Abstract
Rationale
Although cocaine is often abused in social situations, very few animal studies examine the effects of cocaine in the context of social behavior.
Objectives
This review highlights studies investigating the behavioral effects of cocaine in the context of social housing conditions using nonhuman primates. In addition, this review presents recent findings examining the effects of self-administering cocaine on social behavior and the effects of manipulations hypothesized to be stressful or enriching on the interactions between cocaine reinforcement and social rank. The following dependent variables are examined: 1) cocaine-induced changes in social behavior and 2) cocaine self-administration in cynomolgus monkeys of varying social ranks. The independent variables examined include several environmental and pharmacological manipulations.
Conclusions
The studies reviewed here indicate that several variables can differentially affect cocaine self-administration when studied in a social context, rather than in individually housed animals. These variables include the social rank and sex of the individual, drug history, the nature of the “fear”-inducing manipulation, and the reliability of cortisol as an appropriate measure of “stress.” While the inclusion of socially housed animals necessitates larger sample sizes, animal models incorporating social behavior are more homologous to the human condition and should be implemented when possible.
Keywords: aggression, submission, social interactions, social consequences, social rank, cocaine self-administration, nonhuman primates
INTRODUCTION
This review highlights the use of animal models of social behavior to better understand the behavioral, physiological and neurobiological consequences of cocaine use in humans. While there are excellent models of social behavior in rodents (e.g. Duncan et al. 2006; Quadros and Miczek 2009; Caldji et al. 2011; Smith 2012), this review will primarily focus on the use of nonhuman primates. There are several advantages to using nonhuman primates to study human diseases, including the close phylogeny and physiology of humans and nonhuman primates, the similarity in brain structure and function, pharmacokinetics, and neurochemistry. Furthermore, their complex social and behavioral repertoire allows for hypothesis-driven, longitudinal studies of disease states (Weerts et al. 2007; Kaplan et al. 2010). In addition, the ability to study the same subjects for years allows for within-subject assessments of multiple independent variables (Nader et al. 2010; Czoty et al. 2011; Howell and Murnane 2011; Gould et al. 2012).
When describing research involving social behavior and drugs, the dependent variables can be unconditioned (e.g. drug-elicited aggression) or conditioned (e.g. drug effects on operant behavior in dominant or subordinate animals) behaviors. Both are discussed in this review. As it relates to operant behavior, behavioral pharmacologists primarily consider three main factors: a discriminative stimulus, a response, and the presentation of another stimulus (see Morris 1992 for an excellent historical description of this 3-term contingency). A slightly more comprehensive view would consider the antecedent conditions, characteristics of the organism, and the nature of the consequences. Each of these variables has a level of complexity, including the history of the individual (both behavioral and pharmacological), environmental context, and genetics. Moreover, whether the consequence of the response leads to more (reinforcement) or less (punishment) behavior cannot be known in the absence of studying the behavior.
Regarding environmental context, for most studies, experiments are frequently arranged to control certain organismal variables that can lead to greater variability. Animals are individually housed, lighting conditions remain the same for every experiment and a stable body weight is maintained. Socially housing four animals can change an experiment from N=4 to four “groups” of N=1 (that is, the four different positions in the social dominance hierarchy). Thus, studying socially housed animals necessitates increased cost and complexity to the experiment. However, certain translational questions can only be answered using experimental designs incorporating situations in which animals behave in a social environment. It certainly complicates the experiment but, as described below, these are the most sophisticated models for studying human disease. As Barrett and Miczek (1995) stated:
“[T]here is a particular irony in the current situation in which it appears that as the understanding of molecular events underlying synaptic transmission and neuroregulation has assumed increasing complexity and sophistication, the procedures used to evaluate the relationship of those events to behavior are, in many cases, often rather simple.” (p. 66).
As a minimum requirement, animal models of human disease should have predictive validity with respect to clinical outcomes (Katz and Higgins 2003). For this review, the outcomes of interest are vulnerability to drug use and treatment efficacy. It is reasonable to speculate as to how this increased complexity in experimental design predicts the human condition. Social rank, as determined in the nonhuman primate social dominance hierarchy, represents two ends of a continuum incorporating socially derived stress (in subordinate monkeys) and environmental enrichment (in dominant monkeys). This continuum has tremendous predictive, face, and construct validity (Nader and Czoty 2005) and can influence the behavioral and reinforcing effects of drug.
This review describes some of the important variables that may mediate the interactions between drug effects and social rank in monkey models. First, studies involving physiological indicators, in particular the most commonly used measure of stress responsiveness, cortisol, are described. This is followed by an examination of the effects of various environmental variables hypothesized to be stressful or enriching on cocaine self-administration in dominant and subordinate monkeys, and concludes with examples of differential drug effects in socially housed monkeys.
Social Rank and Cortisol Response
In group-housed monkeys, social subordination has been characterized as stressful (e.g. Kaplan et al. 1986, 1991, 1999). Subordinate monkeys are more susceptible to reproductive dysfunction (Cameron 1997), upper respiratory infection (Cohen et al. 1997), and atherosclerosis (Kaplan and Manuck 1999). Such results are consistent with findings in humans suggesting a relationship between socioeconomic status, social conditions, and susceptibility to disease (Adler and Matthews 1993; Krantz and McCeney 2002). Furthermore, subordinate cynomolgus monkeys have heavier adrenal glands compared to dominant monkeys (Shively and Kaplan 1984). This finding supports the hypothesis that social status differentially affects the physiology of the hypothalamic-pituitary-adrenal (HPA) axis (Henry and Stephens 1977), which is the most frequently studied marker of individual differences in response to stress. Studies in rodents have shown complex relationships between glucocorticoids (in most rodent studies it’s corticosterone), stress and drug reinforcement (reviewed in Goeders 2002, Koob and Kreek 2007).
The identification of biological substrates that vary predictably with stress or cocaine exposure strengthened the hypothesis that stress-induced alterations in the behavioral effects of cocaine were mediated through the HPA axis. Evidence suggests that environmental stressors, by activating the HPA axis, can enhance the reinforcing strength of cocaine by increasing the activity of brain dopamine (DA) systems. For example, acute and chronic stress resulted in increased DA levels in the mesolimbic and mesocortical pathways of rats (Kalivas and Duffy 1989; Sorg and Kalivas 1991). Goeders and Gurein (1994) found that the same stressors used by Kalivas and colleagues (foot shock) could increase rates of acquisition of cocaine self-administration. As it relates to nonhuman primates, social subordination in male cynomolgus monkeys, which is hypothesized to be stressful (Morgan et al. 2000), resulted in greater sensitivity to cocaine reinforcement compared to dominant monkeys (Morgan et al. 2002). However, basal cortisol levels did not differ between dominant and subordinate cynomolgus monkeys.
