Abstract
Objective
To evaluate Juvenile Dermatomyositis (JDM) for duration of untreated disease (DUD) impact on: vascular cell adhesion molecule-1 (VCAM-1) and microRNA (miRNA) expression in muscle biopsy (MBx); soluble VCAM-1 (sVCAM-1) and TNF-α in sera.
Methods
Pediatric controls (n=8) and untreated JDM (n=28) enrolled. Short DUD (n=11, symptoms ≤2 months before MBx); long DUD (n=17, >2 months symptoms). Vascular structures, characterized by immunoflorescence using antibodies against von Willebrand factor (vWF), VCAM-1, and α-smooth muscle actin (SMA), were measured for total area (microns2) and intensity (pixels) (SlideBook 4.2). Circulating sVCAM-1 and TNF-α levels (Mesoscale) were determined (JDM [6 short, 8 long DUD], 5 controls). MiR-126 differential expression in JDM MBx ([3 long, 3 short DUD], 2 controls) was detected by Exiqon’s miRCURY microRNA Array, and confirmed (qRT-PCR) in JDM ([5 short, 5 long], 5 controls).
Results
Short DUD JDM had higher total positive area (p=0.043) and intensity/high power field, (p=0.015) of VCAM-1 expression than long DUD JDM or controls (p=0.004, p=0.001 respectively). vWF:Ag+ vasculature displayed greater VCAM-1 intensity in short DUD compared to long DUD (p=0.001). Circulating sVCAM-1 and TNF-α were higher in JDM short DUD than controls (p=0.013, p=0.048 respectively). MiR-126, a negative regulator of VCAM-1 expression, was decreased by 3.39 fold, p=0.006 in controls vs. short DUD; for controls vs. long DUD, no significant difference (0.145 fold, p=0.548).
Conclusion
In short DUD, miR-126 downregulation is associated with increased VCAM-1 in both muscle and blood, suggesting that VCAM-1 plays a critical role early in JDM disease pathophysiology, augmented by TNF-α.
INTRODUCTION
Although a rare disease, with an incidence of 3.2/million children/year (1), juvenile dermatomyositis (JDM) is one of the more easily recognized pediatric rheumatic conditions. The patient, who is usually about 6–7 years of age or younger (1), develops the characteristic rash (erythema of eye lids, malar area, extensor joint surfaces and, if severe, the trunk) with progressive, symmetrical proximal muscle weakness (2). The systemic vasculopathy is documented by deformation and loss of microvascular structures, reflected in both the muscle histology (3, 4), and in the loss of nailfold capillary end row loops (5, 6), which is associated with decreased gastrointestinal absorption (7). It is well documented that endothelial cell activation and neovasularization are one of the major components of the disease pathophysiology (8), but the effect of untreated chronic inflammation on JDM muscle vasculature is unknown.
Recent epidemiological studies established that disease chronicity had a previously unrecognized impact on the child's phenotype (9, 10), as well as a direct association with loss of end row capillary loops determined by nailfold capillaroscopy and impaired capacity for microvascular regeneration (5). Gene expression profile studies of muscle and peripheral blood of untreated children with JDM demonstrated a florid upregulation of Type I interferon induced genes related to disease severity (11, 12), and after 2 months or more of illness, a dysregulation of genes associated with vascular remodeling (13). This molecular evidence is supplemented by careful studies of the physical structures in muscle which suggest that capillary abnormalities precede other structural changes (14). Despite this observation, assessment of the impact of untreated JDM disease chronicity on vascular associated adhesion molecules in association with miRNA control is unknown.
Adhesion molecule expression, specifically ICAM-1 and VCAM-1, have been inconsistently identified in JDM muscle (15–20). VCAM-1 is expressed on differentiating skeletal muscle (21), but not on adult skeletal muscle fibers (22). VCAM-1 is expressed on activated, but not quiescent endothelial cells, dendritic cells, macrophages and epithelium, and is involved in the recruitment of leukocytes from the blood into almost all tissues (15–25). The ligand for VCAM-1, VLA-4, has been identified on a wide range of cells including leukocytes, hematopoietic progenitors and stem cells, as well as developing myotubes (22). VCAM-1 is released from activated endothelial cells, resulting in soluble VCAM-1(sVCAM-1) (25). The increase in VCAM-1 expression and associated endothelial activation contributes to the promotion of inflammation and tissue damage which is augmented by TNF-α, a pro-inflammatory cytokine (21), found to be elevated in children with JDM (26). Because VCAM-1 plays an integral role in the inflammatory process, it is a key factor in the pathophysiology of several different autoimmune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematous (SLE) and scleroderma (23). VCAM-1 participates in systemic disease activity in SLE (24), as well as the evolution of heart disease, in which this adhesion molecule plays a dominant role in the initial phases of the development of atherosclerosis (27).
