Abstract
Objectives
Most primary central nervous system lymphomas (PCNSLs) and primary vitreoretinal lymphomas (PVRLs) are B-cell lymphomas that produce high levels of interleukin (IL)-10, which is linked to rapid disease progression. The IL-10-1082G→A polymorphism (IL-10 SNP) is associated with improved survival in certain non-CNS lymphoma patients. PDCD4 is a tumor suppressor gene and upstream regulator of IL-10. This study examined the correlation between the IL-10 SNP, PDCD4 mRNA expression, and IL-10 expression (at transcript and protein levels) in these lymphoma cells.
Materials and methods
Single-nucleotide polymorphism (SNP)-typing at IL-10-1082 was performed after micro-dissecting cytospun PVRL cells from 26 specimens. Vitreal IL-10 and IL-6 levels were measured by ELISA. PCNSL cells from 52 paraffin-embedded sections were microdissected and SNP typed on genomic DNA. RT-PCR was performed to analyze expression of IL-10 and PDCD4 mRNA. IL-10-1082 SNP typing was performed on blood samples of 96 healthy controls. We measured IL-10-1082 SNP expression in 26 PVRLs and 52 PCNSLs and examined its relationship with IL-10 protein and gene expression, respectively.
Results
More PVRL patients expressed one copy of the IL-10-1082G→A SNP with the GA genotype compared to controls. The frequencies of the three genotypes (AA, AG, GG) significantly differed in PVRL versus controls and in PCNSL versus controls. In PVRLs, the vitreal IL-10/IL-6 ratio was higher in IL-10-1082 AG and IL-10-1082 AA patients, compared to IL-10-1082 GG patients. IL-10 mRNA expression was higher in IL-10-1082 AG and IL-10-1082 AA PCNSLs, compared to IL-10-1082 GG PCNSLs. No correlation was found between IL-10 and PDCD4 expression levels in 37 PCNSL samples.
Conclusions
PVRL and PCNSL patients had similar IL-10-1082 A allele frequencies, but genotype distributions differed from healthy controls. The findings suggest that the IL-10-1082 A allele is a risk factor for higher IL-10 levels in PVRLs and PCNSLs. Higher IL-10 levels have been correlated with more aggressive disease in both PVRLs and PCNSLs, making this finding an important and potentially clinically significant observation.
Keywords: Primary vitreoretinal lymphoma, Primary CNS lymphoma, Interleukin-10, Single-nucleotide polymorphism, PDCD4 (program cell death 4)
Introduction
Primary central nervous system lymphomas (PCNSLs) are uncommon extranodal non-Hodgkin’s lymphomas (NHLs) that involve only the brain, leptomeninges, spinal cord, and eyes. PCNSL is a rare fatal disease with an incidence of 0.48 in 100,000 persons per year and a 12-month survival of 33 % [1, 2]. The incidence of PCNSL has been increasing over the last 30 years in both immunocompetent and immunodeficient populations [3, 4]. According to the World Health Organization lymphoma classification system, most PCNSLs are diffuse large B-cell lymphomas (DLBCLs) with a heterogeneous population of aggressive malignant B-cells [5, 6]. Primary vitreoretinal lymphoma (PVRL), also known as “primary intraocular lymphoma” (PIOL), is considered a subset of PCNSL that involves the retina, vitreous, and optic nerve head [7–9]. The incidence of PVRL was 0.46 in 100,000 persons per year in the United States from 2004 to 2007 [10], and the median overall survival was 22.5 months [7, 11]. The cell of origin for both PCNSL and PVRL is still unknown. However, it is considered to be a B-cell that has exited the germinal center [12, 13].
PVRLs have been shown to secrete high levels of interleukin-10 (IL-10), and high vitreous IL-10 levels and/or a high vitreous IL-10/IL-6 ratio correspond to a more severe disease course [8, 14–16]. IL-10 levels are also increased in the cerebrospinal fluid and serum of PCNSL patients compared to patients with other CNS tumors and patients who are free of disease [17]. Higher IL-10 levels correlate with radiographic progression of brain lesions in PCNSL, adverse disease features, and poorer prognosis in DLBCL [17, 18]. This is likely due to the stimulatory effect IL-10 has on B-cells [19, 20]. IL-10 also has a strong immunomodulatory effect, and its ability to inhibit inflammation may be a mechanism tumors use to defy immune surveillance and prevent their destruction [21].
Single-nucleotide polymorphisms (SNPs) in the IL-10 gene promoter have been described to contribute to risk for NHL [22–25]. A well-studied SNP in the IL-10 promoter is the IL-10-1082 G →A allele (IL-10-1082) SNP [26–30]. There is in vitro and in vivo evidence linking the IL-10-1082 SNP with IL-10 levels [27, 28, 31, 32].
