Abstract
COUP-TFII is an orphan nuclear receptor that acts as a transcriptional activator or repressor in a cell type-dependent manner. Best characterized for its role in the regulation of angiogenesis during mouse development, COUP-TFII also plays important roles in glucose metabolism and cancer. Expression of COUP-TFII is altered in various endocrine conditions. Cell type-specific functions and the regulation of COUP-TFII expression result in its varying physiological and pathological actions in diverse systems. Evidence will be reviewed for oncogenic and tumor suppressive functions of COUP-TFII, with roles in angiogenesis, metastasis, steroidogenesis, and endocrine sensitivity of breast cancer described. The applicability of current data to our understanding of the role of COUP-TFII in cancer will be discussed.
Keywords: COUP-TFII, cancer, nuclear receptor
Introduction
Steroid hormones and nuclear receptor ligands play critical roles in cancer initiation and progression and their antagonists have proven efficacy in the treatment and prevention of cancers. This is most notable in breast and prostate cancers and the use of all-trans retinoic acid for acute promyelocytic leukemia (Risbridger, et al. 2010; Siddikuzzaman, et al. 2011). Steroid/nuclear receptors act as ligand-activated transcription factors to either positively or negatively regulate gene expression (Ahmad and Kumar 2011; Stanisic, et al. 2010). Activation of nuclear receptors occurs through binding a variety of ligands including hormones and vitamins/retinoids. Nuclear receptors (NR) have physiological roles to modulate gene expression during development and growth. As alteration of basal gene expression leads to many pathogenic outcomes - including cancer, maintenance of normal gene expression by nuclear receptors is vital. One such critical nuclear receptor is chicken ovalbumin upstream promoter transcription factor II (COUP-TFII). From the time of the identification of the COUP-TF family in 1986 (Sagami, et al. 1986), the many functions of COUP-TFs have continued to be explored. The role of COUP-TFII in cancer is widely debated with evidence linking COUP-TFII to both tumor suppressive and oncogenic functions. This review will explore both the regulation and function of COUP-TFII and its connections to cancer.
COUP-TFI and COUP-TFII
The COUP-TF family consists of two highly homologous subtypes, COUP-TFI and COUP-TFII, located on human chromosomes 5 and 15, respectively (Figure 1). COUP-TFs have been previously reviewed (Lin, et al. 2011; Tsai and Tsai 1997), but not in the specific context of separating COUP-TFI and COUP-TFII in cancer. COUP-TFs are ancient NRs and are located close to retinoid X receptors (RXRs) in the evolutionary tree (Thornton 2001; Thornton, et al. 2003). As evolutionarily conserved transcription factors, COUP-TFs have major roles in development. The importance of COUP-TFII expression is evidenced by studies in knockout mice (Pereira, et al. 1999). Homozygous mutation of COUP-TFII leads to embryonic lethality due to impaired angiogenesis and heart defects, resulting in hemorrhage and edema. These effects may in part be explained by the reduction in angiopoietin-1 expression in COUP-TFII- null mice (Pereira et al. 1999). Other important embryonic roles for COUP-TFII include regulation of limb growth and muscle development (Lee, et al. 2004). COUP-TFII-null mice display a reduction in expression of Lbx1, a protein required for proper muscle precursor cell migration, and in myogenin, which is necessary for muscle cell differentiation (Lee et al. 2004; Vasyutina and Birchmeier 2006).
Figure 1. Comparison of COUP-TFI and COUP-TFII protein homology.
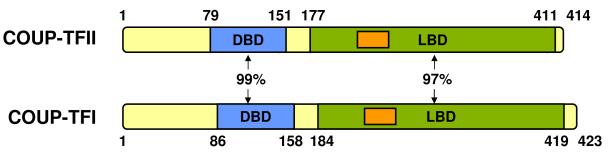
COUP-TFI (NP_005645.1) and COUP-TFII (NP_066285.1) amino acid sequences were obtained from the National Center for Biotechnology Information. The COUP-TF DNA binding domains (DBD) and ligand binding domains (LBD) share 99% and 97% amino acid homology, respectively. Positions noted as important in coactivator recognition are shown in orange.
Based on the high sequence identity in their DNA binding domains DBDs (Figure 1), we anticipate that COUP-TFI and COUP-TFII regulate the same genes. However, this has not been empirically tested and it is worth noting that the N-terminus is divergent (Figure 1) and immunoprecipitation studies indicate differences in proteins interacting with COUP-TFI (Zhang, et al. 2009) and COUP-TFII (Litchfield, et al. 2012), although, again, this has not been systematically studied in cells in which both are expressed. COUP-TFI and COUP-TFII may have divergent functions in certain contexts as well. Differences in COUP-TFI and COUP-TFII function in breast cancer endocrine sensitivity, for example, have also been identified (Riggs, et al. 2006). This review will focus specifically on COUP-TFII.
COUP-TFII regulation of gene expression
Mechanisms of regulation
COUP-TFII can activate or repress gene expression in both a tissue-specific and gene- specific manner through mechanisms involving direct binding to DNA response elements or binding to other transcription factors. Through binding to 5′-AGGTCA-3′ direct repeats (DR) with variable spacing (Kliewer, et al. 1992), COUP-TFII modulates the expression of target genes. Specific genes upon which COUP-TFII activates transcription include retinoic acid receptor β2 (RARβ2, RARB2) (Lin, et al. 2000; Litchfield et al. 2012), phosphoenolpyruvate carboxykinase (PEPCK, PCK1) (De Martino, et al. 2004b), NGFI-A (Kruse, et al. 2008; Pipaon, et al. 1999), and cholesterol 7a-hydroxylase (CYP7A1) (Stroup and Chiang 2000). COUP-TFII action may be potentiated by interaction with coactivators such as steroid receptor coactivator family members SRC-1/NCOA1, SRC-2/NCOA2, and SRC-3/NCOA3 (Kruse et al. 2008; Pipaon et al. 1999), as well as PGC1α (Kruse et al. 2008), p300/CBP (Pipaon et al. 1999), orphan receptor coactivator (ORCA) (Marcus, et al. 1996), and nucleolin (Litchfield et al. 2012). DNA binding of COUP-TFII can promote the binding of a second transcription factor, further activating gene transcription. This occurs for both the PEPCK and CYP7A1 genes, where COUP-TFII binding to the promoter recruits binding of glucocorticoid receptor (GR) to enhance gene expression (De Martino, et al. 2004a; De Martino et al. 2004b). COUP-TFII can also bind to Sp1 sites to cooperatively activate gene expression, as was reported for regulation of Otx2 expression during morphogenesis in the mouse eye (Tang, et al. 2010).
Alternatively, binding of COUP-TFII to DRs may result in repression of gene expression. In the mechanism of “active repression,” COUP-TFII binding results in recruitment of corepressors, i.e., nuclear corepressor (NCoR) (Bailey, et al. 1997) and silencing mediator of retinoid and thyroid receptors (SMRT) (Okamura, et al. 2009a; Shibata, et al. 1997), resulting in repressed chromatin structure and a corresponding blockade of target gene transcriptional activation. COUP-TFII interaction with SMRT represses PPARγ1 and PPARγ2 expression to suppress adipogenesis (Okamura et al. 2009a). Repression of the human oxytocin promoter by COUP-TFII binding has also been reported (Chu and Zingg 1997). COUP-TFII represses Pax2 expression in the retina via binding to a DR1 site (TGTTCACAGTCCA) (Tang et al. 2010).
Through an alternative mechanism of transrepression, COUP-TFII can interact with other nuclear receptors and transcription factors to inhibit their normal transcriptional activity. Examples of this include inhibition of ER- and GR-induced gene expression in a gene-specific manner (De Martino et al. 2004b; Klinge, et al. 1997). COUP-TFII can also repress AP-1 signaling through interaction with c-Jun (Lin, et al. 2002). Interaction of COUP-TFII with Runx2 inhibits osteoblast differentiation via blocking Runx2 binding to the osteocalcin promoter (Lee, et al. 2012). Other mechanisms of repression involve the modulation of ER, RXR, PPAR, and VDR activity by competing for DNA response element-binding or heterodimerization with the class II heterodimeric partner RXR (Cooney, et al. 1993).