In his review on social hierarchy and primate health, Sapolsky (2005) differentiated between physical stressors (external challenges to homeostasis) and psychosocial stressors, which he defined as “anticipation, justified or not, that a challenge to homeostasis looms” (p. 648). Sapolsky (2005) pointed out that it is not necessarily the case that lower dominance rank is associated with greater stress. In fact, a recent study (Gesquiere et al. 2011) examined the relationship between social rank and cortisol response in a natural population of savannah baboons and reported a clear inverse relationship between social rank and cortisol response for ranks 2–14+, just as one would hypothesize. What was striking though was that the cortisol concentration of the #1-ranked baboon was as high as the #14-ranked animal and significantly higher than the #2-ranked baboon. This suggests that simply assigning social ranks and assuming stress-responsiveness will not necessarily lead to orderly relationships.
It is clear that circulating cortisol concentrations are both influenced by prevailing environmental conditions and sensitive to neuroendocrine adaptations to long-term exposure to social stress. In addition, other factors can complicate the correlation between cortisol and health, including testosterone concentrations, age and physical condition (cf. Archie et al. 2012). In a study of wound healing in baboons, Archie et al. (2012) found that despite having high glucocorticoid concentrations, alpha males recovered from injury significantly faster than low-ranking male baboons. These investigators pointed out that the high glucocorticoid concentrations in alpha males were caused by energetic stress (i.e., related to reproductive effort) while high concentrations in subordinate animals were due to social stressors. Just as we’ll describe below, Archie et al. (2012) noted that different types of stressors can lead to different immune responses.
The latter observations were based almost exclusively on the study of feral animals in their natural habitat and may not generalize to socially housed nonhuman primates in captivity (see Goymann and Wingfield 2004). In fact, basal cortisol concentrations have not been shown to be orderly predictors of social rank in captive monkeys (Vellucci 1990). Abbott et al. (2003) performed a meta-analysis on social rank and cortisol concentrations across several Old World and New World monkeys and concluded that social rank is not uniform in meaning across different primate species. They reported large species differences in basal cortisol concentrations, with subordinate monkeys having either higher, lower or no difference than dominant monkeys (Abbott et al. 2003). For example, subordinate cynomolgus monkeys had approximately 27% higher basal cortisol concentrations compared to dominant monkeys, while subordinate marmosets had 50% lower cortisol concentrations compared to dominant marmosets. Abbott et al. (2003) determined that in species in which social subordination is accompanied by higher rates of physical and psychological stressors, along with having fewer resources available, the low-ranking monkeys had higher cortisol concentrations compared to the higher-ranking individuals.
In our initial studies, neither basal cortisol nor the magnitude of cortisol response to an ACTH challenge predicted eventual social rank (Morgan et al. 2000). In a more recent study, cortisol concentrations in male cynomolgus monkeys were assessed during the early stages of social hierarchy formation (Czoty et al. 2009). At the start of the study all the monkeys were individually housed. Neither basal cortisol concentrations nor HPA axis response following dexamethasone suppression and ACTH challenge was predictive of eventual social rank. The stress response to new social group formations was rapid, transient, and dependent on the eventual rank of the monkeys. During the first three days of social hierarchy formation, cortisol concentrations were significantly higher in monkeys that would eventually become subordinate. By day 4 and for the remainder of the study (12 weeks), there were no differences in cortisol concentrations as a function of social rank. At the end of the 12 weeks, when social hierarchies were deemed stable, basal cortisol concentrations were significantly higher in dominant monkeys, while the cortisol response to ACTH challenge following dexamethasone suppression was greater in subordinate monkeys.
What is particularly striking about the conclusion regarding whether a social rank is stressful is that it appears to vary according to the dependent variable under study. If the subordinate monkeys and not the dominant animals had higher cortisol concentrations, the elevated basal cortisol concentrations would be considered evidence of living in stressful conditions. The results though are strikingly similar to the findings reported in feral baboons for the most dominant animals (Gesquiere et al. 2011). The dominant monkeys in the Czoty et al. (2009) study accounted for 30% of the aggression in the social groups and submitted infrequently (5% of the total submissions), so it is possible that the maintenance of the social rank resulted in greater cortisol concentrations. Alternatively, it is possible that the relatively lower circulating cortisol concentrations in subordinate monkeys was the result of neuroendocrine adaptations that occurred due to chronic exposure to social stress. Supporting this idea is the finding that subordinate animals had greater adrenal responsiveness to ACTH challenge compared to dominant monkeys. As described below, this hyper-responsiveness to stressful conditions led to the hypothesis that subordinate monkeys would be more sensitive to other environmental stressors than dominant- or intermediate-ranked monkeys in the social group.
Whereas the study of social subordination has served as a model of chronic stress for decades, an under-appreciated facet of the linear hierarchy involves the other end of the continuum involving dominant macaques. In male cynomolgus monkeys, following the formation of social groups, DA D2-like receptor availability significantly increased in animals that became dominant (Morgan et al. 2002). One interpretation of this elevation is that D2-like receptor changes resulted from exposure to an enriching environment. Dominant macaques freely move throughout the pen without fear of aggression, are groomed and submitted to more frequently, and have first access to food or treats within the pen (Morgan et al. 2000).
Several studies in rodents have attempted to characterize the neurobiological effects of environmental enrichment (e.g. Bowling et al. 1993; Howes et al. 2000; see Smith and Lynch 2011 for recent review). In many of these studies, individually housed rats are compared to rats housed in social groups with enrichment objects placed in the cage. Although these studies often do not examine social hierarchies in the rodent groups, the data have provided relevant information regarding effects of environmental enrichment on the brain. For example, Bowling et al. (1993) and Hall et al. (1998) reported that rats living in an enriched environment had lower concentrations of DA in the nucleus accumbens compared to individually housed rats. In related work, environmentally enriched rats self-administered d-amphetamine at lower rates than individually housed rats (Bardo et al. 2001; Green et al. 2002). Overall, the effects seen in enriched rats (i.e., lower DA levels) could partially account for the higher D2-like receptor binding potentials observed in dominant monkeys because the D2 receptor radiotracer competes with DA for the receptor; less DA in dominant monkeys would result in higher binding potentials. Furthermore, the behavioral data suggest that environmental enrichment attenuated or retarded the acquisition of stimulant self-administration in rodents.
