMed] [Google Scholar]
- 106.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122(3160):157–158. [PubMed] [Google Scholar]
- 107.Simson PC, Booth DA. Effect of CS-US interval on the conditioning of odour preferences by amino acid loads. Physiol Behav. 1973;11(6):801–808. doi: 10.1016/0031-9384(73)90274-6. [DOI] [PubMed] [Google Scholar]
- 108.Rogers QR, Wigle AR, Laufer A, Castellanos VH, Morris JG. Cats select for adequate methionine but not threonine. J Nutr. 2004;134(8 Suppl):2046S–2049S. doi: 10.1093/jn/134.8.2046S. [DOI] [PubMed] [Google Scholar]
- 109.Meliza LL, Leung PM, Rogers QR. Effect of anterior prepyriform and medial amygdaloid lesions on acquisition of taste-avoidance and response to dietary amino acid imbalance. Physiol Behav. 1981;26(6):1031–1035. doi: 10.1016/0031-9384(81)90205-5. [DOI] [PubMed] [Google Scholar]
- 110.Gietzen DW, Erecius LF, Rogers QR. Neurochemical changes after imbalanced diets suggest a brain circuit mediating anorectic responses to amino acid deficiency in rats. J Nutr. 1998;128(4):771–781. doi: 10.1093/jn/128.4.771. [DOI] [PubMed] [Google Scholar]
- 111.Dardou D, Datiche F, Cattarelli M. Fos and Egr1 expression in the rat brain in response to olfactory cue after taste-potentiated odor aversion retrieval. Learn Mem. 2006;13(2):150–160. doi: 10.1101/lm.148706. doi:10.1101/lm.148706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inui-Yamamoto C, Yoshioka Y, Inui T, Sasaki KS, Ooi Y, Ueda K, Seiyama A, Ohzawa I. The brain mapping of the retrieval of conditioned taste aversion memory using manganese-enhanced magnetic resonance imaging in rats. Neuroscience. 2010;167(2):199–204. doi: 10.1016/j.neuroscience.2010.02.027. doi:10.1016/j.neuroscience.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 113.Riley AL, Tuck DL. Conditioned food aversions: a bibliography. Ann N Y Acad Sci. 1985;443:381–437. doi: 10.1111/j.1749-6632.1985.tb27087.x. [DOI] [PubMed] [Google Scholar]
- 114.Aja S, Sisouvong S, Barrett JA, Gietzen DW. Basolateral and central amygdaloid lesions leave aversion to dietary amino acid imbalance intact. Physiol Behav. 2000;71(5):533–541. doi: 10.1016/s0031-9384(00)00378-4. [DOI] [PubMed] [Google Scholar]
- 115.Fromentin G, Feurte S, Nicolaidis S, Norgren R. Parabrachial lesions disrupt responses of rats to amino acid devoid diets, to protein-free diets, but not to high-protein diets. Physiol Behav. 2000;70(3–4):381–389. doi: 10.1016/s0031-9384(00)00275-4. [DOI] [PubMed] [Google Scholar]
- 116.Overmann SR, Woolley DE, Bornschein RL. Hippocampal potentials evoked by stimulation of olfactory basal forebrain and lateral septum in the rat. Brain Res Bull. 1980;5(4):437–449. doi: 10.1016/s0361-9230(80)80014-1. [DOI] [PubMed] [Google Scholar]
- 117.Leung PM, Rogers QR. Effects of hippocampal lesions on adaptive intake of diets with disproportionate amounts of amino acids. Physiol Behav. 1979;23(1):129–136. doi: 10.1016/0031-9384(79)90132-x. [DOI] [PubMed] [Google Scholar]
- 118.Beverly JL, 3rd, Gietzen DW, Rogers QR. Protein synthesis in the prepyriform cortex: effects on intake of an amino acid-imbalanced diet by sprague-dawley rats. J Nutr. 1991;121(5):754–761. doi: 10.1093/jn/121.5.754. [DOI] [PubMed] [Google Scholar]
- 119.Torii K, Kondoh T, Mori M, Ono T. Hypothalamic control of amino acid appetite. Ann N Y Acad Sci. 1998;855:417–425. doi: 10.1111/j.1749-6632.1998.tb10601.x. [DOI] [PubMed] [Google Scholar]
- 120.Markison S, Gietzen DW, Spector AC. Essential amino acid deficiency enhances long-term intake but not short-term licking of the required nutrient. J Nutr. 1999;129(8):1604–1612. doi: 10.1093/jn/129.8.1604. [DOI] [PubMed] [Google Scholar]