Several human malignancies downregulate programmed cell death 4 (PDCD4), a pro-inflammatory tumor suppressor gene that prevents transformation and inhibits cellular proliferation. The PDCD4 protein inhibits protein synthesis by suppressing translation initiation. PDCD4 is downregulated in different tumors, including lung cancer, hepatocellular carcinoma, breast carcinoma, gliomas, and esophageal carcinoma [33, 34]. Mice deficient in PDCD4 develop spontaneous lymphomas and have a short life span [35]. It is via NF-κB that PDCD4 suppresses IL-10 translation [35, 36].
PCNSLs and PVRLs are extremely rare, and tissue is rarely obtainable for research investigation because of the current modalities of disease treatment. The prevalence of the IL-10-1082 SNP in PCNSL or PVRL has not been previously reported, and a genotype-phenotype correlation of an IL-10 SNP with IL-10 expression in PCNSL cells or vitreal IL-10 levels in PVRL has not previously been documented. The objectives of the present study, based on the availability of the specimens, were to: (a) compare the IL-10-1082 SNP in PVRL and PCNSL; (b) determine the correlation of the IL-10-1082 genotype and the vitreous IL-10/IL-6 ratio in PVRLs; (c) examine for an association between the IL-10-1082 genotype and IL-10 mRNA levels in PCNSLs; and (d) identify any correlation between PDCD4 and IL-10 mRNA levels in PCNSL.
Materials and methods
Sample collection
The study design was approved by the Institutional Review Boards (IRBs) of the National Eye Institute (NEI) and the University of Liverpool. All participants from the NEI and the University of Liverpool signed informed consent forms. All included patients were HIV negative and had no history of transplantation. The Oregon Health & Science University (OHSU) IRB determined that the research did not involve human subjects, and for this reason, consent was not required or obtained at OHSU. The research adhered to the tenets of the Declaration of Helsinki. The clinic-based cases included 27 patients diagnosed with PVRL at the NEI between 1993 and 2009. The cytology slides of the vitrectomy specimens and vitreous cytokine information from these patients were obtained. Slides from a total of 59 paraffin-embedded brain biopsies containing PCNSL were obtained from Oregon Health & Science University and the University of Liverpool. DNA samples of 98 healthy age- and gender-matched subjects were obtained from the NIH blood bank. Study group populations are summarized in Table 1.
Table 1.
Demography and clinical information
| a) PVRL patients | ||||
| Specimen origin (no.) | Age mean (SD) | Gender (♂/♀) | Vitreous IL-10 (pg/ml) mean (SD) | Vitreous IL-10/IL-6 mean (SD) |
| Eye (n=26) | 64 (14) | 12/14 | 1,666 (2,632) | 37 (82) |
| b) PCNSL patients | ||||
| Specimen origin (no.) | Age mean (SD) | Gender (♂/♀) | ||
| Brain (n=52) | 56 (15) | 34/18 | ||
| c) Healthy controls | ||||
| Specimen origin (no.) | Age mean (SD) | Gender (♂/♀) | ||
| Peripheral blood (n=96) | 44 (13) | 72/24 | ||
Vitrectomy and vitreal cytokine measurement
Vitreous specimens from 27 patients subsequently diagnosed with PVRL were obtained through a standard three-port pars plana vitrectomy [37]. The vitrectomy specimens were delivered for cytopathological analysis according to previously described methods [37]. Vitreous supernatant (100 μl per case) was submitted for IL-10 and IL-6 measurement in pg/ml by ELISA (Quantikine; R&D Systems, Abingdon, UK).
Cytology and histology
The vitreous from the 27 PVRL patients was cytocentrifuged (Wescor, Inc. Cytopro 7620, Logan, Utah) at 443 g for 8 min to concentrate cells and cytospun onto glass slides that were subsequently air dried and stained with Giemsa. Brain biopsy tissue with PCNSL was removed surgically, fixed in 10 % neutral buffered formalin, and embedded in paraffin. Neuropathology was determined on 3- or 5-μm-thick sections stained with hematoxylin and eosin (H&E). CD20 positivity was evaluated using routine immunohistochemistry on the PCNSL cases [9].
Microdissection
Using previously described methods [38], PVRL cells were microdissected from vitreous cytological slides that were stored at 4°C in a refrigerator for months or years, and PCNSL cells were microdissected from the brain pathological slides that were stored at room temperature for one or several years. Briefly, the buffered formalin-fixed paraffin sections of PCNSL were stained with H&E. Fifty-five of the 59 PCNSL cases were deparaffinized, as four PCNSL cases contained insufficient tissue for further analysis. Lymphoma cells were selected by visualization under a light microscope and collected using a 30-gauge needle as previously described [38]. The PVRL cases collected were all characterized to be B-cell lymphomas based on molecular identification of the IgH gene rearrangement as previously described [39]. For 27 PVRL cases, lymphoma cells on the Giemsa-stained cytological slides were gently lifted and collected with a 30-gauge needle under a light microscope as previously described [40].