One of the difficult questions that studying socially housed animals poses is how an investigator defines an event as enriching or stressful. As described above, analyses of cortisol concentrations as a physiological measure of the response to stress frequently do not differentiate between dominant and subordinate monkeys; cortisol concentrations may in fact be higher in dominant animals under certain conditions. There are no obvious physiological measures of enrichment short of measuring endorphin levels in brain. For the purposes of this review, and as it relates to drug abuse, environmental enrichment is defined as “conditions that decrease cocaine self-administration, shifting the cocaine dose-response curve to the right”, in a manner similar to the effects of a pharmacological antagonist. An environmental stressor is operational defined as “conditions that increase cocaine self-administration, shifting the cocaine dose-response curve to the left”, in a manner similar to the effects of a pharmacological agonist. Physiological endpoints such as cortisol concentrations can be measured but, in the end, the most relevant dependent variable in assessing social enrichment vs. social stress is the behavior. With this definition, it is important to point out that an environmental event that does not shift the cocaine dose-response curve to the left (and operationally defined as not being a stressor), may be considered a stressor under other conditions. For behavioral pharmacologists, this is no different than stating that under a particular set of conditions, a stimulus can maintain responding and under another set of conditions that stimulus can punish responding (e.g. Barrett and Glowa 1977; Spealman 1979).
Effects of Social Environment on Cocaine Reinforcement
Epidemiological studies have demonstrated relationships between socioeconomic status (SES) and substance abuse (e.g. Wohlfarth and Van Den Brink 1998; Amick et al. 2002; Goodman and Huang 2002). Although SES affects emotional and cognitive development to varying degrees, growing up in a family with low SES has been associated with poor health and impaired cognitive development (cf. Hackman et al. 2010). Data discussed in the next section indicate that social status can prominently influence the abuse-related behavioral effects of cocaine. In translating these findings from monkeys to humans, however, it is clear that equating social rank and SES is overly simplistic; people of all socioeconomic strata can and do experience stress and enrichment to varying degrees and may become addicted to drugs. The dominance hierarchy is conceptualized as a continuum consisting of chronic social stress at the lower end and environmental enrichment at the upper end (Nader and Czoty 2005). The research question addressed in this section is how individuals of varying social ranks respond to changes in their environment.
In the studies described in this section, social status was determined for each cynomolgus monkey according to the outcomes of agonistic encounters as described previously (Kaplan et al. 1982; Morgan et al. 2000; Czoty et al. 2009). Initially, aggressive, submissive, and affiliative behaviors were recorded for individual monkeys in each pen during 45-min observation sessions. The animal that aggressed towards, and elicited submissive behaviors from, all others was designated the dominant monkey. The subordinate monkey received aggression from all others and rarely aggressed.
In these experiments, cynomolgus monkeys living in groups of four in which stable dominance hierarchies had been established self-administered cocaine under a food-cocaine choice procedure (Czoty and Nader 2012). Under this procedure, in each session ascending doses of cocaine were made available as an alternative to one food pellet. These reinforcers were delivered as a consequence of responding on one of two photo-optic switches under a fixed-ratio (FR) schedule of reinforcement. Ascending cocaine doses (0.0, 0.003, 0.01, 0.03 and 0.1 mg/kg per injection cocaine) were available in separate, consecutive components throughout the session; components lasted until monkeys made a total of 10 choices or 20 min elapsed. As observed previously (Paronis et al. 2002; Negus 2003), there was a dose-related increase in allocation of responding towards the cocaine-associated lever. That is, when the alternative to a food pellet was no injection, monkeys emitted nearly all responses on the food-associated lever, whereas monkeys chose drug injections exclusively when choosing between a high cocaine dose and a food pellet. When responding was deemed stable, a variety of interventions occurred as described below.
We hypothesized that stressful events would shift the cocaine-choice curve to the left (i.e., monkeys would choose more cocaine compared to baseline conditions), whereas enriching manipulations would shift the cocaine-choice curve to the right. A primary question was whether dominant and subordinate monkeys would respond similarly to a given manipulation. In general, a monkey was considered “affected” by a manipulation if the ED50 for cocaine choice was shifted by 0.25 log units or more in either direction. One conclusion that was true across all manipulations was that not every individual was affected by a given manipulation. This result is reminiscent of the clinical situation in which a drug treatment or other intervention is not universally effective. Nonetheless, several conclusions could be drawn with respect to the interaction between chronic living conditions and acute exposure to environmental stimuli.
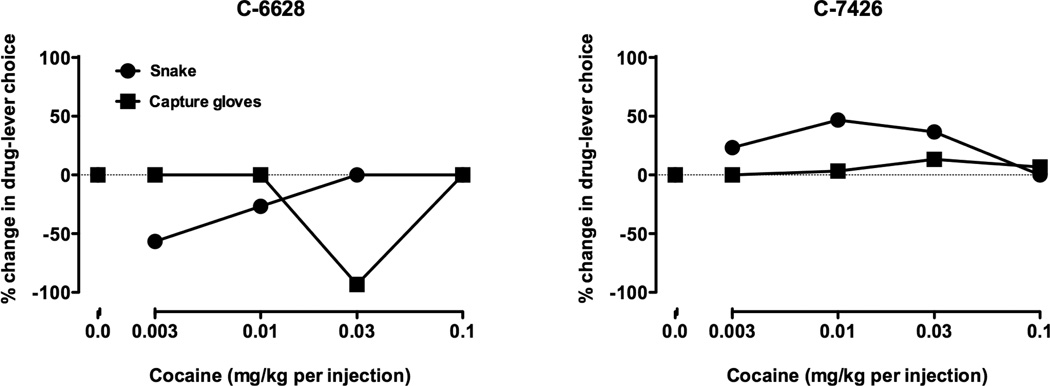
Some examples of environmental manipulations hypothesized to shift the curve to the left (stressors) and to the right (enrichers) are provided. Initial studies examined brief, unexpected exposure to a rubber snake in the monkeys’ home cage prior to a cocaine self-administration session (Czoty and Nader 2012). Exposure to a rubber snake has been shown to elicit fear responses in monkeys comparable to those elicited by real snakes (Kalin et al. 2001; Prather et al. 2001; Nelson et al. 2003). All monkeys clearly exhibited a fear-like reaction to the presence of the snake, retreating to the opposite end of the test cage. Despite this similarity, the presence of the snake only altered cocaine self-administration in half of the 12 monkeys. Data from two socially dominant monkeys are shown in Figure 1. For one monkey (C-7426), exposure to the rubber snake increased cocaine preference at the lower cocaine doses, when choice was primarily for food reinforcement, suggesting exposure to a stressor. For the other monkey (C-6628), cocaine preference decreased from baseline, suggesting exposure to an environmental enricher.