The potential role of microRNAs (miRNAs) as regulators of VCAM-1 in JDM inflammatory cascade is unknown. Modulation of key miRNA levels can affect several physiological and pathological functions, offering a new prototype for therapeutic intervention (28). MiRNAs are non-coding RNAs usually 18–25bp long that regulate several messenger RNAs (mRNAs) simultaneously by mechanisms such as incomplete base pairing and post-transcriptional gene silencing (29). MiRNAs control a network of genes involved in endothelial cell function, vascular disease and angiogenesis. The purpose of this study was to evaluate the impact of the duration of untreated disease (DUD) in children with definite/probable JDM on: VCAM-1 and miRNA expression in the diagnostic muscle biopsies, and on the concentrations of sVCAM-1 and TNF-α in the patient’s sera.
MATERIALS and METHODS
Juvenile Dermatomyositis Patients and Pediatric Controls
Muscle biopsies, sliced 6 µm thick, from 28 definite/probable JDM patients, Bohan-Peter criteria (2), were obtained before the start of therapy, (mean age = 7.07 ± 3.75 yrs; 24 girls, 4 boys), after their families gave their age-appropriate, informed consent (IRB# 10778). Muscle biopsies were derived from areas of inflammation as defined by an MRI image (T2 weighted, fat suppressed), usually the vastus lateralis. For the JDM patients, 17 children were characterized as having a long duration of untreated disease (long DUD, > 2 months) and 11 as a short duration of untreated disease (short DUD, ≤ 2 months). The duration of untreated disease (DUD) was defined as the amount of time from first symptom (rash and/or weakness) until muscle biopsy. Three to four muscle pieces were obtained from the MRI-directed biopsy. One of the samples was wrapped, placed in a sterile container, and transported on wet ice to the Neuromuscular Laboratory at Northwestern University Medical School for diagnosis. The remaining muscle pieces were snap-frozen in liquid nitrogen and transferred to a −180°C freezer until molecular studies (miRNA and q-RT PCR) were performed. Fourteen of the children with JDM who had the TNF-α-308 A allele (AA/AG) were age, DUD matched with 14 JDM who were TNF-α-308 GG positive. Eight control muscle biopsies (taken from areas affected in inflammatory myopathies, trunk and proximal muscles) were obtained from healthy pediatric patients undergoing orthopedic surgery; all 8 controls were positive for TNF-α-308 GG, the most common allelic representation, and had no evidence of inflammation in their biopsies. For the microRNA expression profiling, 6 definite/probable untreated JDM girls (mean age = 5.8 ± 2.13) muscle biopsies were tested; 3 children defined as short duration (≤ 2.4 months) and 3 with long duration (> 7 months). The controls were two healthy, age and race-matched females. The miRNA data were validated by testing with q RT-PCR, in an independent group of age-matched MBxs from 5 untreated girls with JDM with short DUD, 5 JDM girls with long DUD and 5 age-matched healthy girls.
VCAM-1 determined by Triple Immunoflorescence Staining
Frozen muscle slides were fixed with Pipes buffer and 3.7% formaldehyde in a 2.1:1 ratio. The slides were blocked with 10% donkey serum and incubated overnight in a 4°C humidified chamber with two primary antibodies: mouse anti-VCAM-1 (1:100, R&D BBA5) and rabbit anti-α-SMA (1:50, Abcam ab5694). After using 1 X PBS with 0.1% saponin as the wash, the slides were incubated with secondary antibodies, Cy3-conjugated anti-mouse Ig and Cy5-conjugated anti-rabbit Ig (1:100, both from Jackson ImmunoResearch Laboratory, 715-165-152 and 711-175-152, respectively) for one hour at room temperature. After washing, and a second round of fixation and blocking, the Zenon technique was used to couple a mouse monoclonal antibody against vWF (1:100, Abcam ab20435) with Fab-Alexa Fluor 488 (Invitrogen Z-25002) following the instructions from manufacturers. The slides were washed, mounted with fluorosave and cover slipped.
Image Capturing and Analysis
Images of the triple stained tissue sections were acquired within 48 hours using Openlab computer software 4.04 (Improvision Inc., Lexington, MA) and a Leica DMR-HC microscope (Leica Microsystems GmbH, Wetzlar, Germany) coupled to a Photometric Cool Snap charge-coupled device camera. SlideBook 4.2 was used to measure the positive fluorescence of the area (microns2) and intensity (pixels) of VCAM-1, SMA and vWF. VCAM-1 expression on arterioles was defined as strong positive SMA, positive vWF and positive VCAM-1. VCAM-1 expression on venules was defined as minimally positive or negative SMA, positive vWF and positive VCAM-1. Capillaries were defined as negative SMA, positive vWF and negative VCAM-1.
Determination of TNF-α-308 Polymorphism
A single base pair substitution of an A for the more common G at TNF-α-308 promoter site was analyzed using PCR as previously described (26). Digestion of amplified product with NcoI restriction enzyme confirmed genotype of the JDM samples as GG, GA or AA (26).