SNP typing
Genomic DNA samples were collected from PVRL cells on the cytospun slides from 27 NEI patients and the PCNSL cells on the pathology slides from 55 cases obtained through OHSU and the University of Liverpool. Ninety-eight blood bank samples from the NIH were also obtained as controls. All samples were amplified using the illustra GenomiPhi V2 DNA Amplification Kit (Amersham Biosciences). Genotyping of rs1800896 was performed (Taqman SNP Genotyping Assays C__1747360_10; Applied Biosystems, Foster City, CA). This procedure was successful for 26 PVRL samples, 35 PCNSL cases, and 96 controls.
RNA isolation and quantitative real-time reverse transcriptase polymerase chain reaction
Total RNA from the microdissected cells was isolated using the Paradise™ Sample Quality Assessment Kit (MDS Analytical Technology, Sunnyvale, CA). Universal human RNA was purchased from BD Biosciences (Palo Alto, CA) and used for assay normalization. The cDNA synthesis was performed using SuperScript™ II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed using a Stratagene Mx3000 Real-Time PCR System and Brilliant SYBR Green QPCR Master Mix (Stratagene, CA). The primers for IL-10 and PDCD4 were synthesized by SA Biosciences (Frederick, MD) and supplied as the quantitative RT-PCR Gene Expression Analysis kit. Following PCR, a thermal melt profile was performed for amplicon identification. To determine the Ct, the threshold level of fluorescence was set manually in the early phase of PCR amplification. The 2–ΔΔCt analysis method was used to determine relative amounts of product using β-actin as an endogenous control. Due to the limited number of microdissected cells, only one common and reliable housekeeping gene, β-actin, was selected. The transcript expression was normalized by the level of β-actin from the same cDNA sample. The average relative expression due to gene manipulation was again normalized to the transcript level of the universal human RNA and presented graphically. Each sample was analyzed twice. Samples without template and samples without PCR mixtures were used as negative controls.
Statistics
Data were analyzed using GraphPad Prism v5. The χ2-test was used to detect statistical differences between the genotypic distribution and allelic frequencies of the IL-10-1082 SNP. The non-parametric Wilcoxon t test was performed to detect statistical differences between vitreous IL-10 levels, vitreous IL-10/IL-6 ratios, and IL-10 mRNA transcript expression. When three or more groups were compared, the ANOVA test was used. The spearmen correlation coefficient (r) was used to evaluate correlation of PDCD4 and IL-10 mRNA expression. Statistical significance was defined as p<0.05.
Results
Demography
The PVRL patient cases from the NEI varied in age from 32 to 81 years, with a mean age of 64 years. All patients were HIV negative and did not have a history of transplantation. The PCNSL patient cases from the Oregon Health & Science University and the University of Liverpool varied in age from 20 to 80 years, with a mean age of 56 years. Demographic information was available for all 26 PVRL patients, 52 PCNSL patients, and 96 control patients (Table 1).
Allele and genotype frequencies at IL-10-1082 in PCNSL and PVRL
Genotyping of the IL-10 promoter SNP was successful in 26 of 27 PVRL, 35 of 59 PCNSL patients, and 96 of 98 healthy subjects. There was no significant difference in allele frequencies of the IL-10-1082 A allele or the IL-10-1082 G allele in patients with PCNSL or PVRL when compared to controls (Table 2). More PVRL patients expressed one copy of the IL-10-1082G→A SNP with the GA genotype compared to controls (p=0.048), while this was not significant in PCNSL patients (p=0.063). There was no statistical difference in genotype frequencies between PCNSL and PVRL. However, there was a statistically significant difference in the frequencies of the three genotypes (AA, AG, GG) in PVRL versus controls (p=0.0001) and in PCNSL versus controls (p=0.005). Genotypic distributions were in Hardy-Weinberg equilibrium for blood bank controls, PVRLs, and PCNSLs.
Table 2.