Figure 1.
Individual differences in effects of “stressful” environmental stimuli on cocaine self-administration. Data for two dominant monkeys are shown following exposure to a toy snake in the home cage (circles) and presentation of capture gloves while they are seated in a primate chair in another room (squares). Each point represents the percent change in cocaine choice from baseline. The cocaine dose-response curve was determined in one session.
In these same two monkeys, another manipulation hypothesized to be stressful was examined. Each monkey was exposed to capture gloves while seated in a primate chair in an unfamiliar room. Capture gloves have been used as an aversive stimulus in laboratory studies (Bowers et al. 1998; Machado et al. 2009) and, when monkeys are in the home cage, the appearance of an individual wearing these gloves elicits vocalizations and other fear-related behaviors. In the present experiment, there was some evidence of fear-related behaviors, primarily yawning and vocalization. In monkey C-7426, while the toy snake appeared stressful, exposure to the capture glove did not impact cocaine choice. The same capture glove manipulation in the monkey in which the toy snake decreased cocaine choice (C-6628), also decreased choice, but at a higher cocaine dose.
Given these findings, what is different about C-7426 and C-6628 that could account for such disparate results? Both monkeys are dominant in their social group and there were no differences in their cocaine-food choice baseline behavior. Cortisol concentrations, measured immediately before the self-administration sessions, did not differentiate monkeys either. Monkey C-7426 appears to be particularly sensitive to stimuli in his home cage (the snake), but not to stimuli presented in a novel context (the capture gloves in another room), while C-6628 appears to be affected in an entirely different manner by both manipulations. In this animal stress may have altered choice by making the food more salient. As with people, in monkeys stress may manifest itself in different ways. While some individuals may be more vulnerable to drug abuse, others may seek an increase in caloric intake or palatable food substances.
While the previous set of studies was designed to evaluate “stress-induced” increases in cocaine self-administration, manipulations that decrease the reinforcing effects of cocaine by providing environmental enrichment may more directly inform treatment approaches. In a recent study, two environmental manipulations hypothesized to serve as enrichment was examined in socially housed monkeys (Czoty and Nader 2012). The manipulations were providing food treats prior to the cocaine-food choice session and allowing individual monkeys to live in a large enclosure for the weekend. Below, we describe data from the large enclosure and a third manipulation involving pair-housing a male monkey with a female.
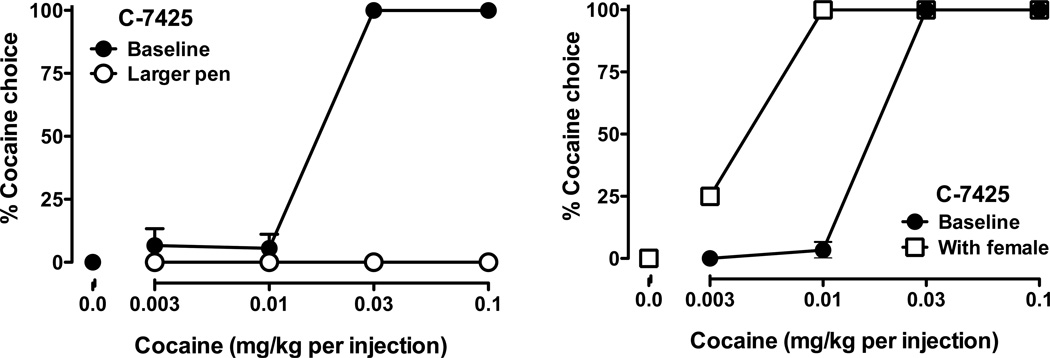
In one experiment, monkeys were allowed to live in their entire pen in the absence of their cage-mates for three days. Increasing monkeys’ living space has previously been shown to decrease abnormal behaviors (Draper and Bernstein 1963; Paulk et al. 1977; Kaufman et al. 2004), while increasing density of individuals per cage has been shown to have deleterious physiological and behavioral effects in rodents (e.g. Brown and Grunberg 1996; Gadek-Michalska and Bugajski 2003; Botelho et al. 2007). Data from a subordinate male monkey (C-7425) are shown in Figure 2. When this monkey was allowed to self-administer cocaine in the context of the choice paradigm after spending 3 days living in the large pen without his 3 cage mates, he chose only food throughout the session (Fig. 2, left panel). A few weeks later this monkey was retested with another manipulation hypothesized to be enriching – pair housing him with a female for a 3-day period. In contrast to the hypothesis that this manipulation was enriching, pair-housing this monkey with a female resulted in his cocaine dose-response curve shifting to the left (Fig. 2, right panel); the manipulation appeared to be an environmental stressor. These data reinforce the observation that environmental events cannot be defined as stressful or enriching in the absence of behavior and, more importantly, the same event may have different effects in different individuals.
Figure 2.
Individual differences in effects of environmental “enrichers” on cocaine selfadministration. Data are for cocaine vs. food choice from a subordinate monkey following two 3-day manipulations. Left: following a 3-day period in an enlarged space without cage mates (red symbols). Right: following a 3-day period pair-housed with a female cynomolgus monkey (open circle). Black symbols represent baseline (mean from the preceding 3 sessions).