Measurement of Serum sVCAM-1, TNF-α, IL-1 β , IL-6 and IFN-γ Levels
Five of the children with long DUD had a corresponding fasting, serum sample obtained at the time of the muscle biopsy. An additional 3 untreated long DUD JDM (total =8) serum samples, 6 untreated short DUD JDM serum samples and 6 pediatric control serum samples were tested. A MSD Multi-Spot 96-well 4 Spot Human VCAM-1 plate (Mesoscale, K151EQC-1) and MSD Multi-Spot 96-well Human Proinflammatory I 4-plex Assay (Mesoscale, K15009C-1) were used to measure concentrations of sVCAM-1, TNF-α, IL-1β, IL-6 and IFN-γ in sera. The manufacturer’s protocol was followed and data were analyzed using MSD Workbench. To test for a potential inhibitor of IL-1β, plasma from 10 JDM (5 with a short duration of untreated disease and 5 with a long duration) and 5 healthy children were spiked with two concentrations of IL-1β (62.5 pg/mL and 15.6 pg/mL) and assessed with both negative and positive controls, using the Mesoscale® technology. There was no evidence of an inhibitor of IL-1β in any of the children's plasma—either from JDM or controls.
MiRNA Expression Profiling
Total RNA was extracted using miRCURY™ RNA Isolation Kit (Exiqon, Vedbaek, Denmark). RNA quality control was performed using 2100 Bioanalyzer (Agilent – Santa Clara, CA). The 2100 Bioanalyzer Expert software was used to generate a RNA Integrity Number (RIN) that provides a numerical assessment of the integrity of RNA. Following quality control, RNA samples were labeled using miRCURY™ LNA microRNA Power Labeling Kit according to Exiqon’s protocol. Array experiments were conducted as double-channel Hy3/Hy5 experiments in triplicates on Exiqon’s miRCURY LNA microRNA Array, v.11.0. Hybridization of labeled RNA to the array was performed on Tecan HS4800™ Pro automated hybridization stations and scanned with Agilent G2505B Microarray Scanners. The obtained microarray images were analyzed using GenepixProTM 4.0 software.
MiR-126 qRT-PCR
Total RNA was extracted from the muscle samples using miRCURY RNA Isolation Kit (Exiqon, Vedbaek, Denmark) according to manufacturer’s instruction. The first strand cDNA and real-time PCR for microRNA were conducted using miRCURY LNA Universal RT miRNA PCR kit (Exiqon, Vedbaek, Denmark). Briefly, for the first strand cDNA synthesis, 20ng of total RNA was used in 20 µl reaction mixture. The reaction was carried out for 60 min at 42 °C followed by heat-inactivation of reverse transcriptase for 5 min at 95 °C. For real-time PCR amplification, cDNA was diluted 80 times; 4 µl of diluted cDNA was used for the real-time PCR reaction. The miR-126 primer set and SYBR Green master mix was purchased from Exiqon, Vedbaek, Denmark, and the two-step PCR program was performed in ABI 7500 Fast thermal cycler with polymerase activation/DNA denatured, 95 °C, 10 min amplification cycles, 40 cycles, 95 °C, 10s, 60 °C, 1 min.
Statistical Analysis
The analysis of the total area and total intensity/hpf of VCAM-1 in diagnostic muscle biopsies from JDM with short DUD, long DUD and the pediatric controls were compared using a one-way ANOVA with Tukey test via SPSS 18.0. The same method was used to compare sera from JDM, both short and long DUD with controls, for sVCAM-1 and the cytokines listed, including TNFα. For miRNA profiling, RNA from muscle samples were hybridized and analyzed against a common reference pool of all eight samples. For these samples the quantified signals (background corrected) were normalized using the global Lowess (LOcallyWEighted Scatterplot Smoothing) regression algorithm. The microRNA expression in JDM with short DUD was compared with long DUD and pediatric controls using Student’s t-tests. Fold-change was calculated and miRNAs fulfilling both criteria of p ≤ 0.05 and FC ≥ 1.5 or FC ≤ −1.5 were selected. Ingenuity Pathways Analysis software (Ingenuity Systems, Inc., Mountain View, CA) and Pub Med were used to search and identify target protein-coding genes potentially regulated by the top differentially expressed mi-RNAs.