Odds ratios and 95 % confidence intervals of primary vitreoretinal lymphoma (PVRL) and primary central nervous system lymphomas (PCNSL) versus unrelated controls at the IL-10 promoter single-nucleotide polymorphism (SNP)
| Sample | IL-10-1082 SNP | Control | Case | p value | Odds ratio (95 % confidence interval) |
|---|---|---|---|---|---|
| PVRL | GG | 27 (28.0 %) | 4 (15.4 %) | 1 | |
| GA | 38 (40.0 %) | 18 (69.2 %) | 0.0480* | 3.197 (0.972–10.52) | |
| AA | 31 (32.0 %) | 4 (15.4 %) | 0.8546 | 0.871 (0.198–3.823) | |
| GA+AA | 69 (72.0 %) | 22 (84.6 %) | 0.1856 | 2.152 (0.678–6.830) | |
| G | 92 (48.0 %) | 26 (50.0 %) | 1 | ||
| A | 100 (52.0 %) | 26 (50.0 %) | 0.7897 | 1.087 (0.589–2.007) | |
| PCNSL | GG | 27 (28.0 %) | 6 (17.1 %) | 1 | |
| GA | 38 (40.0 %) | 22 (62.9 %) | 0.0630 | 2.605 (0.931–7.290) | |
| AA | 31 (32.0 %) | 7 (20.0 %) | 0.9793 | 1.016 (0.304–3.396) | |
| GA+AA | 69 (72.0 %) | 29 (82.9 %) | 0.2001 | 1.891 (0.706–5.067) | |
| G | 92 (48.0 %) | 34 (49.0 %) | 1 | ||
| A | 100 (52.0 %) | 36 (51.0 %) | 0.9252 | 1.027 (0.594–1.775) |
PVRL IL-10-1082 A allele and higher vitreous IL-10/IL-6 ratios
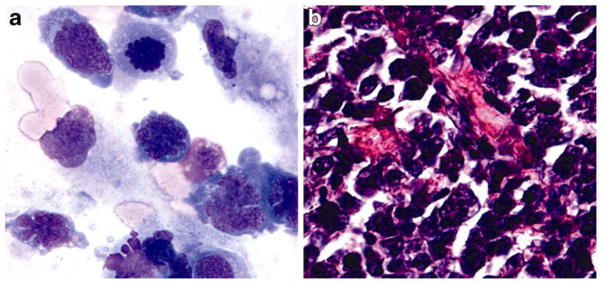
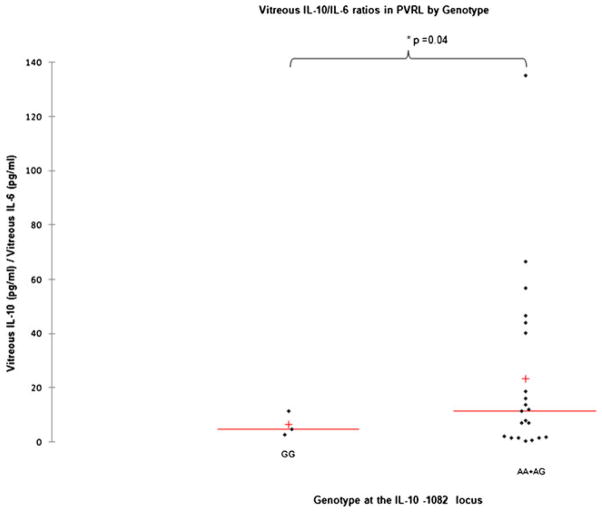
Lymphoma cells from the cytospun slides of 27 PVRL cases were microdissected. A representative image of PVRL cells on a cytospun slide is shown in Fig. 1a. SNP typing was successful in 26 cases. The vitreous IL-10/IL-6 ratio was 6.4±2.6 in patients with IL-10-1082 GG and 23.5±7.1 in patients with IL-10-1082 AG or IL-10-1082 AA (p =0.04), as shown in Fig. 2. Vitreous IL-10 levels (pg/ml) were 724±593 in IL-10-1082 GG patients and 1,794±603 in IL-10-1082 AG, and IL-10-1082 AA patients (p>0.05). PVRLs with at least one IL-10-1082 A allele have higher vitreous IL-10 levels.
Fig. 1.
Photomicrographs of primary vitreoretinal lymphoma (PVRL) cells and primary central nervous system lymphoma (PCNSL) cells. a Retinal lymphoma cells from a cytospun slide (original magnification x640, Giemsa stain). b Central nervous system lymphoma cells from a brain biopsy slide (original magnification x400, Giemsa stain)
Fig. 2.
Vitreous IL-10/IL-6 ratio compared to the IL-10-1082 genotype in primary vitreoretinal lymphoma (PVRL) patients. The mean vitreous IL-10/IL-6 ratios (line) and medians (+) are compared in PVRLs, grouped by genotype at IL-10-1082. The difference between the two groups is statistically significant
PCNSL IL-10-1082 A allele and higher IL-10 mRNA levels
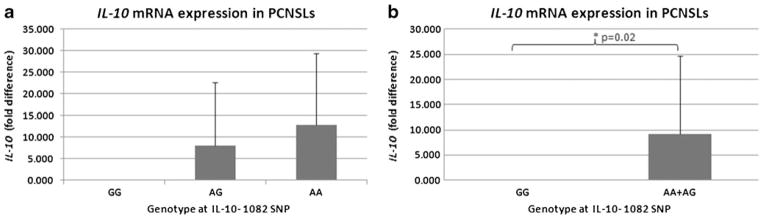
Of the 59 PCNSL cases (Fig. 1b), four cases had insufficient tissue for microdissection. Fifty-five cases were microdissected, and total RNA was extracted from microdissected samples and reverse-transcribed to cDNA. RT-PCR for IL-10 mRNA was successful for 52 cases. Due to technical difficulty of SNP genotype assay on the paraffin-embed, microdissected cells, only 35 of the 55 PCNSL cases were successfully SNP typed. The mean relative expression of the IL-10 transcript was 0.002 in PCNSL cases carrying IL-10-1082 GG and 9.2 for cases carrying IL-10-1082 AG or IL-10-1082 AA (p=0.019), as shown in Fig. 3. PCNSLs with at least one IL-10-1082 A allele have higher IL-10 mRNA levels (Fig. 3b).