Ingenuity Pathway Analysis
As summarized here, COUP-TFII regulates the expression of diverse gene targets. Table 1 contains a list of known COUP-TFII targets as identified using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, www.ingenuity.com). These targets are also displayed in Figure 2. COUP-TFII has varying effects on expression of other nuclear receptors and transcription factors. COUP-TFII increased the expression of HNF-1α (Ktistaki and Talianidis 1997), HNF- 1β (Power and Cereghini 1996), HNF-4α (Perilhou, et al. 2008b), and RARβ (Lin et al. 2000; Litchfield et al. 2012; Wu, et al. 1997), while it decreased the expression of Oct4 (Ben-Shushan, et al. 1995; Rosa and Brivanlou 2011), Dax1 (Yu, et al. 1998), and PPARα (Pineda Torra, et al. 2002). As previously described, COUP-TFII has well known functions in repressing the transcriptional activity of other nuclear receptors and transcription factors. Although COUP- TFII increases HNF-4 expression, other reports highlight the repression of HNF-4 function by COUP-TFII. Specifically, COUP-TFII decreases transcriptional activation of ALDH2 (You, et al. 2002) and retinol binding protein 2 (RBP2) (Nakshatri and Chambon 1994) by HNF-4. The HNF-4 activation of hepatic lipase is suppressed by COUP-TFII (Rufibach, et al. 2006), while lipoprotein lipase expression is induced by COUP-TFII synergistically with PPARγ (Robinson, et al. 1999); part of the many of reported functions of COUP-TFII in the cholesterol processing pathway. A similar response occurs for apolipoproteins A-I, A-IV, and C-III, where COUP-TFII represses the RXRα-mediated expression of APOA-I (Jiang, et al. 1995; Power and Cereghini 1996; Widom, et al. 1992) and HNF4-mediated expression of APOA-IV (Ochoa, et al. 1993; Sauvaget, et al. 2002) and APOC-III (Ktistaki and Talianidis 1997; Lavrentiadou, et al. 1999; Mietus-Snyder, et al. 1992; Power and Cereghini 1996). HNF-4 and COUP-TFII binding to the sex hormone binding globulin (Shbg) promoter was reported in murine Sertoli cells (Selva, et al. 2005). SHBG expression is increased by HNF-4 and suppressed by COUP-TF in HepG2 hepatoblastoma cells (Janne and Hammond 1998). Decreased SHBG expression is indicative of metabolic syndrome and may result in increased plasma androgen and estrogen levels, though the precise connection of COUP-TFII to these phenotypes has not been investigated (Hammond 2011).
Table 1.
List of COUP-TFII-regulated genes identified by Ingenuity Pathway Analysis
| Gene (protein) |
Name | Location | Family | Regulation by COUP-TFII |
Reference |
|---|---|---|---|---|---|
| ALDH2 | Aldehyde dehydrogenase 2 family (mitochondrial) |
Cytoplasm | Enzyme | Decrease | (You et al. 2002) |
| ANGPT1 | Angiopoietin 1 | Extracellular Space |
Growth factor | Increase | (Pereira et al. 1999) |
| APOA1 | Apolipoprotein A-I | Extracellular Space |
Transporter | Decrease | (Jiang et al. 1995; Power and Cereghini 1996; Widom et al. 1992) |
| APOA4 | Apolipoprotein A-IV | Extracellular Space |
Transporter | Decrease | (Ochoa et al. 1993; Sauvaget et al. 2002) |
| APOC3 | Apolipoprotein C-III | Extracellular Space |
Transporter | Decrease | (Ktistaki and Talianidis 1997; Lavrentiadou et al. 1999; Mietus-Snyder et al. 1992; Power and Cereghini 1996) |
| CYP11B2 | Aldosterone synthase, cytochrome P450, family 11, subfamily B, polypeptide 2 |
Cytoplasm | Enzyme | Increase | (Kurihara et al. 2005; Shibata et al. 2004) |
| CYP7A1 | Cholesterol 7 alpha- hydroxylase, cytochrome P450, family 7, subfamily A, polypeptide 1 |
Cytoplasm | Enzyme | Increase | (Stroup and Chiang 2000) |
| GATA6 | GATA binding protein 6 | Nucleus | Transcription regulator |
Decrease suggested |
(Kyrmizi et al. 2006) |
| HBE1 | Hemoglobin, epsilon 1 | Cytoplasm | Transporter | Decrease | (Liberati et al. 2001; Tanimoto, et al. 2000) |
| HNF1A | HNF1 homeobox A | Nucleus | Transcription regulator |
Increase | (Ktistaki and Talianidis 1997; Kyrmizi et al. 2006) |
| HNF1B | HNF1 homeobox B | Nucleus | Transcription regulator |
Increase | (Power and Cereghini 1996) |
| HNF4A | Hepatocyte nuclear factor 4, alpha |
Nucleus | Transcription regulator |
Increase | (Perilhou et al. 2008b) |
| KDR | VEGFR-2; kinase insert domain receptor (a type III receptor tyrosine kinase) |
Plasma Membrane |
Kinase | Decrease | (Kang et al. 2010) |
| LIPC | Lipase, hepatic | Extracellular Space |
Enzyme | Decrease | (Rufibach et al. 2006) |
| LPL | Lipoprotein lipase | Cytoplasm | Enzyme | Increase | (Robinson et al. 1999) |
| LTF | Lactotransferrin | Extracellular Space |
Peptidase | Decrease | (Lee et al. 1995) |
| NPPA | Natriuretic peptide A | Extracellular Space |
Other | Increase | (Huggins et al. 2001) |
| NR0B1 | Dax1, nuclear receptor subfamily 0, group B, member 1 |
Nucleus | Ligand- dependent nuclear receptor |
Decrease | (Yu et al. 1998) |
| NR1H4 | FXR, nuclear receptor subfamily 1, group H, member 4 |
Nucleus | Ligand- dependent nuclear receptor |
Unknown | (Kyrmizi et al. 2006) |
| NR1I2 | PXR, nuclear receptor subfamily 1, group I, member 2 |
Nucleus | Ligand- dependent nuclear receptor |
Unknown | (Kyrmizi et al. 2006) |
| NR5A2 | LRH-1, nuclear receptor subfamily 5, group A, member 2 |
Nucleus | Ligand- dependent nuclear receptor |
Unknown | (Kyrmizi et al. 2006) |
| NRP1 | Neuropilin 1 | Plasma Membrane |
Transmembrane receptor |
Decrease | (Kang et al. 2010) |
| PCK1 | Phosphoenolpyruvate carboxykinase 1 (soluble) |
Cytoplasm | Kinase | Decrease | (Eubank et al. 2001) |
| POU5F1 | Oct 4; POU class 5 homeobox 1 |
Nucleus | Transcription regulator |
Decrease | (Ben-Shushan et al. 1995; Rosa and Brivanlou 2011) |
| PPARA | Peroxisome proliferator- activated receptor alpha |
Nucleus | Ligand- dependent nuclear receptor |
Decrease | (Pineda Torra et al. 2002) |
| RARB | Retinoic acid receptor, beta | Nucleus | Ligand- dependent nuclear receptor |
Increase | (Lin et al. 2000; Litchfield et al. 2012; Nakshatri and Chambon 1994; Wu et al. 1997) |
| RBP2 | Retinol binding protein 2, cellular |
Cytoplasm | Transporter | Decrease | (Nakshatri and Chambon 1994) |
| SHBG | Sex hormone-binding globulin |
Extracellular Space |
Other | Decrease | (Janne and Hammond 1998; Selva et al. 2005) |
| SLC9A1 | Solute carrier family 9, subfamily A (NHE1, cation proton antiporter 1), member 1 |
Plasma Membrane |
Ion channel | Increase | (Li et al. 2002) |
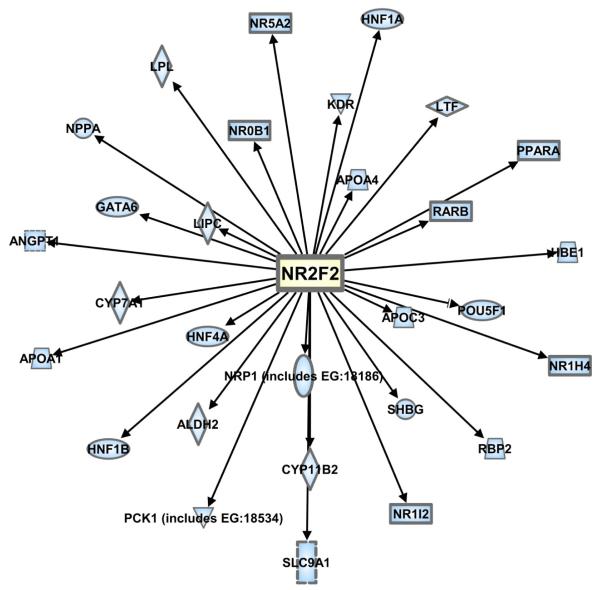
Figure 2. COUP-TFII target genes.