RESULTS
Expression of VCAM-1 in Muscle Biopsies from Untreated Children
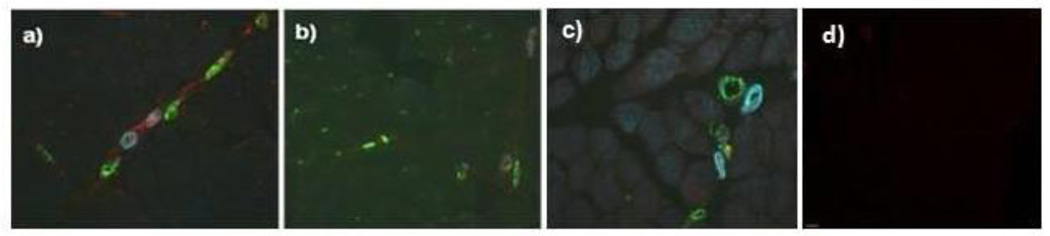
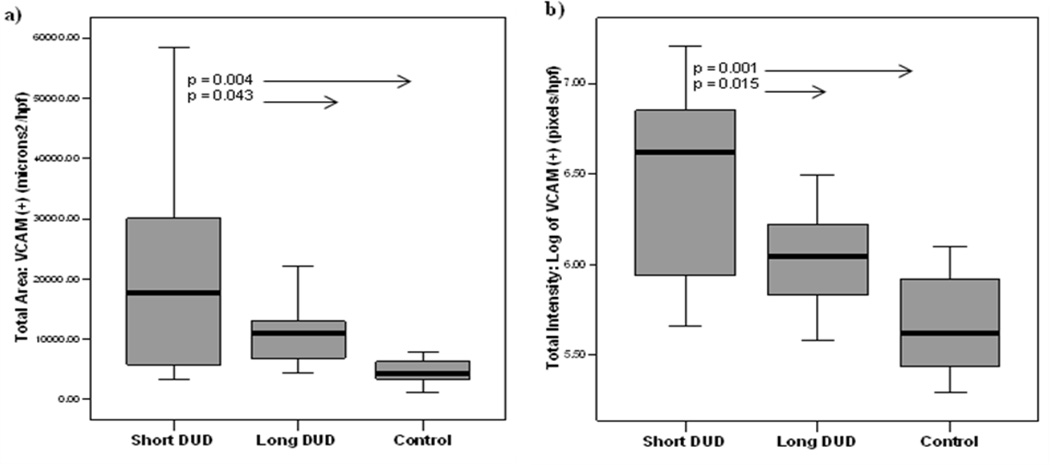
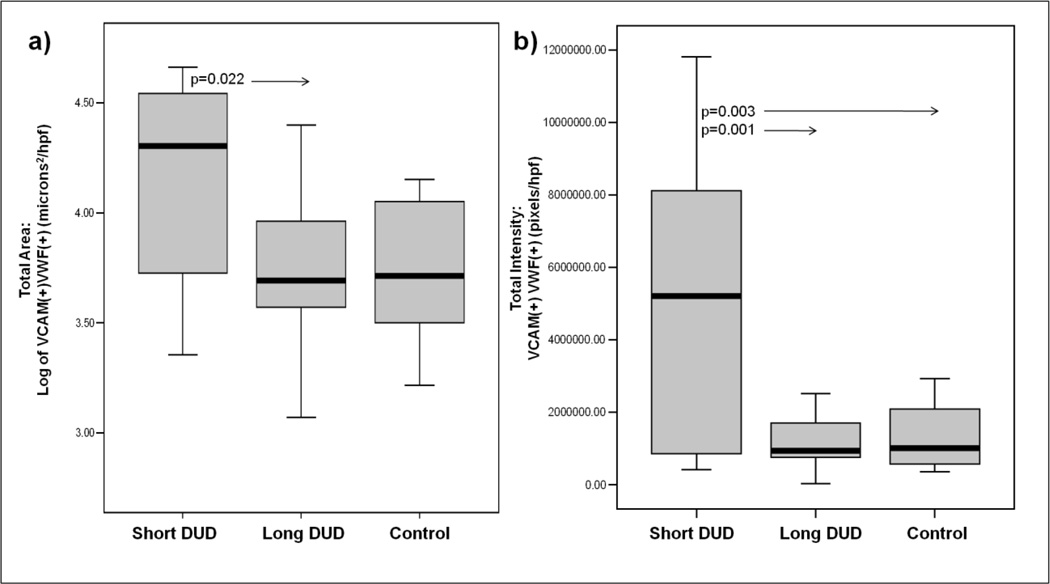
Figure 1 presents representative images of the vascular system in the muscle of: a) untreated JDM of short DUD, b) long DUD, c) pediatric control, and d) isotype control. In the JDM of short DUD, the capillaries, positive for vWF:Ag, are negative for VCAM-1 (red) and smooth muscle actin (SMA) (blue), which defines the arterioles and some venules compared with the negative control studies The analysis of the JDM diagnostic MBx tissue as a whole documented that children with a short DUD displayed a significantly higher total area of VCAM-1 expression compared to both long DUD (p=0.043, Figure 2a) and controls (p=0.004), (Figure 2a). The intensity of VCAM-1 expression/hpf was also increased in JDM of short DUD compared to long DUD (p=0.015) and short DUD compared with controls (p=0.001), (Figure 2b). Analysis of VCAM-1 expression in vWF positive blood vessels, similarly showed a significant increase in total area/hpf in JDM children with short DUD compared to long DUD (p=0.022), (Figure 3a), while intensity/hpf was also higher in vWF positive blood vessels in MBx of short disease duration compared with long DUD (p=0.001) and controls (p=0.003, Figure 3b).
Figure 1. Immunohistochemistry of untreated Juvenile Dermatomyositis muscle and vasculature: VCAM-1, smooth muscle actin and von Willebrand factor antigen.
Triple immunofluorescence stained image (20X) of MBx from untreated children with a) Short DUD JDM, b) Long DUD JDM, c) age, gender matched control, d) isotype negative controls. VCAM-1 is shown in red, α-smooth muscle actin is shown in blue and von Willebrand factor in green.
Figure 2. Increased total area and intensity of VCAM-1 positive staining in diagnostic muscle biopsies from untreated children with Juvenile Dermatomyositis of short disease duration.
a) Total area (microns²/hpf) and b) total intensity (pixels/hpf) of VCAM-1 positive expression in muscle biopsies increased in JDM short DUD compared with JDM of long DUD and controls. The rectangular box plot represents 50 percent of the data with the median value indicated by the line. The whiskers represent the smallest and largest values. JDM population: Short DUD, n=11 (less than 2 months), Long DUD (greater than 2 months), n=17 and age-matched controls, n=8.