Fig. 3.
IL-10 relative expression levels in primary central nervous system lymphoma (PCNSL) cells. a The mean IL-10 mRNA fold differences ± standard deviations are compared in PCNSL cells grouped by genotype at IL-10-1082. b PCNSLs with at least one IL-10 SNP allele have significantly higher IL-10 expression
Expression of PDCD4 and IL-10 in PCNSLs
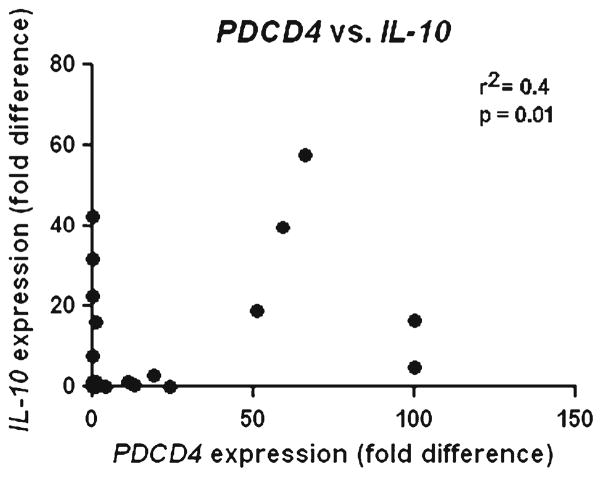
RT-PCR for IL-10 and PDCD4 mRNA was successful in 52 microdissected PCNSL cases. IL-10 and PDCD4 mRNA expression levels were compared in the same cases. When PDCD4 mRNA levels were plotted against IL-10 mRNA levels (Fig. 4), a weak positive correlation was found: r2=0.36, with 95 % confidence interval of 0.093 to 0.58 (p=0.008).
Fig. 4.
Comparison of PDCD4 and IL-10 mRNA relative expression. No correlation was detected between PDCD4 and IL-10 mRNA expression levels in primary central nervous system lymphoma (PCNSL) cells
Discussion
IL-10 gene promoter polymorphisms have been considered risk factors for NHL [22–25]. There is some evidence suggesting that the IL-10-1082 A allele is a risk allele in lymphoma patients [26, 41]. One group observed that the frequency of the IL-10-1082 AA allele was higher in patients with aggressive systemic lymphomas compared to controls [26]. In another study, the frequency of the IL-10-1082 G allele was higher in patients with DLBCL compared to controls [41]. The IL-10-1082 A allele has been shown to confer greater overall survival in patients with multiple myeloma and chronic lymphocytic leukemia [27], and the G allele has been associated with a better survival in melanoma patients [42]. Genotype-phenotype studies have measured serum IL-10 levels and IL-10-1082 SNP expression in vitro after endotoxin stimulation in autoimmune disease and Hodgkin’s lymphoma [27–29, 31, 32, 43]. To our knowledge, this is the first study providing a genotype-phenotype correlation for an IL-10 SNP in PCNSL or PVRL.
In this study, we measured the expression of the IL-10-1082 SNP, IL-10, and PDCD4 in the same PCNSL cases, and therefore were able to make a potential genotype-phenotype correlation. In the PVRL cases, we correlated IL-10-1082 genotype and vitreous IL-10 levels, which are considered to be a relatively sensitive and specific biomarker of lymphoma cell IL-10 production [14, 44, 45]. Recently, elevation of IL-10 levels in the vitreous has been repeatedly associated with poorer prognosis [16, 46, 47]. A direct relationship cannot be predicted between vitreous IL-10 levels and CSF IL-10 levels, as PVRL cells secrete IL-10 directly into the vitreous, while PCNSL cells may secrete IL-10 remotely from CSF. However, this is a potential area of investigation in future prospective clinical trials such as the International Extranodal Lymphoma Study Group (IELSG) trail in Europe.
This study demonstrated a similar IL-10-1082 genotype distribution in PVRLs and PCSNLs, which differed significantly from that observed in healthy controls. Compared to controls, more PVRLs were found to have one copy of the IL-10-1082 SNP. However, allelic frequency of the IL-10-1082 A and IL-10-1082 G alleles was similar in PVRLs, PCNSLs, and controls, supporting evidence of the genetic resemblance of these to non-Hodgkin’s lymphomas [48]. This finding differs from a previous study that demonstrates an increased prevalence of the IL-10-1082 G allele in DLBCL patients. In our genotype-phenotype study, we noted that PVRLs with at least one copy of the IL-10-1082 A allele had a significantly higher vitreous IL-10/IL-6 ratio than PVRLs that were IL-10-1082 GG. This finding was replicated for PCNSLs, in which we found a significant association between the IL-10-1082 A allele and higher IL-10 mRNA levels. A dose effect of the IL-10-1082 A allele seems to be present in both PVRL and PCNSL. Elevated IL-10 levels have been associated with more aggressive disease in PVRL, PCNSL, and NHL [16, 17, 49–51]. It is possible that the presence of the IL-10-1082 SNP might predict a worse prognosis for PVRL and PCNSL patients; however, mortality data is necessary to confirm this hypothesis.