COUP-TFII has been reported to modulate the expression of a variety of target genes both positively and negatively. A list of COUP-TFII target genes and corresponding network pathway were generated using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, www.ingenuity.com).
Although COUP-TFII is classically known for its role in transrepression, COUP-TFII may also enhance the effect of a second nuclear receptor. Induction of cytochrome P450 family members cholesterol 7 -hydroxylase CYP7A1 (Stroup and Chiang 2000) and aldosterone synthase CYP11B2 (Kurihara, et al. 2005; Shibata, et al. 2004) by COUP-TFII was reported, with COUP-TFII and HNF-4 acting to synergistically activate CYP7A1 (Stroup and Chiang 2000). CYP7A1 catalyzes the first step in the conversion of cholesterol to bile acid (Stroup and Chiang 2000), while CYP11B2 catalyzes the final steps of aldosterone synthesis (Kurihara et al. 2005), implying that COUP-TFII transcriptional activation would increase the production of bile acid and aldosterone.
As shown in Table 1 and Figure 2, COUP-TFII opposes PPARγ/RXR activation of PEPCK transcription in predadipocytes/fibroblasts, a result that was proposed to suppress adipogenesis (Eubank, et al. 2001). COUP-TF also inhibited 9-cis retinoic acid/RXR-induced activation of the lactotransferrin promoter in transiently transfected ZR-75-1 and Hs578T breast cancer cells apparently by competing for DNA binding to a composite RARE/ERE in the gene promoter (Lee, et al. 1995). Concurrent binding of COUP-TFII and NF-Y to the hemoglobin epsilon promoter leads to a repression of gene expression (Liberati, et al. 2001). In addition to the targets identified by IPA, COUP-TF was reported to play a dual regulatory role in the transcriptional regulation of the mitochondrial HMG-CoA synthase gene: alone COUP-TFI stimulated reporter gene activity from the HMG-CoA synthase promoter in transiently transfected HepG2 human hepatoma and rat Leydig tumor R2C cells, but it inhibited PPARα- stimulated transcriptional activity by competing for the same DNA binding site (Rodriguez, et al. 1997).
Some of the IPA-identified COUP-TFII target gene relationships and mechanisms remain to be fully elucidated. In a study of the transcriptional regulation of murine hepatic development, COUP-TFII occupancy of GATA-6, FXR, PXR, and LRH-1 promoters, as determined by chromatin immunoprecipitation (ChIP) assay, was reported during the postnatal period (Kyrmizi, et al. 2006). While an inhibitory relationship was suggested for the effect of COUP-TFII on GATA-6, the effect on FXR, PXR, and LRH-1 expression is not yet known (Kyrmizi et al. 2006). Several other target genes have been identified that highlight the critical function of COUP-TFII in the vascular system. These include an increase in angiopoietin 1 (Pereira et al. 1999) and natriuretic peptide A (Huggins, et al. 2001) by COUP-TFII, and a decrease in VEGFR-2 and neuropilin 1 (Kang, et al. 2010). COUP-TFII enhances expression of the NHE1 solute exchanger (Fernandez-Rachubinski and Fliegel 2001; Li, et al. 2002).
In summary, as indicated by the IPA (Figure 2) and consistent with previous reports, COUP-TFII plays a role in many downstream pathways and may either activate or suppress gene expression.
Role in the retinoic acid pathway
COUP-TFs are classified as orphan members of the NR superfamily, because their endogenous ligand(s) is not known. However, Kruse et al demonstrated in silico binding of all- trans (atRA) and 9-cis (9cRA) retinoic acid to the crystal structure of the COUP-TFII LBD (Kruse et al. 2008). RA released the COUP-TFII LBD from the autorepressed conformation. While the investigators did not directly test binding of all-trans or 9-cis RA to COUP-TFII, they demonstrated that treatment with atRA or 9cRA increased COUP-TFII interaction with the coactivator SRC-3, with an EC50 of 10-30 μM. In agreement with this data, addition of 20 μM atRA or 9cRA led to COUP-TFII’s activation of a NGFI-A-luciferase reporter (Kruse et al. 2008). Although these concentrations of atRA and 9cRA are greater than the physiological concentration of these retinoids, this finding provides novel insight into the ligand binding ability of COUP-TFII. Indeed, the function of this activation can be seen in the regulation of RARβ2 by COUP-TFII, as COUP-TFII activatation of RARβ2 expression is increased with the addition of all-trans retinoic acid (Lin et al. 2000; Litchfield et al. 2012). Treatment of MCF-7 breast cancer cells with atRA also increased COUP-TFII-binding to the RARB2 promoter in a ChIP assay (Litchfield et al. 2012). Retinoic acid induces the expression of COUP-TFII in certain breast cancer cell lines (e.g. T47D and ZR-75) but not others (e.g. MCF-7 and MDA-MB-231) (Figure 3) (Litchfield et al. 2012; Nakshatri, et al. 2000). This indicates a potential feed forward loop, as treatment with retinoic acid may increase both the expression and activation of COUP- TFII, with downstream effects on retinoic acid receptor.
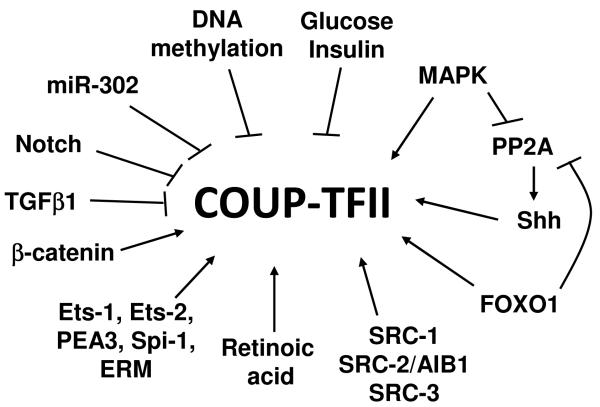
Figure 3. Regulation of COUP-TFII expression.
COUP-TFII expression has been shown to be modulated both transcriptionally and post-transcriptionally by a variety of transcription factors, signaling pathways, and various molecules, as diagramed here.
Regulation of COUP-TFII expression
Tissue-specific regulation in humans
COUP-TFII has a widespread tissue distribution, with detectable expression in every human tissue type examined (Suzuki, et al. 2000a). The regulation of COUP-TFII expression is tissue and cell-type specific, and can be modulated both transcriptionally and post- transcriptionally (Figure 3). Hyperinsulinemia is a risk for breast cancer (Ferguson, et al. 2012; Gunter, et al. 2009). COUP-TFII expression was repressed by insulin and glucose in the liver and pancreas of C57BL6/J mice and in mouse primary hepatic and pancreatic cell culture (Perilhou, et al. 2008a). In contrast, we found that insulin treatment had no effect on COUP-TFII expression in MCF-7 and T47D breast cancer cells (Figure 4). The lack of alteration in COUP- TFII expression with insulin in breast cancer cells highlights the importance of cell-specific regulation of COUP-TFII expression. There are currently no reports on the effect of insulin on COUP-TFII expression in other cancers.
Figure 4. Insulin treatment does not affect COUP-TFII expression in human breast cancer cells.
MCF-7 and T47D human breast cancer cells were grown as in described in (Litchfield et al., 2012). As T47D growth media contains 6 mg/ml insulin, T47D cells were either grown in normal media (with insulin) or in insulin-free media to determine if this affected outcome. Prior to treatment with insulin, all cells were “starved” in low glucose media (5 mM glucose) for 24 h (Perilhou et al., 2008). Cells were treated for 6 h with the indicated concentrations of insulin. QRT-PCR was performed to measure NR2F2 expression relative to GAPDH as a reference gene, as described in (Litchfield et al., 2012). Insulin treatment had no statistically significant effect on COUP-TFII expression in these cell lines.
miRNA regulation
MicroRNA (miRNA) expression is altered in a variety of conditions and disease states, including cancer, and results in important post-transcriptional regulation of crucial proteins (Lovat, et al. 2011). While 115 miRNAs are predicted to target NR2F2 (http://cometa.tigem.it/site/index.php), only one miRNA has been verified. miRNA-302 directly represses COUP-TFII expression in human embryonic stem cells (Rosa and Brivanlou 2011). Regulation of COUP-TFII expression by miRNA has not yet been reported in cancer cells.