Whereas the manipulations described above were expected to function as either stressors or enrichers in all monkeys regardless of social rank, two other interventions were conducted that were hypothesized to have differential effects depending on the social rank of the monkey. In one experiment, competition over food occurred by placing a treat into the cage while monkeys were socially housed. This represented a novel situation for the monkeys because standard operating procedure is to separate monkeys for approximately 90 min each day for feeding to assure that all monkeys in the pen receive adequate food. The presence of a preferred treat would create conflict as a dominant monkey retrieved the treat, aggressing towards lower-ranked monkeys if necessary. In each of the four pens tested, the treat was retrieved by a dominant monkey. In one of these dominant monkeys, the cocaine choice curve was shifted to the right. Other dominant- and intermediate-ranked monkeys were unaffected. In 3 of 4 pens, the most subordinate (#4-ranked) monkey chose significantly more cocaine than under baseline conditions. Thus, this manipulation clearly produced differential effects according to social rank. One possible interpretation of these findings is that whereas most monkeys are unaffected by the manipulation, subordinate monkeys find the experience stressful and consequently self-administer more cocaine when given the opportunity.
In all the studies described in this section, the same environmental stimulus or experience did not affect all monkeys identically with respect to cocaine self-administration. These individual differences in the effects of social/environmental stimuli on sensitivity to cocaine are of clear clinical relevance. In male cocaine-dependent individuals, experiencing a high number of “daily hassles” is associated with greater cocaine use (Waldrop et al. 2007) and recent studies reporting “real-time” data have demonstrated positive relationships between momentary stress and craving for cocaine (Preston and Epstein 2011). Such results support the view that, whereas most individuals can manage the many minor inconveniences we all encounter on a daily basis, such experiences appear to be much more stressful in certain people which subsequently makes them more likely to abuse cocaine. Moreover, there are predictive relationships between reactivity to stress, craving, and relapse to cocaine and alcohol use (e.g. Kosten 1992; Brown et al. 1995; Back et al. 2010; Higley et al. 2011). Understanding the neurobiological mechanisms that underlie this variability may provide insight into the biological basis for differences in individuals’ ability to remain abstinent in the face of social stress and in vulnerability/resilience to becoming addicted in the first place.
Interactions of Drug Effects with Social Behavior
The studies described above examined the effects of environmental manipulations on cocaine self-administration in socially housed monkeys. There is also a growing body of literature examining the direct and indirect effects of psychoactive drugs on social behaviors (e.g. Knobbout et al. 1996). One of the earliest reports (Crowley et al. 1974) demonstrated that ethanol, methamphetamine, pentobarbital and morphine produced dose-dependent changes on social behavior in group-housed macaques. The psychomotor stimulant methamphetamine, for example, increased locomotion and stereotypies and decreased food foraging and aggression. The effects of other stimulants (such as cocaine and amphetamine) on agonistic (primarily, aggressive) behaviors have been examined in several species of monkeys in various experimental situations. Crowley et al. (1992) showed that cocaine produced dramatic decreases in various affiliative measures without altering rates of aggression in an all-male group of macaques (8 monkeys in the group). When larger macaque groups were studied (38 stumptail macaques, some female and adolescent), cocaine or amphetamine increased aggression in dominant and subordinate monkeys (Smith and Byrd 1894, 1985; Martin et al. 1990). Miczek and colleagues studied the effects of these drugs on aggression in group-housed squirrel monkeys in their home environment and in situations designed to increase levels of aggressive activity such as introduction of an “intruder” (e.g. Miczek et al. 1981; Miczek and Yoshimura 1982; Miczek and Gold 1983). In general, cocaine and amphetamine decreased most forms of social behavior, including aggression. These investigators also found that, at high doses, dominant monkeys change from primary initiators of aggression to recipients of aggression (Miczek and Gold 1983). Taken together, these data demonstrate that drugs do not have a unitary effect on social behavior, but are influenced by the social rank of the individual and the social context.
The neurobiology of aggression and the interactions between genetics and social behavior have great relevance to understanding human behavior (Miczek et al. 1984, 2004; Miczek and de Wit 2008; Miczek and de Almeida 2012; Takahashi et al. 2012). Recent studies have extended the pioneering studies of Miczek, Crowley and colleagues examining drug effects on social behavior to incorporate cocaine self-administration (as opposed to experimenter-administered drug administration). Evidence has unequivocally shown that drug effects on several endpoints, including behavior, are different depending on whether the drug is experimenter-administered or self-administered (Dworkin et al. 1995; Bradberry 2000; Howell et al. 2010). The goals of these studies were to (1) examine the effects of social consequences (i.e., increases or decreases in aggression) on the reinforcing effects of cocaine and (2) to determine if the social consequences of self-administering cocaine were dependent on the social rank of the monkey.
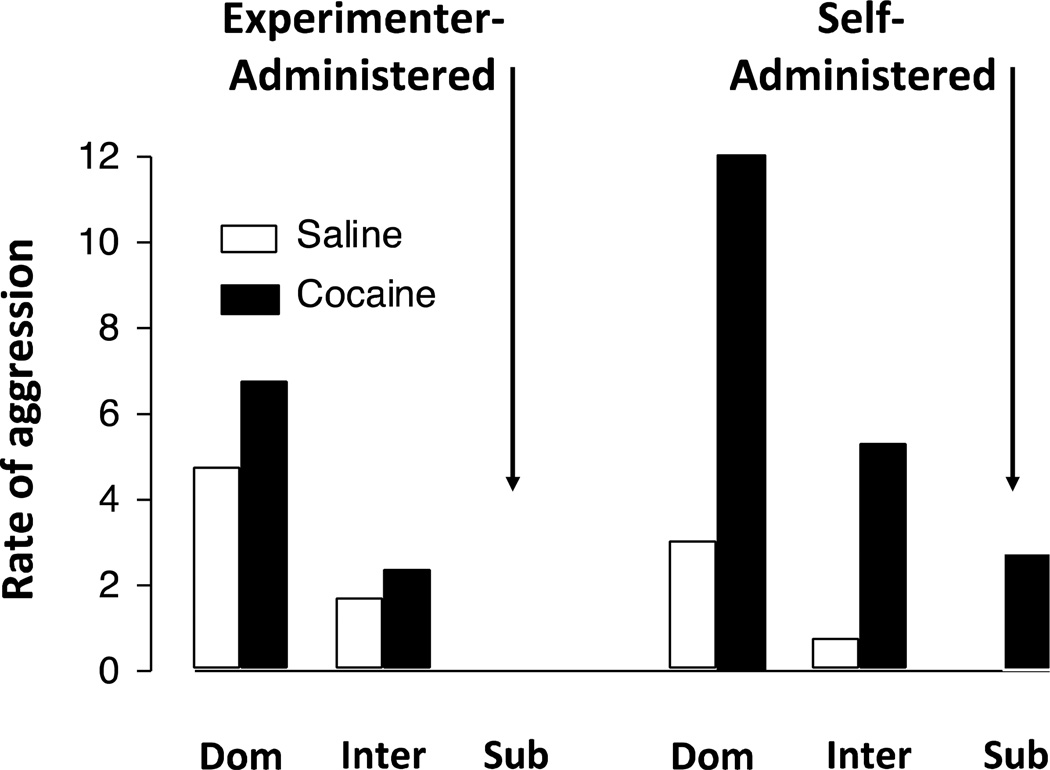
In a preliminary study in one social group, the effects on social behavior of a non-contingent injection of cocaine to a subordinate cynomolgus monkey were compared with the effects of self-administered cocaine in that same animal (Fig. 3). Under baseline conditions, the subordinate monkey showed no signs of aggression. When this subordinate monkey received a non-contingent injection of 0.3 mg/kg cocaine, aggression by the dominant and intermediate-ranked monkeys increased, but the subordinate monkey himself did not increase aggression (Fig. 3, left panel). In contrast, when the subordinate monkey was the only animal in the pen permitted to self-administer cocaine (10 injections of 0.03 mg/kg/injection cocaine), his aggression increased, as did the other monkeys (Fig. 3, right panel).