DLBCLs may have low PDCD4 levels and higher IL-10 levels. In turn, PDCD4 expression has not been previously reported in PCNSL or PVRL [35, 36, 52]. The tumor suppressor PDCD4 is a pro-inflammatory protein that promotes activation of the transcription factor NF-κB and suppresses interleukin-10 (IL-10). Given that PDCD4 suppresses IL-10, we expected a negative correlation between PDCD4 and IL-10 transcript levels. However, no such relationship was found in 52 PCSNLs. Other factors such as conserved non-coding sequences 9 (CNS-9) may regulate IL-10 in PCNSL [53, 54].
Despite the rarity of these diseases, a large number of PCNSL (n=52) and PVRL (n=26) cases were analyzed. The IL-10-1082 A allele may not be a direct risk allele for PVRL or PCNSL. However, our study has shown that PVRL patients are more likely to have the IL-10-1082 SNP than controls and that PVRL and PCNSL patients have an IL-10-1082 genotype distribution that differs from controls. The IL-10-1082 A allele is significantly associated with higher IL-10 mRNA levels in PCNSL and a higher vitreous IL-10/IL-6 ratio in PVRL.
Acknowledgments
We would like to thank the National Eye Institute Intramural Research Program, Howard Hughes Medical Institute, Research to Prevent Blindness’s unrestricted grant to Casey Eye Institute, the Schnitzer-Novack Foundation, and the Eye Tumour Research Fund for financial support. We thank Dr. Nussenblatt and the NEI clinical fellows for the contribution of their patients’ vitreous specimen to this study. We also appreciate the physicians from other institutions who provided vitreous specimens.
Footnotes
Commercial or financial interests None.
Conflict of Interest The authors declare that they have no conflicts of interest.
Contributor Information
Hema L. Ramkumar, Immunopathology Section, Laboratory of Immunology National Institutes of Health, 10 Center Drive, 10/10 N103, NIH/NEI, Bethesda, MD 20892-1857, USA. Howard Hughes Medical Institute, Chevy Chase, MD, USA
De Fen Shen, Immunopathology Section, Laboratory of Immunology National Institutes of Health, 10 Center Drive, 10/10 N103, NIH/NEI, Bethesda, MD 20892-1857, USA.
Jingsheng Tuo, Immunopathology Section, Laboratory of Immunology National Institutes of Health, 10 Center Drive, 10/10 N103, NIH/NEI, Bethesda, MD 20892-1857, USA.
Rita M. Braziel, Department of Surgical Pathology, Oregon Health & Science University, Portland, OR, USA
Sarah E. Coupland, Department of Cellular & Molecular Pathology, University of Liverpool, Liverpool, UK
Justine R. Smith, Casey Eye Institute & Department of Cell & Developmental Biology, Oregon Health & Science University, Portland, OR, USA
Chi-Chao Chan, Email: chanc@nei.nih.gov, Immunopathology Section, Laboratory of Immunology National Institutes of Health, 10 Center Drive, 10/10 N103, NIH/NEI, Bethesda, MD 20892-1857, USA.
References
- 1.O’Neill BP. Lymphoma of the nervous system. Butterworth-Heinemann; Boston: 2004. Epidemiology of primary CNS lymphoma; pp. 1–207. [Google Scholar]
- 2.Pulido JS, Vierkant RA, Olson JE, Abrey L, Schiff D, O’Neill BP. Racial differences in primary central nervous system lymphoma incidence and survival rates. Neuro Oncol. 2009;11:318–322. doi: 10.1215/15228517-2008-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson JE, Janney CA, Rao RD, Cerhan JR, Kurtin PJ, Schiff D, Kaplan RS, O’Neill BP. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- 4.Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Schmitt D. Advances in experimental medicine and biology. Vol. 2 Plenum Press; New York: 1995. Dendritic cells in fundamental and clinical immunology; pp. 1–572. [Google Scholar]
- 6.Kleihues PC, WK . World Health Organization classification of tumors. Lyon; France: 2000. Pathology and genetics of tumours of the nervous system; pp. 1–314. [Google Scholar]
- 7.Chan CC, Gonzales JA. Primary intraocular lymphoma. World Scientific Publishing Co Pte Ltd; Hackensack, NJ: 2007. pp. 1–200. [Google Scholar]
- 8.Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR, Batchelor TT, Pulido JS. Primary vitreoretinal lymphoma: a report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist. 2011;16:1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coupland SE, Damato B. Understanding intraocular lymphoma. Clin Experiment Ophthalmol. 2008;36:564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 10.Fast stats: Statistics stratified by cancer site. National Cancer Institute. Surveillance, Epidemiology, and End Results; [Accessed May 8, 2011]. [Google Scholar]