DNA methylation
Methylation at CpG islands can result in suppression of gene transcription, and is known to be a hallmark of cancer progression. DNA methylation may also occur at intragenic and intergenic sites, as well as at the promoter (Deaton and Bird 2011; Shenker and Flanagan 2012). Specifically, COUP-TFII has been found to be methylated in many cancers, including mantle cell lymphoma, acute myeloid leukemia, salivary gland adenoid cystic carcinoma, pancreatic adenocarcinoma, colon cancer, breast cancer ductal carcinoma in situ, as well as a tamoxifen- resistant breast cancer cell line (Bell, et al. 2011; Bullinger, et al. 2010; Enjuanes, et al. 2011; Fan, et al. 2006; Irizarry, et al. 2009; Tommasi, et al. 2009; Vincent, et al. 2011). NR2F2 gene hypermethylation was associated with a concordant reduction in mRNA expression in mantle cell lymphoma, pancreatic cancer, and tamoxifen-resistant breast cancer cells (Enjuanes et al. 2011; Fan et al. 2006; Vincent et al. 2011). Whether this indicates a general trend of reduced COUP-TFII expression due to epigenetic modification across cancer types remains to be seen. Contrary to these reports, high levels of COUP-TFII mRNA expression were found in all cell lines in the NCI60 panel of human cancer cell lines (Holbeck, et al. 2010).
Regulation by other transcription factors
COUP-TFII and Ets-1 have overlapping expression patterns in mesenchymal cells of the mouse gut, spleen, lungs, and other tissues (Petit, et al. 2004). Members of the ETS family (Ets- 1, Ets-2, ETV, PEA3, Spi-1, and ERM) increased murine COUP-TFII-promoter activity in HeLa cells. Steroid receptor coactivators SRC-1/NCOA1, TIF2/SRC-2/NCOA2, and RAC3/SRC- 3/NCOA3 enhanced the activation of the COUP-TFII promoter (Petit et al. 2004). In agreement with this data, SRC-3 and RARα increased COUP-TFII-promoter activity in HepG2 human hepatocellular carcinoma cells with atRA treatment. Reciprocally, siRNA knockdown of SRC-3 repressed COUP-TFII expression (Ma, et al. 2011). We observed that the protein expression (by immunohistochemical staining) of AIB1/SRC-3/NCOA3, PEA3, and SRC-1/NCOA1 were correlated with COUP-TFII in breast cancer patient samples (Litchfield et al. 2012).
Regulation by altered kinase activity and other signaling pathways
Several factors were reported to alter COUP-TFII expression in pathogenic states. More et al reported that expression of COUP-TFII, but not COUP-TFI, is stimulated by activation of the MAP kinase (MAPK) pathway. Breast cancer cell lines with increased MAPK activity, i.e., SKBR3, had a concomitant increase in COUP-TFII expression (More, et al. 2003). In contrast to the idea that MAPK activation increases COUP-TFII expression, MAPK has also been shown to phosphorylate and inactivate PP2A (protein phosphatase 2A), leading to a suppression of COUP- TFII expression in human peripheral blood CD34+ cells (Aerbajinai, et al. 2009). Inactivation of PP2A also inhibits sonic hedgehog-induced COUP-TFII expression in P19 cells (Krishnan, et al. 1997). PP2A is inhibited by the FOXO transcription factors, including FOXO1 (Ni, et al. 2007). COUP-TFII expression is induced by FOXO1 in pancreatic beta cells and hepatocytes (Perilhou et al. 2008a), highlighting the highly cell type-specific nature of these pathways. MAPK activity may lead to increased COUP-TFII expression in certain conditions, while it may alternatively repress COUP-TFII in others. Taken together, these data suggest a possible feedback loop in certain cell types (Figure 3).
In addition to MAPK activation, Notch signaling is also dysregulated in many types of cancer. Increased Notch signaling has been implicated in carcinogenesis and metastasis and is also involved in regulation of endothelial cell proliferation and angiogenesis (Garcia and Kandel 2012; Gu, et al. 2012). In breast cancer, Notch and its ligand Jagged1 upregulate the expression of Slug, a transcriptional repressor of E-cadherin important in metastatic progression (Leong, et al. 2007). Notch signaling has also been implicated in the amplification of HER2 and survival of tumor initiating cells (Magnifico, et al. 2009) and cancer stem cells (Gu et al. 2012; Harrison, et al. 2010; Pannuti, et al. 2010). Activation of the Notch pathway confers cancer-like properties and apoptosis-resistance to normal breast epithelial cells (Stylianou, et al. 2006). Regulation of COUP-TFII by Notch signaling has been reported in endothelial cells of both arterial and venous origin and in mouse studies (Kang et al. 2010; Srinivasan, et al. 2010; You, et al. 2005). Notch can suppress COUP-TFII and Prospero-related homeobox domain 1 (Prox1), leading to an arterial rather than lymphatic phenotype in endothelial cells (Francois, et al. 2011; Kang et al. 2010; Srinivasan et al. 2010). COUP-TFII, in turn, can also suppress Notch signaling to result in vein rather than artery formation (You et al. 2005). Transforming growth factor-β1 (TGFβ1) suppresses COUP-TFII expression in keratinocytes and fibroblasts leading to induction of collagen type VII (COL7A1) expression (Calonge, et al. 2004) and in vascular progenitor cells to negatively regulate lymphvasculogenesis (Vittet, et al. 2012). Whether COUP-TFII is regulated via Notch and TGFβ1 signaling has not yet been explored in cancer.
Amplification of Wnt/β-catenin signaling has been widely reported in cancer (Incassati, et al. 2010). In normal tissues, β-catenin signaling is controlled through signals leading to its phosphorylation by a multiprotein destruction complex and subsequent degradation. In breast and other cancers, increased expression of Wnt ligands leads to maintenance of β-catenin activation by preventing its degradation (Incassati et al. 2010). β-catenin signaling has many outcomes, such as normal mammary morphogenesis and ductal maturation; however, sustained activation, through a variety of mechanisms, leads to carcinogenesis (Incassati et al. 2010). ChIP assays demonstrated that β-catenin/TCF7L2 (T-cell factor 7-like 2 or transcription factor 7-like 2) bind the promoter of COUP-TFII to activate expression, resulting in suppression of adipocyte differentiation (Okamura, et al. 2009b). COUP-TFII is expressed in mouse liver and pancreatic β-cells and plays roles in the maintenance of glucose homeostasis and insulin sensitivity (Bardoux, et al. 2005; Perilhou et al. 2008a). Boutant et al also reported that β-catenin/TCF7L2 induces COUP-TFII expression in the pancreas, and that COUP-TFII expression was necessary for normal β-cell function and glucose tolerance in mice (Boutant, et al. 2012). The influence of β-catenin signaling on COUP-TFII expression in cancer has yet to be examined.
Role of COUP-TFII in cancer
Angiogenesis
Many studies of COUP-TFII involve its regulation of the angiogenesis pathway. Under normal conditions, angiogenesis is not active after the time of vasculature development during embryogenesis. However, upon progression of a tumor’s growth, activation of angiogenesis leads to the formation of new blood vessels to support the tumor (Hanahan and Weinberg 2011). COUP-TFII is necessary during normal development for angiogenesis and lymphangiogenesis, as evidenced by the impaired vessel formation and embryonic lethality in COUP-TFII knockout mice (Lin, et al. 2010; Pereira et al. 1999). The expression of many pro-angiogenic factors is modulated by COUP-TFII, including members of the vascular endothelial growth factor (VEGF) family and their receptors. VEGF induces angiogenesis and lymphangiogenesis by activating tyrosine kinase receptors and upregulates endothelial cell proliferation and migration (Hoeben, et al. 2004). In a model of pancreatic islet tumorigenesis, ablation of COUP-TFII increased VEGFR-1 expression, impairing VEGFR-2 signaling and reducing angiogenesis (Qin, et al. 2010b). Metastasis to regional lymph nodes was reduced as a result, implying that COUP-TFII may have a pro-angiogenic, pro-metastatic role in pancreatic cancer (Qin et al. 2010b). Similarly, ablation of COUP-TFII decreased tumorigenesis in B16-F10 melanoma and Lewis lung carcinoma mouse xenografts, and reduced tumorigenesis and metastasis in a spontaneous mouse mammary tumor model. These effects were attributed to a decrease in blood vessel density in COUP-TFII-deficient mice (Qin, et al. 2010a).
In addition to regulating VEGFR expression, COUP-TFII can also affect angiogenesis via regulation of angiopoetin-1 (Ang-1), through binding to an Sp1 site in the promoter region. The induction of Ang-1 is partially responsible for the effects of COUP-TFII, as overexpression of Ang-1 allowed for recovery of angiogenesis in COUP-TFII-deficient mice (Qin et al. 2010a).