Figure 3. Increased VCAM-1 positive areas and intensity is localized to von Willebrand factor antigen positive vasculature in untreated JDM muscle biopsies of short disease duration.
a) Total area (microns²/hpf) and b) total intensity (pixels/hpf) of VCAM-1 positive and vWF positive expression in blood vessels of muscle biopsies of JDM short DUD (n=11), JDM long DUD (n=17) and controls (n=8). The rectangular box plot represents 50 percent of the data with median value indicated by the line. The whiskers represent the smallest and largest values. The additional circle outside the range indicates an outlier.
Serological Studies
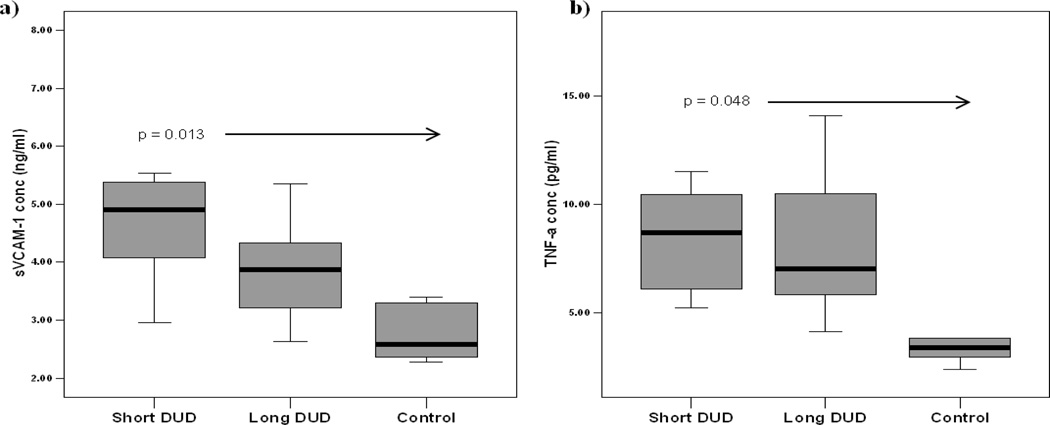
A similar pattern was observed with sVCAM-1 levels in JDM sera when compared to pediatric controls. JDM children with short DUD had significantly higher sVCAM-1 levels, compared to controls (p = 0.013, Figure 4a). In parallel, the levels of TNF-α in JDM of short DUD were higher than the controls, (p=0.048), (Figure 4b). The other pro-inflammatory cytokines, IL-1β, IL-6, and IFN-γ, were not significantly different when short DUD, long DUD and control were compared. Although the IL-1β levels were below the level of detection (data not shown), the controlled experiments using the addition of two concentrations (62.5 pg/mL and 15.6 pg/mL) of IL-1β did not demonstrate the presence of an inhibitor.
Figure 4. Elevated circulating levels of soluble VCAM-1 (sVCAM-1) and TNF-α in untreated JDM with short disease duration compared with long disease duration and controls.
a) sVCAM-1 levels (ng/ml), and b) TNF-α levels (pg/ml) in sera from untreated JDM short DUD (n=6), less than 2 months of symptoms, JDM long DUD (n=8), symptoms for greater than 2 months, and pediatric controls (n=5). The rectangle box plot represents 50 percent of the data with the median value indicated by the line. The whiskers represent the smallest and largest values.
MiRNA Expression Profiles and qRT-PCR
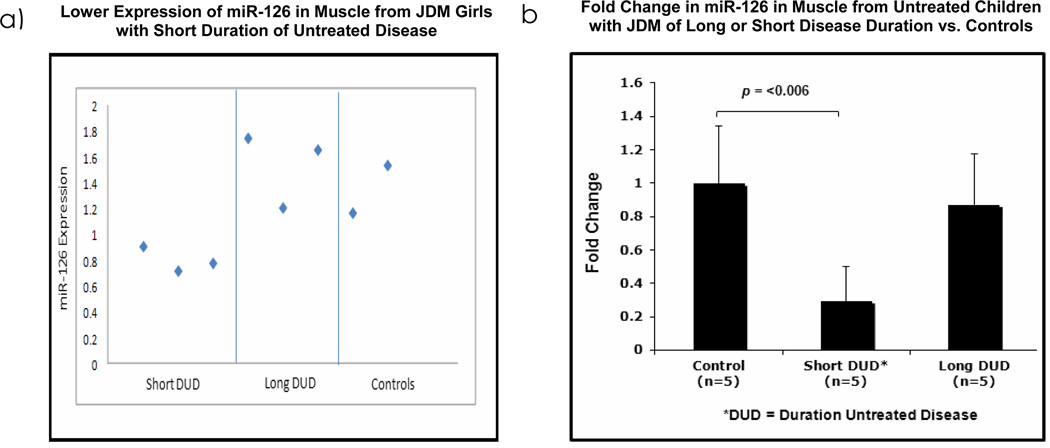
195 out of 841 human miRNAs represented in the array were expressed across all the samples. The greatest downregulated miRNA in muscle biopsies of patients was miR-126, which was decreased by 1.92 fold, (p=0.014) in short DUD compared to long DUD, (Figure 5a). To confirm these findings, miR-126 qPCR was performed in an independent set of untreated JDM patients (5 short, 5 long and 5 healthy controls. MiR-126 expression was lower in short DUD compared to controls, (3.39 fold, p=0.006), while expression in controls vs. long DUD, were not significantly different (0.145 fold, p=0.548), as seen in Figure 5b. These data provide confirmatory evidence that expression of miRNA-126 is lower in muscle of JDM with a short DUD compared with JDM of long untreated DUD and pediatric controls.