- 11.Faia LJ, Chan CC. Primary intraocular lymphoma. Arch Pathol Lab Med. 2009;133:1228–1232. doi: 10.5858/133.8.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 13.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, Powell J, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Campo E, Jaffe ES, Delabie J, Smeland EB, Rimsza LM, Fisher RI, Weisenburger DD, Chan WC, Staudt LM. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassoux N, Giron A, Bodaghi B, Tran TH, Baudet S, Davy F, Chan CC, Lehoang P, Merle-Beral H. Il-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120:671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 16.Sen HN, Chan CC, Byrnes G, Fariss RN, Nussenblatt RB, Buggage RR. Intravitreal methotrexate resistance in a patient with primary intraocular lymphoma. Ocul Immunol Inflamm. 2008;16:29–33. doi: 10.1080/09273940801899764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmaggi A, Eoli M, Corsini E, Gelati M, Frigerio S, Silvani A, Boiardi A. Cerebrospinal fluid interleukin-10 levels in primary central nervous system lymphoma: a possible marker of response to treatment? Ann Neurol. 2000;47:137–138. [PubMed] [Google Scholar]
- 18.Lech-Maranda E, Bienvenu J, Michallet AS, Houot R, Robak T, Coiffier B, Salles G. Elevated IL-10 plasma levels correlate with poor prognosis in diffuse large B-cell lymphoma. Eur Cytokine Netw. 2006;17:60–66. [PubMed] [Google Scholar]
- 19.Banchereau J, Briere F, Liu YJ, Rousset F. Molecular control of B lymphocyte growth and differentiation. Stem Cells. 1994;12:278–288. doi: 10.1002/stem.5530120304. [DOI] [PubMed] [Google Scholar]
- 20.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin-10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099–2105. [PubMed] [Google Scholar]
- 22.Lan Q, Zheng T, Rothman N, Zhang Y, Wang SS, Shen M, Berndt SI, Zahm SH, Holford TR, Leaderer B, Yeager M, Welch R, Boyle P, Zhang B, Zou K, Zhu Y, Chanock S. Cytokine polymorphisms in the Th1/Th2 pathway and susceptibility to non-Hodgkin lymphoma. Blood. 2006;107:4101–4108. doi: 10.1182/blood-2005-10-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lech-Maranda E, Baseggio L, Bienvenu J, Charlot C, Berger F, Rigal D, Warzocha K, Coiffier B, Salles G. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood. 2004;103:3529–3534. doi: 10.1182/blood-2003-06-1850. [DOI] [PubMed] [Google Scholar]
- 24.Purdue MP, Lan Q, Kricker A, Grulich AE, Vajdic CM, Turner J, Whitby D, Chanock S, Rothman N, Armstrong BK. Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis. 2007;28:704–712. doi: 10.1093/carcin/bgl200. [DOI] [PubMed] [Google Scholar]
- 25.Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, De Sanjose S, Cocco P, Berndt SI, Brennan P, Brooks-Wilson A, Wacholder S, Becker N, Hartge P, Zheng T, Roman E, Holly EA, Boffetta P, Armstrong B, Cozen W, Linet M, Bosch FX, Ennas MG, Holford TR, Gallagher RP, Rollinson S, Bracci PM, Cerhan JR, Whitby D, Moore PS, Leaderer B, Lai A, Spink C, Davis S, Bosch R, Scarpa A, Zhang Y, Severson RK, Yeager M, Chanock S, Nieters A. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol. 2006;7:27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham LM, Chapman C, Dunstan R, Bell MC, Joske DJ. Polymorphisms in the interleukin 10 gene promoter are associated with susceptibility to aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44:251–255. doi: 10.1080/1042819021000035590. [DOI] [PubMed] [Google Scholar]
- 27.Domingo-Domenech E, Benavente Y, Gonzalez-Barca E, Montalban C, Guma J, Bosch R, Wang SS, Lan Q, Whitby D, Fernandez de Sevilla A, Rothman N, de Sanjose S. Impact of interleukin-10 polymorphisms (-1082 and -3575) on the survival of patients with lymphoid neoplasms. Haematologica. 2007;92:1475–1481. doi: 10.3324/haematol.11350. [DOI] [PubMed] [Google Scholar]
- 28.Hohaus S, Giachelia M, Massini G, Vannata B, Criscuolo M, Martini M, D’Alo F, Voso MT, Larocca LM, Leone G. Clinical significance of interleukin-10 gene polymorphisms and plasma levels in Hodgkin lymphoma. Leuk Res. 2009;33:1352–1356. doi: 10.1016/j.leukres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Hohler T. Differential regulation of interleukin-10 production by genetic and environmental factors–a twin study. Genes Immun. 2002;3:407–413. doi: 10.1038/sj.gene.6363920. [DOI] [PubMed] [Google Scholar]
- 30.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 31.Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, Kimberly RP. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166:3915–3922. doi: 10.4049/jimmunol.166.6.3915. [DOI] [PubMed] [Google Scholar]