Lymphangiogenesis can also contribute to metastasis by allowing the spread of tumor cells to lymph nodes (Achen, et al. 2005; Tobler and Detmar 2006). COUP-TFII regulates tumor lymphangiogenesis via inducing expression of VEGF-C and neuropilin-2, a coreceptor for VEGF-C (Lin et al. 2010; Nagasaki, et al. 2009). In a murine model of pancreatic islet tumorigenesis, COUP-TFII deletion resulted in impaired lymphangiogenesis and reduced metastasis (Qin et al. 2010b). Concordant with a role for COUP-TFII in lymphangiogenesis, Kang et al reported that Notch suppresses COUP-TFII expression, along with Prox1, in human primary dermal lymphatic endothelial cells to signal for arterial rather than lymphatic differentiation (Kang et al. 2010). Suppression of COUP-TFII resulted in an increase in VEGF signaling by activating expression of VEGFR-2, a VEGF receptor whose signaling can feedback to increase activation of Notch signaling (Kang et al. 2010).
COUP-TFII induction by 9cRA was also shown to promote network formation but not cell fusion in SKBR3 breast cancer cells, suggesting a role in the endothelial transdifferentiation pathway as a necessary part of vascular formation (Prahalad, et al. 2010). Taken together, these data indicate that COUP-TFII may regulate angiogenesis and lymphangiogenesis, primarily through modulation of VEGF and its receptor in a cell context-dependent manner.
Invasion and metastasis
In addition to stimulation of angiogenesis, COUP-TFII may have other distinct roles in regulation of tumor growth and metastasis. Transfection with COUP-TFII in A549, H520, and H441 lung cancer cells and MDA-MB-231 breast cancer cells was reported to increase migration and invasion (Navab, et al. 2004). Navab et al found that COUP-TFII upregulated the expression of extracellular matrix-degrading proteinases matrix metalloproteinase 2 (MMP2) and urokinase-type plasminogen activator (uPA) (Navab et al. 2004). MMP2 and uPA are known to play critical roles in cancer, particularly in angiogenesis and metastasis (Annecke, et al. 2008). High levels of uPA are predictive of recurrence but also of a favorable response to adjuvant chemotherapy in breast cancer patients (Harbeck, et al. 2004). Interestingly, it has also been reported that uPA expression is dependent on Notch signaling in MDA-MB-231, MDA-MB-468, and HCC1143 breast cancer cells (Shimizu, et al. 2011). COUP-TFII and MMP2 expression were also positively correlated in a breast tumor microarray (Litchfield et al. 2012), further indicating a potential relationship between COUP-TFII and extracellular matrix degradation. In contrast, COUP-TFII decreased cell motility when transfected into LY2 tamoxifen-resistant breast cancer cells, while having no significant effect on invasion (Riggs et al. 2006).
Estrogen receptor and clinical outcome
Nagasaki et al demonstrated that COUP-TFII expression was correlated with ERα status and indices of poor clinical outcome (clinical stage, lymph node status, histological grade) in human breast tumor samples, indicating COUP-TFII may play a role in cancer progression (Nagasaki et al. 2009). We also found that COUP-TFII and ERα expression were correlated in a human breast tissue/tumor microarray, but instead noted an inverse relationship between COUP- TFII expression and TNM (tumor, node, metastasis) classification (Litchfield et al. 2012). Similar findings were observed at the mRNA level by examining breast tumor mRNA transcriptomes in Oncomine (Litchfield et al. 2012). COUP-TFII expression was significantly higher in ERα+ breast cancer samples and significantly lower in metastatic samples (Litchfield et al. 2012). These findings indicate a function for COUP-TFII in inhibiting tumor progression. A positive correlation with ERα is consistent with a previous report that siRNA knockdown of ERα in MCF-7 breast cancer cells decreased COUP-TFII expression and treatment with estradiol increased the expression of COUP-TFII (Riggs et al. 2006). ERα is a positive prognostic factor in breast tumors and is the target of endocrine-targeted cancer therapeutics such as the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene (Jordan 2009). COUP-TFII, but not COUP-TFI, is reduced in tamoxifen-resistant human breast cancer cells, and re- expression of COUP-TFII can restore tamoxifen-sensitivity (Riggs et al. 2006). As ERα expression is important in keeping breast cancer cells responsive to treatment, the correlation of COUP-TFII and ERα further demonstrates a beneficial role for COUP-TFII, highlighting its potential importance in maintaining differentiation and endocrine sensitivity.
In contrast to a role for COUP-TFII in maintaining antiestrogen sensitivity, Holbeck et al reported that cancer cells in the NCI60 panel expressing low levels of COUP-TFII showed higher sensitivity to microtubule-targeting drugs vinblastine, colchicines, and taxol (Holbeck et al. 2010). These data demonstrate that both cell type-specific as well as drug-specific mechanisms may determine the role of COUP-TFII in influencing treatment response.
Steroidogenesis
COUP-TFII expression was reported to be high in aldosteroma, with an inverse correlation to adrenal steroidogenesis (Suzuki, et al. 2000b). These data also indicated an inverse correlation between COUP-TFII expression and CYP17A1 expression, with COUP-TFII inhibiting CYP17A1 in aldosteroma (Suzuki et al. 2000b). COUP-TFII competed with SF-1 for binding to overlapping sites within the promoters of the CYP17A1 (Bakke and Lund 1995; van den Driesche, et al. 2012), CYP11A1, and STARD1 genes in rat Leydig cells and to suppress testosterone production (van den Driesche et al. 2012). COUP-TFI and COUP-TFII both repressed angiotensin II-stimulated STARD1 (StAR) in bovine adrenal glomerulosa cells in primary culture (Buholzer, et al. 2005). COUP-TFII also competed with SF-1 for the human aromatase P450 promoter II in primary endometriotic stromal cells and suppressed aromatase expression.(Zeitoun, et al. 1999). Overexpression of SF-1 in primary endometriotic stromal cells outcompeted the normal protective effect of COUP-TF (whether COUP-TFI or COUP-TFII was involved was unclear since both were equally expressed at the mRNA level) resulting in high local aromatase expression in endometriosis (Zeitoun et al. 1999). COUP-TFII was reported to bind the S1 silencer region of the human aromatase gene and suppress transcription in MCF-7 cells (Yang, et al. 2002). Indeed, the decreases in COUP-TFI, EARγ, EAR-2, Snail and Slug in breast cancer were suggested to increase aromatase expression (Chen, et al. 2005). Thus, the downregulation of COUP-TFII expression that we observed in endocrine-resistant breast cancer cells (Riggs et al. 2006) would be expected to increase aromatase and thus increase local estrogen production. However, whether increased COUP-TFII suppresses local androgen or estrogen biosynthesis in breast tissue is unknown. Local conversion of adrenal androgens to estrogens by aromatase is the target of AI therapy for post-menopausal women. However, there are androgen metabolites, e.g., 3β-adiol, that bypass aromatase which activate ERα and ERβ and may play a role in AI resistance (Sikora, et al. 2009; Sikora, et al. 2012). Overall, the literature supports a negative role for COUP-TFII in regulating steroid hormone synthesis and further studies addressing COUP-TFII regulation of aromatase gene expression in local estrogen production in breast (Bulun, et al. 2012) and lung (Marquez-Garban, et al. 2009) adenocarcinomas would be of merit.
Conclusions
The studies reviewed here indicate that COUP-TFII is regulated and is functionally active to regulate target gene transcription in a cell type-dependent manner. There is evidence that COUP-TFII may perform both pro- and anti-tumorigenic roles. COUP-TFII has been reported to increase angiogenesis and lymphangiogenesis, both increase and decrease tumor metastasis, lead to favorable and unfavorable therapeutic outcome in cancer therapy, and suppress steroidogenesis. Qin et al reported that COUP-TFII was not expressed in tumor cells, but rather was instead found in high concentration in the surrounding blood vessels that support tumor growth and spread (Qin et al. 2010a). This indicates a crucial point of consideration about the nature of COUP-TFII in cancer formation and progression: the function of COUP-TFII within cancer cells versus in the surrounding tumor microenvironment and other cell types. Tissue type is clearly an important determinant in deciphering the oncogenic or tumor-suppressive nature of COUP-TFII. Many studies published to date involve the regulation and role of COUP-TFII during development and in non-cancerous disease states. The full applicability of these studies to our knowledge of the role of COUP-TFII in carcinogenesis and cancer progression remains to be seen. Future studies are necessary to elucidate the complex nature of this vital nuclear receptor.
Acknowledgments
Funding
This work was supported by a grant from Susan G. Komen for the Cure KG080365 to CMK. LML is supported by a fellowship from NIEHS T32 ES011564.