Figure 3.
Effects of non-contingent vs. contingent cocaine presentation on aggression in socially housed monkeys. In this pilot study, a subordinate monkey received experimenter administered cocaine (left) or self-administered cocaine (right) and the effects on aggression (frequency) in all monkeys was examined. Open bars represent baseline (no cocaine administered) and filled bars represent aggression when the subordinate monkey received cocaine.
Of particular interest for future studies is to examine how the social consequences of self-administered cocaine subsequently affect the future likelihood of self-administering cocaine. As shown under baseline conditions in Fig. 3, subordinate monkeys rarely aggress towards cagemates and hierarchies are typically very stable. One could hypothesize that if cocaine-induced aggression by the subordinate monkey changed his position in the hierarchy, this may initially increase the reinforcing effects of cocaine (because the change in social rank is a consequences associated with cocaine use). In a recently completed study, it was found that under conditions in which only one monkey in the pen self-administers cocaine, the effects over consecutive sessions differ depending on the rank of the monkey. However, irrespective of which monkey in the pen self-administered cocaine, the subordinate monkeys never aggressed. Perhaps what was most striking was that the most affected monkeys, both in terms of increases in cocaine intake and increases in cocaine-induced aggression, were the intermediate-ranked monkeys. The social experiences of intermediate-ranked monkeys, and the effects of those experiences on physiology and behavior, are almost completely overlooked in favor of focusing on the most dominant and most subordinate monkeys, but it is clear that they are extremely relevant and should be studied in greater detail. As a final point related to the importance of social conditions and drug self-administration, a recent study of interactions between social rank, social separation and ethanol consumption in squirrel monkeys (McKenzie-Quirk and Miczek 2008) reported an inverse relationship between social rank and alcohol consumption. In addition, male monkeys drank more alcohol when removed from the social context. Thus, alcohol intake appeared to be influenced by the social environment. Overall, these examples remind us of other tenets within the field of behavioral pharmacology and it seems appropriate to conclude this section with the following observation based on maintaining events and environmental context:
“This experiment and others … indicate that the reinforcing or punishing properties of environmental events are not invariant features of the event, but depend also on characteristics of the schedule under which that event is presented. Such other factors as the behavioral history of the organism and the environmental context in which behavior occurs also play an important role in determining the effects of various consequences on behavior.” (Barrett and Katz, 1981, p. 128)
Conclusions: Interactions of Social Behavior and Treatment Drugs on Cocaine Self- Administration
This review has highlighted several examples of dominant and subordinate animals being differentially sensitive to the behavior-altering effects of drugs. A better understanding of the neurobiological mechanisms underlying these differences may provide insight into treatment strategies (Miczek and de Almeida 2012). Positron emission tomography studies in socially housed cynomolgus monkeys suggest that subordinate animals have higher basal levels of synaptic dopamine and/or lower levels of dopamine D2-like receptors (Grant et al. 1998; Morgan et al. 2002; Nader et al. 2012). In terms of translational science, a similar finding has recently been reported in humans (Martinez et al. 2010). If cocaine produced qualitatively different effects on social interactions as a function of social rank because of these differences in dopaminergic functioning, it may be that the susceptibility and vulnerability to the reinforcing effects of dopaminergic agents, such as cocaine, would also be influenced by these social variables.
The data, at least in male subjects, suggest that low measures of dopamine D2 receptor availability is associated with greater vulnerability to stimulant abuse (Volkow et al. 1999; Morgan et al. 2002; Martinez et al. 2004; Nader et al. 2006; Dalley et al. 2007). Importantly, it appears that chronic cocaine exposure results in decreases in dopamine D2-like receptor availability and densities (e.g. Volkow et al. 1993; Nader et al. 2002, 2006). Thus, one pharmacological strategy could focus on treatments that ultimately increase dopamine D2-like receptor availability. However, based on the studies in monkeys described in this review, it is clear that the pharmacological consequences of treatment drugs may be different depending on the social experiences of the individual. Such individual differences need to be factored into the experimental designs in preclinical studies of potential pharmacotherapies for cocaine addiction. In a recent study, it was found that the relationship between dopamine D2-like receptor availability and vulnerability to cocaine abuse may be opposite in female monkeys (Nader et al. 2012). That is, although like males, dominant female monkeys had higher D2-like receptor measures than subordinate females, the dominant females were more sensitive to the reinforcing effects of cocaine. This implies that treatment strategies for females (with a direct relationship between D2-like receptor availability and cocaine reinforcement) and males (with an inverse relationship between D2-like receptor availability and cocaine reinforcement) may be different.
Typically, using individually housed subjects, researchers generate a cocaine self-administration dose-response curve and when responding is stable, examine the behavioral effects of a potential treatment drug. However, as highlighted in this review, the social context in which self-administration occurs can have a dramatic effect on behavior and the behavioral effects of drugs. Thus, a more homologous model of the human condition should require that the treatment drug be administered in a social context and with the understanding that the drug may have different effects depending on the social rank of the subject.