Figure 5. MiR-126 is decreased in muscle biopsies from untreated children with Juvenile Dermatomyositis of short disease duration compared to long disease duration and controls.
a) Intensity plot of the expression levels of miR-126 in muscle biopsies of untreated JDM girls with short duration of untreated disease, DUD, (<2.4 months, n=3), compared with long DUD (>7.0 months, n=3) and controls (n=2). MiR-126 is down regulated in samples from short DUD compared to long DUD (FC=-1.92, p=0.014). b) qRT-PCR data from a different set of muscle biopsies from JDM girls with short DUD (n=5) with long DUD (n=5) and healthy controls (n=5). These data confirm that miR-126 is down regulated in samples from JDM with short DUD, decreased by 3.39 fold (p=0.006) compared with data from normal control vs. long DUD, which is not significantly decreased (0.145 fold, p=0.548).
DISCUSSION
We believe this is the first investigation to evaluate the intensity and localization of VCAM-1 expression in the muscle of untreated children with JDM, classified by the duration of untreated disease in conjunction with assessment of miRNA expression. Our results show an increase in VCAM-1 protein in muscle and vasculature from patients with JDM who had a short DUD compared to children who had JDM with a long DUD and pediatric controls. In addition, our results implicate miR-126 as an important early regulating factor of VCAM-1 expression, suggesting that this miRNA may play a critical role in developing JDM pathophysiology.
Past studies have provided conflicting results concerning VCAM-1 expression in muscle of patients with dermatomyositis, but neither the age of the donor, or the duration of untreated disease at the time of biopsy has been consistently considered. It is important to remember that normal endothelium, as in our pediatric controls, expresses little or no VCAM-1. Sallum et al. did not find a difference in VCAM-1 expression when 27 JDM and control muscle biopsies were compared, but the mean DUD for their JDM patients was 8 months, range 1–64 months (18). Subsequently, the same group reported that muscle biopsies from patients with JDM differed from brachial muscle biopsies from adults with DM, PM, or IBM, with respect to variations in expression of ICAM-1, but not of VCAM-1, leading to their conclusion that VCAM-1 did not play a major role in JDM (19); little to no expression of VCAM-1 was identified in adult Dermatomyositis samples (19). In contrast, Tews et al., in a study of patients with inflammatory myopathy (age and DUD not stated), documented an increase in VCAM-1 expression in blood vessels and described detectable VCAM-1 expression in areas of the muscle without an obvious inflammatory infiltrate (20), while also recognizing inflammatory cell associated VCAM-1 staining. Cid et al. reported that VCAM-1 was increased on vWF:Ag positive microvasculature in DM patients (average age 57 years) compared to controls (15).
There are conflicting data concerning VCAM-1 expression on muscle fibers. Our study of young untreated children documented a significantly higher expression of VCAM-1 in vWF:Ag positive, smooth muscle antigen (SMA) negative blood vessels as well as in the muscle fibers themselves. These findings are consistent with the data from Iademarco et al., who showed that VCAM-1 was present in the basal lamina of muscle cells, prominent in regenerating muscle cells, and appeared to be constitutively expressed, but not usually induced by cytokines, thus differing from the obligatory cytokine-induced VCAM-1 expression on endothelial cells (21). This observation could explain the varied reports of VCAM-1 expression on muscle fibers found in adult patient samples of DM, and provides evidence for the importance of controlling not only duration of untreated disease and the age of the patient studied, but also identifying the specific location of VCAM-1 expression. Our study solidifies the data pertaining to the effect of time on the untreated inflammatory process in the muscle of children with JDM. Furthermore, these data document the impressive differences in VCAM-1 expression when a defined length of time was established between onset of symptoms the date that the muscle biopsy was obtained.
Soluble adhesion molecules have been studied in other related rheumatic, autoimmune diseases, such as SLE, RA and localized scleroderma (32–35). In treated RA adult patients, sVCAM-1 levels were significantly higher compared to controls (33). sVCAM-1 levels were also higher in SLE patients compared to controls and these levels were correlated with SLEDAI scores and inversely related to C3 levels (34). Bloom et al., compared soluble adhesion molecules in sera from children with SLE, mixed connective tissue disease (MCTD), vasculitis and JDM. They found that JDM and MCTD had elevated levels of sVCAM-1 compared to the other rheumatic diseases and controls (36). In the present study, the significantly higher concentration of sVCAM-1 in the sera of JDM children with a short DUD is paralleled by the increased VCAM-1 expression in the muscle of JDM with short DUD confirming that circulating sVCAM-1 could be used as an accessible indicator of the inflammatory process within the muscle.