Footnotes
Declaration of interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Aerbajinai W, Zhu J, Kumkhaek C, Chin K, Rodgers GP. SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII. Blood. 2009;114:187–194. doi: 10.1182/blood-2008-07-170712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Kumar R. Steroid hormone receptors in cancer development: a target for cancer therapeutics. Cancer Lett. 2011;300:1–9. doi: 10.1016/j.canlet.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Annecke K, Schmitt M, Euler U, Zerm M, Paepke D, Paepke S, von Minckwitz G, Thomssen C, Harbeck N. uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Adv Clin Chem. 2008;45:31–45. doi: 10.1016/s0065-2423(07)00002-9. [DOI] [PubMed] [Google Scholar]
- Bailey PJ, Dowhan DH, Franke K, Burke LJ, Downes M, Muscat GE. Transcriptional repression by COUP-TF II is dependent on the C-terminal domain and involves the N-CoR variant, RIP13delta1. J Steroid Biochem Mol Biol. 1997;63:165–174. doi: 10.1016/s0960-0760(97)00079-4. [DOI] [PubMed] [Google Scholar]
- Bakke M, Lund J. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3′,5′-monophosphate-responsive sequence in the bovine CYP17 gene. Mol Endocrinol. 1995;9:327–339. doi: 10.1210/mend.9.3.7776979. [DOI] [PubMed] [Google Scholar]
- Bardoux P, Zhang P, Flamez D, Perilhou A, Lavin TA, Tanti JF, Hellemans K, Gomas E, Godard C, Andreelli F, et al. Essential role of chicken ovalbumin upstream promoter-transcription factor II in insulin secretion and insulin sensitivity revealed by conditional gene knockout. Diabetes. 2005;54:1357–1363. doi: 10.2337/diabetes.54.5.1357. [DOI] [PubMed] [Google Scholar]
- Bell A, Bell D, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117:2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, Sharir H, Pikarsky E, Bergman Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Molecular and cellular biology. 1995;15:1034–1048. doi: 10.1128/mcb.15.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutant M, Ramos OH, Tourrel-Cuzin C, Movassat J, Ilias A, Vallois D, Planchais J, Pegorier JP, Schuit F, Petit PX, et al. COUP-TFII controls mouse pancreatic beta-cell mass through GLP-1-beta-catenin signaling pathways. PLoS One. 2012;7:e30847. doi: 10.1371/journal.pone.0030847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buholzer CF, Arrighi JF, Abraham S, Piguet V, Capponi AM, Casal AJ. Chicken ovalbumin upstream promoter-transcription factor is a negative regulator of steroidogenesis in bovine adrenal glomerulosa cells. Molecular Endocrinology. 2005;19:65–75. doi: 10.1210/me.2004-0061. [DOI] [PubMed] [Google Scholar]
- Bullinger L, Ehrich M, Dohner K, Schlenk RF, Dohner H, Nelson MR, van den Boom D. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood. 2010;115:636–642. doi: 10.1182/blood-2009-03-211003. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonge MJ, Seoane J, Massague J. Opposite Smad and chicken ovalbumin upstream promoter transcription factor inputs in the regulation of the collagen VII gene promoter by transforming growth factor-beta. J Biol Chem. 2004;279:23759–23765. doi: 10.1074/jbc.M402178200. [DOI] [PubMed] [Google Scholar]
- Chen S, Ye J, Kijima I, Kinoshita Y, Zhou D. Positive and negative transcriptional regulation of aromatase expression in human breast cancer tissue. J Steroid Biochem Mol Biol. 2005;95:17–23. doi: 10.1016/j.jsbmb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Chu K, Zingg HH. The nuclear orphan receptors COUP-TFII and Ear-2 act as silencers of the human oxytocin gene promoter. Journal of molecular endocrinology. 1997;19:163–172. doi: 10.1677/jme.0.0190163. [DOI] [PubMed] [Google Scholar]
- Cooney AJ, Leng X, Tsai SY, O’Malley BW, Tsai MJ. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. The Journal of biological chemistry. 1993;268:4152–4160. [PubMed] [Google Scholar]
- De Martino MU, Alesci S, Chrousos GP, Kino T. Interaction of the glucocorticoid receptor and the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII): implications for the actions of glucocorticoids on glucose, lipoprotein, and xenobiotic metabolism. Annals of the New York Academy of Sciences. 2004a;1024:72–85. doi: 10.1196/annals.1321.006. [DOI] [PubMed] [Google Scholar]
- De Martino MU, Bhattachryya N, Alesci S, Ichijo T, Chrousos GP, Kino T. The glucocorticoid receptor and the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II interact with and mutually affect each other’s transcriptional activities: implications for intermediary metabolism. Molecular endocrinology. 2004b;18:820–833. doi: 10.1210/me.2003-0341. [DOI] [PubMed] [Google Scholar]
- Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes A, Fernandez V, Hernandez L, Navarro A, Bea S, Pinyol M, Lopez-Guillermo A, Rosenwald A, Ott G, Campo E, et al. Identification of methylated genes associated with aggressive clinicopathological features in mantle cell lymphoma. PLoS One. 2011;6:e19736. doi: 10.1371/journal.pone.0019736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubank DW, Duplus E, Williams SC, Forest C, Beale EG. Peroxisome proliferator-activated receptor gamma and chicken ovalbumin upstream promoter transcription factor II negatively regulate the phosphoenolpyruvate carboxykinase promoter via a common element. J Biol Chem. 2001;276:30561–30569. doi: 10.1074/jbc.M103019200. [DOI] [PubMed] [Google Scholar]
- Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer research. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- Ferguson R, Novosyadlyy R, Fierz Y, Alikhani N, Sun H, Yakar S, LeRoith D. Hyperinsulinemia enhances c-Myc-mediated mammary tumor development and advances metastatic progression to the lung in a mouse model of type 2 diabetes. Breast Cancer Research. 2012;14:R8. doi: 10.1186/bcr3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Rachubinski F, Fliegel L. COUP-TFI and COUP-TFII regulate expression of the NHE through a nuclear hormone responsive element with enhancer activity. Eur J Biochem. 2001;268:620–634. doi: 10.1046/j.1432-1327.2001.01915.x. [DOI] [PubMed] [Google Scholar]
- Francois M, Harvey NL, Hogan BM. The transcriptional control of lymphatic vascular development. Physiology (Bethesda) 2011;26:146–155. doi: 10.1152/physiol.00053.2010. [DOI] [PubMed] [Google Scholar]
- Garcia A, Kandel JJ. Notch: a key regulator of tumor angiogenesis and metastasis. Histol Histopathol. 2012;27:151–156. doi: 10.14670/hh-27.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, Miele L. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc Cell. 2012;4:7. doi: 10.1186/2045-824X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GYF, Xue X, Anderson GL, et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. Journal of the National Cancer Institute. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85:431–441. doi: 10.1095/biolreprod.111.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Kates RE, Schmitt M, Gauger K, Kiechle M, Janicke F, Thomassen C, Look MP, Foekens JA. Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin Breast Cancer. 2004;5:348–352. doi: 10.3816/cbc.2004.n.040. [DOI] [PubMed] [Google Scholar]
- Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Holbeck S, Chang J, Best AM, Bookout AL, Mangelsdorf DJ, Martinez ED. Expression profiling of nuclear receptors in the NCI60 cancer cell panel reveals receptor-drug and receptor-gene interactions. Molecular endocrinology. 2010;24:1287–1296. doi: 10.1210/me.2010-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins GS, Bacani CJ, Boltax J, Aikawa R, Leiden JM. Friend of GATA 2 physically interacts with chicken ovalbumin upstream promoter-TF2 (COUP-TF2) and COUP-TF3 and represses COUP-TF2-dependent activation of the atrial natriuretic factor promoter. J Biol Chem. 2001;276:28029–28036. doi: 10.1074/jbc.M103577200. [DOI] [PubMed] [Google Scholar]
- Incassati A, Chandramouli A, Eelkema R, Cowin P. Key signaling nodes in mammary gland development and cancer: beta-catenin. Breast Cancer Res. 2010;12:213. doi: 10.1186/bcr2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janne M, Hammond GL. Hepatocyte nuclear factor-4 controls transcription from a TATA-less human sex hormone-binding globulin gene promoter. J Biol Chem. 1998;273:34105–34114. doi: 10.1074/jbc.273.51.34105. [DOI] [PubMed] [Google Scholar]