As researchers delve deeper into individual subject comparisons, important findings will surely arise. For example, how a pharmacological agent affects receptor availability may depend on the baseline levels of the receptor prior to drug treatment. It has been noted that the effects of the D2 receptor partial agonist aripiprazole on D2-like receptor availability were dependent on the monkey’s initial levels of receptor occupancy (Czoty et al., under review). That is, chronic aripiprazole elevated D2-like receptor availability in monkeys with lower initial D2 measures, and decreased D2-like receptor availability in monkeys with higher baseline levels. Similar to the effects of environmental variables, the consequences of drug treatments are dependent on several “organismal” variables and are not an inherent property of the drug.
Although such experiments are certainly more expensive to conduct and more time consuming, the combination of social behavior, chronic drug treatment and manipulation of environmental variables makes studying drug effects in socially housed animals the most homologous animal model of human drug abuse. There are parallels within the field of behavioral pharmacology that may provide important insights into the neuropharmacology of social behavior. For example, we have noted in socially housed cynomolgus monkeys that the behavioral effects of DA receptor agonists appear to be influenced by social rank, while those of DA antagonists are not. It also appears that serotonin (5-HT) agonists (in our case, 8-OH-DPAT a 5-HT1A agonist) did not differentiate between social ranks (Czoty et al. 2005). One of the premises of behavioral pharmacology is that the effects of drugs on behavior maintained by different events can be differentially affected by some drugs, but not by all drugs (Barrett and Katz, 1981). Surely there are CNS mechanisms to account for these differences, but the important point is that individual differences in the behavioral effects of drugs should not be considered “variable” or “equivocal”. The studies reviewed here indicate that several variables can differentially affect cocaine self-administration when studied in a social context, rather than in individually housed animals. Attention to variables such as the social rank, the sex of the individual and the nature of the “stress” and “enrichment” manipulation contribute to the generation of homologous animal models of human drug abuse.
Acknowledgements
Preparation of this manuscript was supported by NIDA grants R37 DA10584 and R01 DA017763. The authors thank Robert Gould, Natallia Riddick, Tonya Calhoun, Michael Coller, Mikki Sandridge, Matthew Dickens, Clifford Hubbard, Osric Prioleau, Ciara McCabe and Michelle Icenhower for excellent technical assistance over the years and Kathleen A. Grant, Robert H. Mach, H. Donald Gage and Jay R. Kaplan for our long-standing collaborations.
Footnotes
The authors declare no conflicts of interest.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adler NE, Matthews K. Health physiology: why do some people get sick and some stay well? Ann Rev Psychol. 1993;45:229–259. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- Amick BC, McDonough P, Chang H, Rogers WH, Pieper CF, Duncan G. Relationship between all-cause mortality and cumulative working life course psychosocial and physical exposures in the United States labor market from 1968 to 1992. Psychosomatic Med. 2002;64:370–381. doi: 10.1097/00006842-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proc Natl Acad Sci. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;1–6:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Glowa JR. Reinforcement and punishment of behavior by the same consequent event. Psychol Rep. 1977;40:1015–1021. [Google Scholar]
- Barrett JE, Katz JL. Drug effects on behaviors maintained by different events. In: Thompson T, Dews PB, McKim WA, editors. Advances in Behavioral Pharmacology. Vol. 3. New York: Academic Press; 1981. pp. 119–168. [Google Scholar]
- Barrett JE, Miczek KA. Behavioral techniques in preclinical neuropsychopharmacology research. In: Bloom FE, Kupfer KJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd; 1995. pp. 65–73. [Google Scholar]
- Botelho S, Estanislau C, Morato S. Effects of under- and overcrowding on exploratory behavior in the elevated plus-maze. Behav Processes. 2007;74:357–362. doi: 10.1016/j.beproc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Bowers CL, Crockett CM, Bowden DM. Differences in stress reactivity of laboratory macaques measured by heart period and respiratory sinus arrhythmia. Am J Primatol. 1998;45:245–261. doi: 10.1002/(SICI)1098-2345(1998)45:3<245::AID-AJP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine abuse. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of environmental conditions on food consumption in female and male rats. Physiol Behav. 1996;60:293–297. doi: 10.1016/0031-9384(96)00020-0. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Shuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Caldji C, Hellstrom IC, Zhang T-Y, Diorio J, Meaney MJ. Environmental regulation of the neural epigenome. FEBS Lett. 2011;585:2049–2058. doi: 10.1016/j.febslet.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. [DOI] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psycosom Med. 1997;59:213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, Williams EA, Zerbe GO, Ingersoll NC. Cocaine, social behavior, and alcohol-solution drinking in monkeys. Drug Alcohol Dep. 1992;29:205–223. doi: 10.1016/0376-8716(92)90094-s. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Stynes AJ, Hydinger M, Kaufman IC. Ethanol, methamphetamine, pentobarbital, morphine, and monkey social behavior. Arch Gen Psychiatry. 1974;31:829–838. doi: 10.1001/archpsyc.1974.01760180069009. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT1A agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus. Behav Pharmacol. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Nader MA. Chapter 47: Group Processes: Social Dominance. In: Decety J, Cacioppo J, editors. Handbook of Social Neuroscience. New York, NY: Oxford University Press, Inc; 2011. pp. 716–728. [Google Scholar]
- Czoty PW, Nader MA. Individual differences in the effects of environmental stimuli on cocaine choice in socially housed male cynomolgus monkeys. Psychopharmacology. 2012 doi: 10.1007/s00213-011-2562-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinsin ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper WA, Bernstein WS. Stereotyped behaviour and cage size. Perceptual and Motor Skills. 1963;16:231–234. [Google Scholar]
- Duncan EA, Tamashiro KLK, Nguyen MMN, Gardner SR, Woods SC, Sakai RR. The impact of moderate alcohol consumption on aggression and the formation of dominance hierarchies in rats. Psychopharmacology. 2006;189:83–94. doi: 10.1007/s00213-006-0536-7. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol. 2003;54:449–459. [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MC, Onyango PO, Alberts SC, Altmann J. Life at the top: rank and stress in wild male baboons. Science. 2011;333:357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goodman E, Huang B. Socioeconomic status, depressive symptoms and adolescent substance use. Arch Periatr Adolesc Med. 2002;156:448–453. doi: 10.1001/archpedi.156.5.448. [DOI] [PubMed] [Google Scholar]