We hypothesized that elevated TNF-α and IL-1β, both known to participate in the JDM inflammatory response (26, 37, 38) and potent inducers of VCAM-1 expression might result in differences of VCAM-1 expression between short and long DUD. Examination of our data confirms only part of this conjecture: significant differences in both TNF-α and VCAM-1 expression were found only when short DUD and controls were compared; when JDM of short and long DUD were compared, there were no significant differences in the TNF-α level. In addition, circulating IL-1β was below detection levels, similar to other serum studies (36, 37), and despite specific testing for a possible circulating inhibitor, none was identified. These data suggest that TNF-α may contribute to vascular bed damage in JDM early in the disease course by enhancing the activation of VCAM-1.
Recent studies have shown the importance of miRNA in regulation of endothelial cell activation (28, 29). Specifically, miR-126 exerts an inhibitory regulation on VCAM-1 expression. The significant down regulation of miR-126 early in the course of untreated disease in children with JDM exemplifies the central role that miRNAs appear to play in the regulation of these critical inflammatory pathways (28–30). MiR-126 inhibits ischemia-induced neovascularization (39), also present in JDM, as well as targeting IRS-1 (insulin receptor substrate-1) involved in the development of insulin resistance associated with mitochondrial dysfunction (40). Mitochondrial dysfunction has been previously described in the muscle of children with JDM (12, 41) as has the development of insulin resistance in older patients with JDM (42) which may be associated with observed premature cardiovascular damage (43). The impact of miRNA-126 on both VCAM-1 and endothelial cell structure and function adds another layer of complexity to the pathophysiology of JDM and the evolution of the inflammatory process as defined by the duration of untreated disease.
In summary, we have demonstrated that VCAM-1 expression is increased in diagnostic muscle biopsies, in both the inflammatory muscle tissue, and in the vasculature in untreated children with JDM who have a short DUD (≤ 2 months) compared with those JDM who have a long DUD (> 2 months). This finding is mirrored by elevated levels of sVCAM-1 and TNF-α in the sera of untreated JDM children with short disease duration, and accompanied by consistent down regulation of miR-126 in short disease duration. We conclude, based on these data, that VCAM-1 expression, augmented by TNF-α and regulated by miR-126, may play a critical role in early JDM pathophysiology, thus possibly opening a new avenue of therapeutic intervention.
ACKNOWLEDGEMENTS
The authors acknowledge with great appreciation Dr. John Sarwark and Theresa Phillip for obtaining healthy muscle, the skilled review of Dong Xu, MD, and the technological expertise of Akadia Kachaochana and Jan Caliendo.
Supported by NIH NR012692-01, Macy’s Miracle, and the Cure JM Foundation (to LMP).
REFERENCES
- 1.Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49(3):300–305. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Banker BQ, Victor M. Dermatomyositis (systemic angiopathy) of childhood. Medicine (Baltimore) 1966;45(4):261–289. doi: 10.1097/00005792-196607000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter S, Karpati G, Rothman S, Watters G. The childhood type of dermatomyositis. Neurology. 1976;26(10):952–962. doi: 10.1212/wnl.26.10.952. [DOI] [PubMed] [Google Scholar]
- 5.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58(2):571–576. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31(8):1644–1649. [PubMed] [Google Scholar]
- 7.Rouster-Stevens KA, Gursahaney A, Ngai KL, Daru JA, Pachman LM. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;59(2):222–226. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagaraju K, Rider LG, Fan C, Chen YW, Mitsak M, Rawat R, et al. Endothelial cell activation and neovasularization are prominent in dermatomyositis. J Autoimmune Dis. 2006;3:2. doi: 10.1186/1740-2557-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148(2):247–253. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, et al. RANKL: osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheum. 2007;56(3):977–983. doi: 10.1002/art.22433. [DOI] [PubMed] [Google Scholar]
- 11.Bilgic H, Ytterberg SR, Amin S, McNallan KT, Wilson JC, Koeuth T, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60(11):3436–3446. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 12.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168(8):4154–4163. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 13.Chen YW, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008;9:43. doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Visser M, Emslie-Smith AM, Engel AG. Early ultrastructural alterations in adult dermatomyositis. Capillary abnormalities precede other structural changes in muscle. J Neurol Sci. 1989;94(1–3):181–192. doi: 10.1016/0022-510x(89)90228-1. [DOI] [PubMed] [Google Scholar]
- 15.Cid MC, Grau JM, Casademont J, Tobias E, Picazo A, Coll-Vinent B, et al. Leucocyte/endothelial cell adhesion receptors in muscle biopsies from patients with idiopathic inflammatory myopathies (IIM) Clin Exp Immunol. 1996;104(3):467–473. [PubMed] [Google Scholar]