- Jiang G, Nepomuceno L, Hopkins K, Sladek FM. Exclusive homodimerization of the orphan receptor hepatocyte nuclear factor 4 defines a new subclass of nuclear receptors. Mol Cell Biol. 1995;15:5131–5143. doi: 10.1128/mcb.15.9.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, Choi I, Otu HH, Shin JW, Dotto GP, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–150. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992;89:1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J Biol Chem. 1997;272:31465–31474. doi: 10.1074/jbc.272.50.31465. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Pereira FA, Qiu Y, Chen CH, Beachy PA, Tsai SY, Tsai MJ. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistaki E, Talianidis I. Chicken ovalbumin upstream promoter transcription factors act as auxiliary cofactors for hepatocyte nuclear factor 4 and enhance hepatic gene expression. Mol Cell Biol. 1997;17:2790–2797. doi: 10.1128/mcb.17.5.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Shibata H, Kobayashi S, Suda N, Ikeda Y, Yokota K, Murai A, Saito I, Rainey WE, Saruta T. Ubc9 and Protein Inhibitor of Activated STAT 1 Activate Chicken Ovalbumin Upstream Promoter-Transcription Factor I-mediated Human CYP11B2 Gene Transcription. The Journal of biological chemistry. 2005;280:6721–6730. doi: 10.1074/jbc.M411820200. [DOI] [PubMed] [Google Scholar]
- Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev. 2006;20:2293–2305. doi: 10.1101/gad.390906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrentiadou SN, Hadzopoulou-Cladaras M, Kardassis D, Zannis VI. Binding specificity and modulation of the human ApoCIII promoter activity by heterodimers of ligand-dependent nuclear receptors. Biochemistry. 1999;38:964–975. doi: 10.1021/bi981068i. [DOI] [PubMed] [Google Scholar]
- Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Molecular and cellular biology. 2004;24:10835–10843. doi: 10.1128/MCB.24.24.10835-10843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KN, Jang WG, Kim EJ, Oh SH, Son HJ, Kim SH, Franceschi R, Zhang XK, Lee SE, Koh JT. Orphan nuclear receptor COUP-TFII negatively regulates BMP2-induced osteoblast differentiation through suppressing Runx2 activity. J Biol Chem. 2012 doi: 10.1074/jbc.M111.311878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MO, Liu Y, Zhang XK. A retinoic acid response element that overlaps an estrogen response element mediates multihormonal sensitivity in transcriptional activation of the lactoferrin gene. Mol Cell Biol. 1995;15:4194–4207. doi: 10.1128/mcb.15.8.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Misik AJ, Rieder CV, Solaro RJ, Lowen A, Fliegel L. Thyroid hormone receptor alpha 1 regulates expression of the Na+/H+ exchanger (NHE1) J Biol Chem. 2002;277:28656–28662. doi: 10.1074/jbc.M203221200. [DOI] [PubMed] [Google Scholar]
- Liberati C, Cera MR, Secco P, Santoro C, Mantovani R, Ottolenghi S, Ronchi A. Cooperation and competition between the binding of COUP-TFII and NF-Y on human epsilon- and gamma-globin gene promoters. J Biol Chem. 2001;276:41700–41709. doi: 10.1074/jbc.M102987200. [DOI] [PubMed] [Google Scholar]
- Lin B, Chen GQ, Xiao D, Kolluri SK, Cao X, Su H, Zhang XK. Orphan receptor COUP-TF is required for induction of retinoic acid receptor beta, growth inhibition, and apoptosis by retinoic acid in cancer cells. Mol Cell Biol. 2000;20:957–970. doi: 10.1128/mcb.20.3.957-970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Kolluri SK, Chen GQ, Zhang XK. Regulation of retinoic acid-induced inhibition of AP-1 activity by orphan receptor chicken ovalbumin upstream promoter-transcription factor. The Journal of biological chemistry. 2002;277:21414–21422. doi: 10.1074/jbc.M201885200. [DOI] [PubMed] [Google Scholar]
- Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. The Journal of clinical investigation. 2010;120:1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup d’Etat: An Orphan Takes Control. Endocr Rev. 2011 doi: 10.1210/er.2010-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield LM, Riggs KA, Hockenberry AM, Oliver LD, Barnhart KG, Cai J, Pierce WM, Jr., Ivanova MM, Bates PJ, Appana SN, et al. Identification and Characterization of Nucleolin as a COUP-TFII Coactivator of Retinoic Acid Receptor beta Transcription in Breast Cancer Cells. PLoS One. 2012;7:e38278. doi: 10.1371/journal.pone.0038278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat F, Valeri N, Croce CM. MicroRNAs in the pathogenesis of cancer. Semin Oncol. 2011;38:724–733. doi: 10.1053/j.seminoncol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Ma X, Xu L, Wang S, Cui B, Li X, Xu J, Ning G. Deletion of steroid receptor coactivator-3 gene ameliorates hepatic steatosis. Journal of hepatology. 2011;55:445–452. doi: 10.1016/j.jhep.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- Marcus SL, Winrow CJ, Capone JP, Rachubinski RA. A p56(lck) ligand serves as a coactivator of an orphan nuclear hormone receptor. The Journal of biological chemistry. 1996;271:27197–27200. doi: 10.1074/jbc.271.44.27197. [DOI] [PubMed] [Google Scholar]
- Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietus-Snyder M, Sladek FM, Ginsburg GS, Kuo CF, Ladias JA, Darnell JE, Jr, Karathanasis SK. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992;12:1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More E, Fellner T, Doppelmayr H, Hauser-Kronberger C, Dandachi N, Obrist P, Sandhofer F, Paulweber B. Activation of the MAP kinase pathway induces chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) expression in human breast cancer cell lines. J Endocrinol. 2003;176:83–94. doi: 10.1677/joe.0.1760083. [DOI] [PubMed] [Google Scholar]
- Nagasaki S, Suzuki T, Miki Y, Akahira J, Shibata H, Ishida T, Ohuchi N, Sasano H. Chicken ovalbumin upstream promoter transcription factor II in human breast carcinoma: possible regulator of lymphangiogenesis via vascular endothelial growth factor-C expression. Cancer Science. 2009;100:639–645. doi: 10.1111/j.1349-7006.2008.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H, Chambon P. The directly repeated RG(G/T)TCA motifs of the rat and mouse cellular retinol-binding protein II genes are promiscuous binding sites for RAR, RXR, HNF-4, and ARP-1 homo- and heterodimers. The Journal of biological chemistry. 1994;269:890–902. [PubMed] [Google Scholar]
- Nakshatri H, Mendonca MS, Bhat-Nakshatri P, Patel NM, Goulet RJ, Jr, Cornetta K. The orphan receptor COUP-TFII regulates G2/M progression of breast cancer cells by modulating the expression/activity of p21(WAF1/CIP1), cyclin D1, and cdk2. Biochemical and biophysical research communications. 2000;270:1144–1153. doi: 10.1006/bbrc.2000.2562. [DOI] [PubMed] [Google Scholar]
- Navab R, Gonzalez-Santos JM, Johnston MR, Liu J, Brodt P, Tsao MS, Hu J. Expression of chicken ovalbumin upstream promoter-transcription factor II enhances invasiveness of human lung carcinoma cells. Cancer research. 2004;64:5097–5105. doi: 10.1158/0008-5472.CAN-03-1185. [DOI] [PubMed] [Google Scholar]
- Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa A, Bovard-Houppermans S, Zakin MM. Human apolipoprotein A-IV gene expression is modulated by members of the nuclear hormone receptor superfamily. Biochim Biophys Acta. 1993;1210:41–47. doi: 10.1016/0005-2760(93)90047-d. [DOI] [PubMed] [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009a;106:5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106:5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perilhou A, Tourrel-Cuzin C, Kharroubi I, Henique C, Fauveau V, Kitamura T, Magnan C, Postic C, Prip-Buus C, Vasseur-Cognet M. The transcription factor COUP-TFII is negatively regulated by insulin and glucose via Foxo1- and ChREBP-controlled pathways. Molecular and cellular biology. 2008a;28:6568–6579. doi: 10.1128/MCB.02211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perilhou A, Tourrel-Cuzin C, Zhang P, Kharroubi I, Wang H, Fauveau V, Scott DK, Wollheim CB, Vasseur-Cognet M. The MODY1 gene for hepatocyte nuclear factor 4alpha and a feedback loop control COUP-TFII expression in pancreatic beta cells. Molecular and cellular biology. 2008b;28:4588–4597. doi: 10.1128/MCB.01191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit FG, Salas R, Tsai MJ, Tsai SY. The regulation of COUP-TFII gene expression by Ets-1 is enhanced by the steroid receptor co-activators. Mechanisms of ageing and development. 2004;125:719–732. doi: 10.1016/j.mad.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARalpha promoter: identification of a functional nuclear receptor response element. Mol Endocrinol. 2002;16:1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- Pipaon C, Tsai SY, Tsai MJ. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol Cell Biol. 1999;19:2734–2745. doi: 10.1128/mcb.19.4.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power SC, Cereghini S. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol Cell Biol. 1996;16:778–791. doi: 10.1128/mcb.16.