- 32.Kurreeman FA, Schonkeren JJ, Heijmans BT, Toes RE, Huizinga TW. Transcription of the IL10 gene reveals allele-specific regulation at the mRNA level. Hum Mol Genet. 2004;13:1755–1762. doi: 10.1093/hmg/ddh187. [DOI] [PubMed] [Google Scholar]
- 33.Jansen AP, Camalier CE, Stark C, Colburn NH. Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther. 2004;3:103–110. [PubMed] [Google Scholar]
- 34.Lankat-Buttgereit B, Goke R. Programmed cell death protein 4 (pdcd4): A novel target for antineoplastic therapy? Biol Cell. 2003;95:515–519. doi: 10.1016/j.biolcel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W, Goke R, Chen YH. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol. 2006;177:8095–8102. doi: 10.4049/jimmunol.177.11.8095. [DOI] [PubMed] [Google Scholar]
- 36.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 37.Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen DF, Zhuang Z, LeHoang P, Boni R, Zheng S, Nussenblatt RB, Chan CC. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 39.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 40.Levy-Clarke GA, Byrnes GA, Buggage RR, Shen DF, Filie AC, Caruso RC, Nussenblatt RB, Chan CC. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a sub-retinal lesion. Retina. 2001;21:281–284. doi: 10.1097/00006982-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Lech-Maranda E, Baseggio L, Charlot C, Rigal D, Berger F, Jamroziak K, Warzocha K, Coiffier B, Salles G. Genetic polymorphisms in the proximal IL-10 promoter and susceptibility to non-Hodgkin lymphoma. Leuk Lymphoma. 2007;48:2235–2238. doi: 10.1080/10428190701615926. [DOI] [PubMed] [Google Scholar]
- 42.Vuoristo MS. The polymorphisms of interleukin-10 gene influence the prognosis of patients with advanced melanoma. Cancer Genet Cytogenet. 2007;176:54–57. doi: 10.1016/j.cancergencyto.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Eskdale J, McNicholl J, Wordsworth P, Jonas B, Huizinga T, Field M, Gallagher G. Interleukin-10 microsatellite polymorphisms and IL-10 locus alleles in rheumatoid arthritis susceptibility. Lancet. 1998;352:1282–1283. doi: 10.1016/S0140-6736(05)70489-X. [DOI] [PubMed] [Google Scholar]
- 44.Coupland SE, Chan CC, Smith J. Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm. 2009;17:227–237. doi: 10.1080/09273940903168696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Shen D, Wang VM, Sen HN, Chan CC. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci. 2011;12:5684–5697. doi: 10.3390/ijms12095684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamura H, Yasuda N, Kakinoki M, Sawada T, Sawada O, Ohji M. Interleukin-10 and interleukin-6 in aqueous humor during treatment of vitreoretinal lymphoma with intravitreally injected methotrexate. Ophthalmic Res. 2009;42:172–174. doi: 10.1159/000230879. [DOI] [PubMed] [Google Scholar]
- 47.Sou R, Ohguro N, Maeda T, Saishin Y, Tano Y. Treatment of primary intraocular lymphoma with intravitreal methotrexate. Jpn J Ophthalmol. 2008;52:167–174. doi: 10.1007/s10384-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 48.Coupland SE, Hummel M, Stein H, Willerding G, Jahnke K, Stoltenburg-Didinger G. Demonstration of identical clonal derivation in a case of “oculocerebral” lymphoma. Br J Ophthalmol. 2005;89:238–239. doi: 10.1136/bjo.2004.047001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, Banchereau J, Favrot MC. Serum interleukin-10 in non-Hodgkin’s lymphoma: a prognostic factor. Blood. 1993;82:2169–2174. [PubMed] [Google Scholar]
- 50.Rajagopal R, Harbour JW. Diagnostic testing and treatment choices in primary vitreoretinal lymphoma. Retina. 2011;31:435–440. doi: 10.1097/IAE.0b013e31820a6743. [DOI] [PubMed] [Google Scholar]
- 51.Whitcup SM, Stark-Vancs V, Wittes RE, Solomon D, Podgor MJ, Nussenblatt RB, Chan CC. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 52.Allgayer H. Pdcd4, a colon cancer prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol. 2010;73:185–191. doi: 10.1016/j.critrevonc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Lee CG, Kang KH, So JS, Kwon HK, Son JS, Song MK, Sahoo A, Yi HJ, Hwang KC, Matsuyama T, Yui K, Im SH. A distal cis-regulatory element, CNS-9, controls NFAT1 and IRF4-mediated IL-10 gene activation in T helper cells. Mol Immunol. 2009;46:613–621. doi: 10.1016/j.molimm.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Liopeta K, Boubali S, Virgilio L, Thyphronitis G, Mavrothalassitis G, Dimitracopoulos G, Paliogianni F. cAMP regulates IL-10 production by normal human T lymphocytes at multiple levels: a potential role for MEF2. Mol Immunol. 2009;46:345–354. doi: 10.1016/j.molimm.2008.10.025. [DOI] [PubMed] [Google Scholar]