- Gould RW, Porrino LJ, Nader MA. Nonhuman primate models of addiction and PET imaging: Dopamine system dysregulation. In: Dalley JW, Carter CS, editors. Brain Imaging in Behavioral Neuroscience. Springer, Heidelberg, Germany: 2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Rev. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. Stress, Health and the Social Environment. New York: Springer-Verlag; 1977. [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Queool SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory pardigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology. 2011;218:121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Murnane KS. Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther. 2011;337:324–334. doi: 10.1124/jpet.108.136689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology. 2010;208:191–199. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, et al. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Human Reprod. 2010;25:2083–2094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Heise ER, Manuck SB, Shively CA, Cohen S, Rabin BS, Kasprowicz AL. The relationship of agonistic and affiliative behavior patterns to cellular immune function among cynomolgus monkeys (macaca fascicularis) living in unstable social groups. Am J Primatol. 1991;25:157–173. doi: 10.1002/ajp.1350250303. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Phillips-Conroy J, Fontenot MB, Jolly CJ, Fairbanks LA, Mann JJ. Cerebrospinal fluid monoaminergic metabolites differ in wild anubis and hybrid (Anubis hamadryas) baboons: possible relationships to life history and behavior. Neuropsychopharmacology. 1999;20:517–524. doi: 10.1016/S0893-133X(98)00078-5. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kaufman BM, Pouliot AL, Tiefenbacher S, Novak MA. Short and long-term effects of a substantial change in cage size on individually housed, adult male rhesus monkeys (Macaca mulata) Applied Animal Behav Sci. 2004;88:319–330. [Google Scholar]
- Knobbout DA, Ellenbroek BA, Cools AR. The influence of social structure on social isolation in amphetamine-treated Java monkeys (Macaca fascicularis) Behav Pharmacol. 1996;7:417–429. [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA. Can cocaine craving be a medication outcome?: drug craving and relapse in opioid and cocaine dependence. Am J Addictions. 1992;1:230–239. [Google Scholar]
- Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Ann Rev Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SP, Smith EO, Byrd LD. Effects of dominance rank on d-amphetamine-Induced increases in aggression. Pharmacol Biochem Behav. 1990;37:493–496. doi: 10.1016/0091-3057(90)90018-d. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology. 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, III, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM. The case for basic research on the psychopharmacology of aggression. J Clin Psychopharmacol. 2012;32:1–2. doi: 10.1097/JCP.0b013e3182463e0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, de Wit H. Challenges for translational psychopharmacology research – some basic principles. Psychopharmacology. 2008;199:291–301. doi: 10.1007/s00213-008-1198-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Gold LH. d-Amphetamine in squirrel monkeys of different social status: effects on social and agonistic behavior, locomotion, and stereotypies. Psychopharmacology. 1983;81:183–190. doi: 10.1007/BF00427259. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Winslow JT, DeBold JF. Heightened aggressive behavior by animals interacting with alcohol-treated conspecifics: studies with mice, rats and squirrel monkeys. Pharmacol Biochem Behav. 1984;20:349–353. doi: 10.1016/0091-3057(84)90269-7. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Woolley J, Schlisserman S, Yoshimura H. Analysis of amphetamine effects on agonistic and affiliative behavior in squirrel monkeys (Saimiri sciureus) Pharmacol Biochem Behav. 1981;14(suppl. 1):103–107. doi: 10.1016/s0091-3057(81)80017-2. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yoshimura H. Disruption of primate social behavior by d-amphetamine and cocaine: differential antagonism by antipsychotics. Psychopharmacology. 1982;76:163–171. doi: 10.1007/BF00435272. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (macaca fascicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morris EK. The aim, progress, and evolution of behavioral analysis. The Behav Analyst. 1992;15:3–29. doi: 10.1007/BF03392582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging studies of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. Characterizing organism × environment interactions in nonhuman primate models of addiction: PET imaging studies of dopamine D2 receptors. In: Robbins T, Everritt B, Nutt DJ, editors. The Neurobiology of Drug Addiction: New Vistas. Oxford, UK: Oxford University Press; 2010. pp. 187–202. [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:3–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HML, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH. Individual differences in the responses of naïve rhesus monkeys to snakes. Emotion. 2003;3:3–11. doi: 10.1037/1528-3542.3.1.3. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Gasior M, Bergman J. Effects of cocaine under concurrent fixed ratio schedules of food and IV drug availability: a novel choice procedure in monkeys. Psychopharmacology. 2002;163:283–291. doi: 10.1007/s00213-002-1180-5. [DOI] [PubMed] [Google Scholar]
- Paulk HH, Dienske H, Ribbens LG. Abnormal behavior in relation to cage size in rhesus monkeys. J Abnormal Psychology. 1977;86:87–92. doi: 10.1037//0021-843x.86.1.87. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Preston KM, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IMH, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206:109–120. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Smith EO, Byrd LD. Contrasting effects of d-amphetamine on affiliation and aggression in monkeys. Pharmacol Biochem Behav. 1984;20:255–260. doi: 10.1016/0091-3057(84)90252-1. [DOI] [PubMed] [Google Scholar]
- Smith EO, Byrd LD. d-Amphetamine induced changes in social interaction patterns. Pharmacol Biochem Behav. 1985;22:135–139. doi: 10.1016/0091-3057(85)90496-4. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2737-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Frontiers in Psychiatry. 2011;2:1–10. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Behavior maintained by termination of a schedule of self-administered cocaine. Science. 1979;204:1231–1233. doi: 10.1126/science.109920. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, De Almeida RMM, Miczek KA. Behavioral and pharmacogenetics of aggressive behavior. Curr Topics Behav Neurosci. 2012 doi: 10.1007/7854_2011_191. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellucci SV. Primate social behavior--anxiety or depression? Pharmacol Ther. 1990;47:167–180. doi: 10.1016/0163-7258(90)90085-g. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Back SE, Brady KT, Upadhyaya HP, McRae AL, Saladin ME. Daily stressor sensitivity, abuse effects, and cocaine use in cocaine dependence. Addict Behav. 2007;32:3015–3025. doi: 10.1016/j.addbeh.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wohlfarth T, Van Den Brink W. Social class and substance use diosrders: the value of social class as distinct from socioeconomic status. Soc Sci Med. 1998;47:51–58. doi: 10.1016/s0277-9536(98)00011-2. [DOI] [PubMed] [Google Scholar]