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahalad P, Dakshanamurthy S, Ressom H, Byers SW. Retinoic acid mediates regulation of network formation by COUP-TFII and VE-cadherin expression by TGFbeta receptor kinase in breast cancer cells. PLoS One. 2010;5:e10023. doi: 10.1371/journal.pone.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:3687–3692. doi: 10.1073/pnas.0914619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer research. 2010b;70:8812–8821. doi: 10.1158/0008-5472.CAN-10-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs KA, Wickramasinghe NS, Cochrum RK, Watts MB, Klinge CM. Decreased chicken ovalbumin upstream promoter transcription factor II expression in tamoxifen-resistant breast cancer cells. Cancer Res. 2006;66:10188–10198. doi: 10.1158/0008-5472.CAN-05-3937. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nat Rev Cancer. 2010;10:205–212. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- Robinson CE, Wu X, Nawaz Z, Onate SA, Gimble JM. A corepressor and chicken ovalbumin upstream promoter transcriptional factor proteins modulate peroxisome proliferator-activated receptor-gamma2/retinoid X receptor alpha-activated transcription from the murine lipoprotein lipase promoter. Endocrinology. 1999;140:1586–1593. doi: 10.1210/endo.140.4.6653. [DOI] [PubMed] [Google Scholar]
- Rodriguez JC, Ortiz JA, Hegardt FG, Haro D. Chicken ovalbumin upstream-promoter transcription factor (COUP-TF) could act as a transcriptional activator or repressor of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene. Biochemical Journal. 1997;326:587–592. doi: 10.1042/bj3260587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A, Brivanlou AH. A regulatory circuitry comprised of miR-302 and the transcription factors OCT4 and NR2F2 regulates human embryonic stem cell differentiation. EMBO J. 2011;30:237–248. doi: 10.1038/emboj.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufibach LE, Duncan SA, Battle M, Deeb SS. Transcriptional regulation of the human hepatic lipase (LIPC) gene promoter. J Lipid Res. 2006;47:1463–1477. doi: 10.1194/jlr.M600082-JLR200. [DOI] [PubMed] [Google Scholar]
- Sagami I, Tsai SY, Wang H, Tsai MJ, O’Malley BW. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol. 1986;6:4259–4267. doi: 10.1128/mcb.6.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvaget D, Chauffeton V, Citadelle D, Chatelet FP, Cywiner-Golenzer C, Chambaz J, Pincon-Raymond M, Cardot P, Le Beyec J, Ribeiro A. Restriction of apolipoprotein A-IV gene expression to the intestine villus depends on a hormone-responsive element and parallels differential expression of the hepatic nuclear factor 4alpha and gamma isoforms. J Biol Chem. 2002;277:34540–34548. doi: 10.1074/jbc.M206074200. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hogeveen KN, Hammond GL. Repression of the human sex hormone-binding globulin gene in Sertoli cells by upstream stimulatory transcription factors. J Biol Chem. 2005;280:4462–4468. doi: 10.1074/jbc.M409616200. [DOI] [PubMed] [Google Scholar]
- Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer. 2012;106:248–253. doi: 10.1038/bjc.2011.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Kobayashi S, Kurihara I, Suda N, Yokota K, Murai A, Ikeda Y, Saito I, Rainey WE, Saruta T. COUP-TF and transcriptional co-regulators in adrenal steroidogenesis. Endocrine research. 2004;30:795–801. doi: 10.1081/erc-200044042. [DOI] [PubMed] [Google Scholar]
- Shibata H, Nawaz Z, Tsai SY, O’Malley BW, Tsai MJ. Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT) Mol Endocrinol. 1997;11:714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Cohen B, Goldvasser P, Berman H, Virtanen C, Reedijk M. Plasminogen activator uPA is a direct transcriptional target of the JAG1-Notch receptor signaling pathway in breast cancer. Cancer Res. 2011;71:277–286. doi: 10.1158/0008-5472.CAN-10-2523. [DOI] [PubMed] [Google Scholar]
- Siddikuzzaman, Guruvayoorappan C, Berlin Grace VM. All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol. 2011;33:241–249. doi: 10.3109/08923973.2010.521507. [DOI] [PubMed] [Google Scholar]
- Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5alpha-androstane-3beta,17beta-diol (3betaAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora MJ, Strumba V, Lippman ME, Johnson MD, Rae JM. Mechanisms of estrogen-independent breast cancer growth driven by low estrogen concentrations are unique versus complete estrogen deprivation. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- Stroup D, Chiang JY. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1) J Lipid Res. 2000;41:1–11. [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Moriya T, Darnel AD, Takeyama J, Sasano H. Immunohistochemical distribution of chicken ovalbumin upstream promoter transcription factor II in human tissues. Mol Cell Endocrinol. 2000a;164:69–75. doi: 10.1016/s0303-7207(00)00242-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takahashi K, Darnel AD, Moriya T, Murakami O, Narasaka T, Takeyama J, Sasano H. Chicken ovalbumin upstream promoter transcription factor II in the human adrenal cortex and its disorders. J Clin Endocrinol Metab. 2000b;85:2752–2757. doi: 10.1210/jcem.85.8.6730. [DOI] [PubMed] [Google Scholar]
- Tang K, Xie X, Park JI, Jamrich M, Tsai S, Tsai MJ. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development. 2010;137:725–734. doi: 10.1242/dev.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14:2778–2794. doi: 10.1101/gad.822500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- Tommasi S, Karm DL, Wu X, Yen Y, Pfeifer GP. Methylation of homeobox genes is a frequent and early epigenetic event in breast cancer. Breast Cancer Res. 2009;11:R14. doi: 10.1186/bcr2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Tsai M-J. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): Coming of age. Endocrine Rev. 1997;18:229–240. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, Seckl JR, Drake AJ, Smith LB, Anderson RA, et al. Proposed Role for COUP-TFII in Regulating Fetal Leydig Cell Steroidogenesis, Perturbation of Which Leads to Masculinization Disorders in Rodents. PLoS One. 2012;7:e37064. doi: 10.1371/journal.pone.0037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E, Birchmeier C. The development of migrating muscle precursor cells. Anat Embryol (Berl) 2006;211(Suppl 1):37–41. doi: 10.1007/s00429-006-0118-9. [DOI] [PubMed] [Google Scholar]
- Vincent A, Omura N, Hong SM, Jaffe A, Eshleman J, Goggins M. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4341–4354. doi: 10.1158/1078-0432.CCR-10-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittet D, Merdzhanova G, Prandini MH, Feige JJ, Bailly S. TGFss1 inhibits lymphatic endothelial cell differentiation from mouse embryonic stem cells. J Cell Physiol. 2012 doi: 10.1002/jcp.24063. [DOI] [PubMed] [Google Scholar]
- Widom RL, Rhee M, Karathanasis SK. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992;12:3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Li Y, Liu R, Agadir A, Lee MO, Liu Y, Zhang X. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. The EMBO journal. 1997;16:1656–1669. doi: 10.1093/emboj/16.7.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yu B, Zhou D, Chen S. Regulation of aromatase promoter activity in human breast tissue by nuclear receptors. Oncogene. 2002;21:2854–2863. doi: 10.1038/sj.onc.1205386. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- You M, Fischer M, Cho WK, Crabb D. Transcriptional control of the human aldehyde dehydrogenase 2 promoter by hepatocyte nuclear factor 4: inhibition by cyclic AMP and COUP transcription factors. Archives of biochemistry and biophysics. 2002;398:79–86. doi: 10.1006/abbi.2001.2713. [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Jameson JL. The murine Dax-1 promoter is stimulated by SF-1 (steroidogenic factor-1) and inhibited by COUP-TF (chicken ovalbumin upstream promoter-transcription factor) via a composite nuclear receptor-regulatory element. Mol Endocrinol. 1998;12:1010–1022. doi: 10.1210/mend.12.7.0131. [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Molecular endocrinology. 1999;13:239–253. doi: 10.1210/mend.13.2.0229. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Liu X, Gafken PR, Kioussi C, Leid M. A chicken ovalbumin upstream promoter transcription factor I (COUP-TFI) complex represses expression of the gene encoding tumor necrosis factor alpha-induced protein 8 (TNFAIP8) The Journal of biological chemistry. 2009;284:6156–6168. doi: 10.1074/jbc.M807713200. [DOI] [PMC free article] [PubMed] [Google Scholar]