- 121.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416(6877):199–202. doi: 10.1038/nature726. doi:10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 122.Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol. 2011;590(Pt 5):1155–1170. doi: 10.1113/jphysiol.2011.211920. doi:10.1113/jphysiol.2011.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982;6(3):303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- 124.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol. 1973;150(2):217–237. doi: 10.1002/cne.901500208. doi:10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 125.Iwatsuki K, Uneyama H. Sense of taste in the gastrointestinal tract. J Pharmacol Sci. 2012;118(2):123–128. doi: 10.1254/jphs.11r08cp. [DOI] [PubMed] [Google Scholar]
- 126.Negri R, Morini G, Greco L. From the tongue to the gut. J Pediatr Gastroenterol Nutr. 2011;53(6):601–605. doi: 10.1097/MPG.0b013e3182309641. doi:10.1097/MPG.0b013e3182309641. [DOI] [PubMed] [Google Scholar]
- 127.Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454(5):759–776. doi: 10.1007/s00424-007-0247-x. doi:10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. doi:10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. doi:10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 130.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. doi:10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 131.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296(4):E603–E613. doi: 10.1152/ajpendo.91002.2008. doi:10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373(Pt 1):1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ljungdahl PO. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem Soc Trans. 2009;37(Pt 1):242–247. doi: 10.1042/BST0370242. doi:10.1042/BST0370242. [DOI] [PubMed] [Google Scholar]
- 134.Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) system A transporter gene. Biochem J. 2006;395(3):517–527. doi: 10.1042/BJ20051867. doi:10.1042/BJ20051867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Conigrave AD, Hampson DR. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther. 2010;127(3):252–260. doi: 10.1016/j.pharmthera.2010.04.007. doi:10.1016/j.pharmthera.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 136.Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G538–G546. doi: 10.1152/ajpgi.00342.2010. doi:10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Conigrave AD, Mun HC, Lok HC. Aromatic L-amino acids activate the calcium-sensing receptor. J Nutr. 2007;137(6 Suppl 1):1524S–1527S. doi: 10.1093/jn/137.6.1524S. discussion 1548S. [DOI] [PubMed] [Google Scholar]
- 138.Albers A, Broer A, Wagner CA, Setiawan I, Lang PA, Kranz EU, Lang F, Broer S. Na+ transport by the neural glutamine transporter ATA1. Pflugers Arch. 2001;443(1):92–101. doi: 10.1007/s004240100663. doi:10.1007/s004240100663. [DOI] [PubMed] [Google Scholar]
- 139.Armano S, Coco S, Bacci A, Pravettoni E, Schenk U, Verderio C, Varoqui H, Erickson JD, Matteoli M. Localization and functional relevance of system A neutral amino acid transporters in cultured hippocampal neurons. J Biol Chem. 2002;277(12):10467–10473. doi: 10.1074/jbc.M110942200. doi:10.1074/jbc.M110942200. [DOI] [PubMed] [Google Scholar]
- 140.Gietzen DW, Jhanwar-Uniyal M. Alpha 2 noradrenoceptors in the anterior piriform cortex decline with acute amino acid deficiency. Brain Res Mol Brain Res. 1996;35(1–2):41–46. doi: 10.1016/0169-328x(95)00179-v. [DOI] [PubMed] [Google Scholar]
- 141.Sanahuja JC, Harper AE. Effect of amino acid imbalance on food intake and preference. Am J Physiol. 1962;202:165–170. doi: 10.1152/ajplegacy.1962.202.1.165. [DOI] [PubMed] [Google Scholar]