- 16.Figarella-Branger D, Civatte M, Bartoli C, Pellissier JF. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve. 2003;28(6):659–682. doi: 10.1002/mus.10462. [DOI] [PubMed] [Google Scholar]
- 17.Jain A, Sharma MC, Sarkar C, Bhatia R, Singh S, Handa R. Increased expression of cell adhesion molecules in inflammatory myopathies: diagnostic utility and pathogenetic insights. Folia Neuropathol. 2009;47(1):33–42. [PubMed] [Google Scholar]
- 18.Sallum AM, Marie SK, Wakamatsu A, Sachetti S, Vianna MA, Silva CA, et al. Immunohistochemical analysis of adhesion molecule expression on muscle biopsy specimens from patients with juvenile dermatomyositis. J Rheumatol. 2004;31(4):801–807. [PubMed] [Google Scholar]
- 19.Sallum AM, Kiss MH, Silva CA, Wakamatsu A, Vianna MA, Sachetti S, et al. Difference in adhesion molecule expression (ICAM-1 and VCAM-1) in juvenile and adult dermatomyositis, polymyositis and inclusion body myositis. Autoimmun Rev. 2006;5(2):93–100. doi: 10.1016/j.autrev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Tews DS, Goebel HH. Expression of cell adhesion molecules in inflammatory myopathies. J Neuroimmunol. 1995;59(1–2):185–194. doi: 10.1016/0165-5728(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 21.Iademarco MF, McQuillan JJ, Dean DC. Vascular cell adhesion molecule 1: contrasting transcriptional control mechanisms in muscle and endothelium. Proc Natl Acad Sci U S A. 1993;90(9):3943–3947. doi: 10.1073/pnas.90.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69(7):1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- 23.Von Andrian UH, Engelhardt B. α4 Integrins as Therapeutic Targets in Autoimmune Disease. New England Journal of Medicine. 2003;348(1):68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 24.McMurray RW. Adhesion Molecules in Autoimmune Disease. Seminars in Arthiritis and Rheumatism. 1996;25(4):215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 25.Y Lebranchu JFV, Buchler M. Measurement of Soluble Adhesion Molecules in Biological Fluids. New York: Marcel Dekker, Inc; 1997. [Google Scholar]
- 26.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43(10):2368–2377. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budhu A, Ji J, Wang XW. The clinical potential of microRNAs. J Hematol Oncol. 3:37. doi: 10.1186/1756-8722-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18(5):504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105(5):1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charreau B. Molecular regulation of endothelial cell activation: novel mechanisms and emerging targets. Curr Opin Organ Transplant. 16(2):207–213. doi: 10.1097/MOT.0b013e3283446c52. [DOI] [PubMed] [Google Scholar]
- 32.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 79(4):581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 33.Littler AJ, Buckley CD, Wordsworth P, Collins I, Martinson J, Simmons DL. A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br J Rheumatol. 1997;36(2):164–169. doi: 10.1093/rheumatology/36.2.164. [DOI] [PubMed] [Google Scholar]
- 34.Spronk PE, Bootsma H, Huitema MG, Limburg PC, Kallenberg CG. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin during disease exacerbations in patients with systemic lupus erythematosus (SLE); a long term prospective study. Clin Exp Immunol. 1994;97(3):439–444. doi: 10.1111/j.1365-2249.1994.tb06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamane K, Ihn H, Kubo M, Yazawa N, Kikuchi K, Soma Y, et al. Increased serum levels of soluble vascular cell adhesion molecule 1 and E-selectin in patients with localized scleroderma. J Am Acad Dermatol. 2000;42(Pt 1):64–69. doi: 10.1016/s0190-9622(00)90010-0. [DOI] [PubMed] [Google Scholar]
- 36.Bloom BJ, Miller LC, Blier PR. Soluble adhesion molecules in pediatric rheumatic diseases. J Rheumatol. 2002;29(4):832–836. [PubMed] [Google Scholar]
- 37.Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol. 185(7):4457–4469. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabay C, Gay-Croisier F, Roux-Lombard P, Meyer O, Maineti C, Guerne PA, et al. Elevated serum levels of interleukin-1 receptor antagonist in polymyositis/dermatomyositis. A biologic marker of disease activity with a possible role in the lack of acute-phase protein response. Arthritis Rheum. 1994;37(12):1744–1751. doi: 10.1002/art.1780371206. [DOI] [PubMed] [Google Scholar]
- 39.Bai Y, Bai X, Wang Z, Zhang X, Ruan C, Miao J. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp.Mol.Pathol. 2011;91(1):471–477. doi: 10.1016/j.yexmp.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Ryu HS, Park SY, Ma D, Zhang J, Lee W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS.One. 2011 Mar;25:e17343. doi: 10.1371/journal.pone.0017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter S, Karpati G, Rothman S, Watters G. The childhood type of dermatomyositis. Neurology. 1976;26:952–962. doi: 10.1212/wnl.26.10.952. [DOI] [PubMed] [Google Scholar]
- 42.Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A, et al. Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 2008;87(2):70–86. doi: 10.1097/MD.0b013e31816bc604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eimer M, Brickman W, Seshadri R, Smulevitz B, Ramsey-Goldman R, Stone NE, McPherson DD, Pachman LM. Abnormal cardiovascular risk profile in adults with history of Juvenile Dermatomyositis. J Pediatrics. 2011 Jul; doi: 10.1016/j.jpeds.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]