- 142.Naito-Hoopes M, McArthur LH, Gietzen DW, Rogers QR. Learned preference and aversion for complete and isoleucine-devoid diets in rats. Physiol Behav. 1993;53(3):485–494. doi: 10.1016/0031-9384(93)90142-3. [DOI] [PubMed] [Google Scholar]
- 143.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47(1):63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 144.Hrupka BJ, Lin Y, Gietzen DW, Rogers QR. Lysine deficiency alters diet selection without depressing food intake in rats. J Nutr. 1999;129(2):424–430. doi: 10.1093/jn/129.2.424. [DOI] [PubMed] [Google Scholar]
- 145.Murphy ME, Pearcy SD. Dietary amino acid complementation as a foraging strategy for wild birds. Physiol Behav. 1993;53(4):689–698. doi: 10.1016/0031-9384(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 146.Roth FX, Meindl C, Ettle T. Evidence of a dietary selection for methionine by the piglet. J Anim Sci. 2006;84(2):379–386. doi: 10.2527/2006.842379x. [DOI] [PubMed] [Google Scholar]
- 147.Fortes-Silva R, Rosa PV, Zamora S, Sanchez-Vazquez FJ. Dietary self-selection of protein-unbalanced diets supplemented with three essential amino acids in Nile tilapia. Physiol Behav. 2012;105(3):639–644. doi: 10.1016/j.physbeh.2011.09.023. doi:10.1016/j.physbeh.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 148.Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: plasticity and perception. Semin Cell Dev Biol. 2006;17(4):462–470. doi: 10.1016/j.semcdb.2006.04.008. doi:10.1016/j.semcdb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 149.Gloaguen M, Le Floc’h N, Corrent E, Primot Y, van Milgen J. Providing a diet deficient in valine but with excess leucine results in a rapid decrease in feed intake and modifies the postprandial plasma amino acid and alpha-keto acid concentrations in pigs. J Anim Sci. 2012 doi: 10.2527/jas.2011-4956. doi:10.2527/jas.2011-4956. [DOI] [PubMed] [Google Scholar]
- 150.Bellinger LL, Williams FE, Rogers QR, Gietzen DW. Liver denervation attenuates the hypophagia produced by an imbalanced amino acid diet. Physiol Behav. 1996;59(4–5):925–929. doi: 10.1016/0031-9384(95)02168-x. [DOI] [PubMed] [Google Scholar]
- 151.Harper AE. Effect of variations in protein intake on enzymes of amino acid metabolism. Can J Biochem. 1965;43(9):1589–1603. doi: 10.1139/o65-176. [DOI] [PubMed] [Google Scholar]
- 152.Sikalidis AK, Stipanuk MH. Growing rats respond to a sulfur amino acid-deficient diet by phosphorylation of the alpha subunit of eukaryotic initiation factor 2 heterotrimeric complex and induction of adaptive components of the integrated stress response. J Nutr. 2010;140(6):1080–1085. doi: 10.3945/jn.109.120428. doi:10.3945/jn.109.120428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, Shin J, Huypens P, Malloy VL, Plaisance EP, Krajcik RA, Orentreich N, Gettys TW. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R728–R739. doi: 10.1152/ajpregu.00837.2009. doi:10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagao K, Bannai M, Seki S, Kawai N, Mori M, Takahashi M. Voluntary wheel running is beneficial to the amino acid profile of lysine-deficient rats. Am J Physiol Endocrinol Metab. 2010;298(6):E1170–E1178. doi: 10.1152/ajpendo.00763.2009. doi:10.1152/ajpendo.00763.2009. [DOI] [PubMed] [Google Scholar]
- 155.Baumeister A, Hawkins WF, Cromwell RL. Need states and activity level. Psychol Bull. 1964;61:438–453. doi: 10.1037/h0047136. [DOI] [PubMed] [Google Scholar]
- 156.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Smith AD, Drevon CA, Refsum H, Perrone CE. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res. 2011;52(1):104–112. doi: 10.1194/jlr.M010215. doi:10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]