Abstract
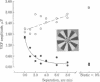
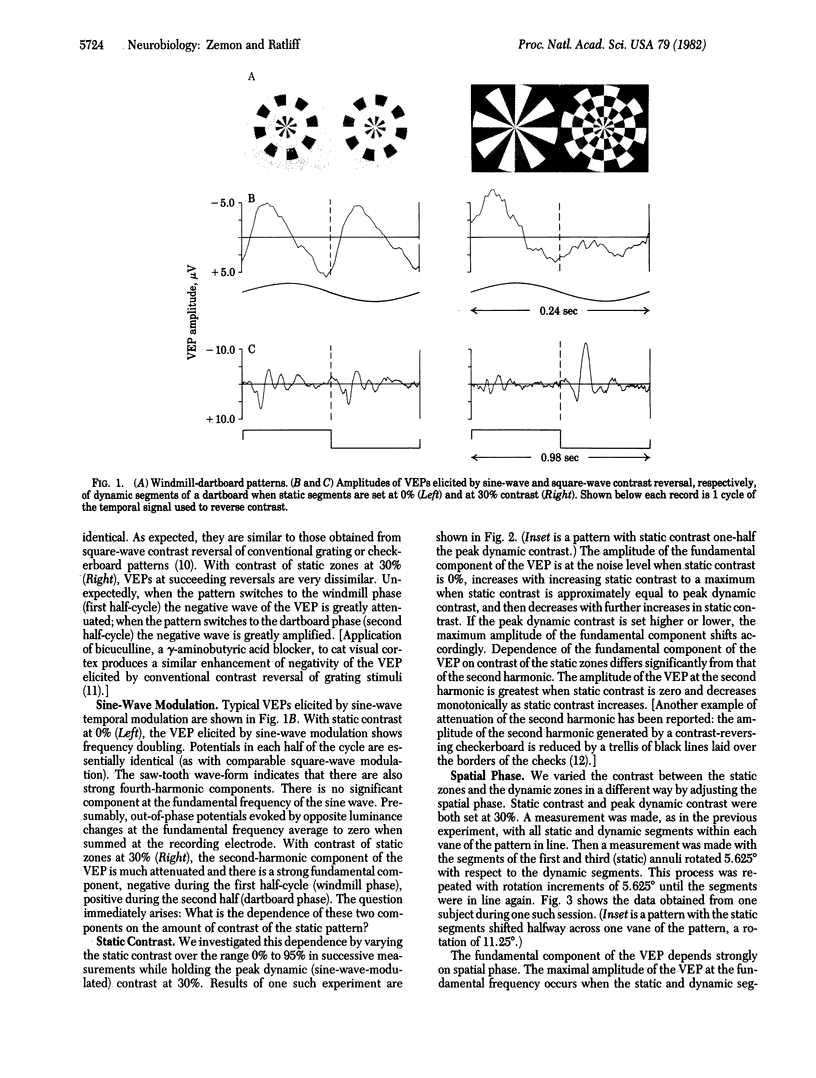
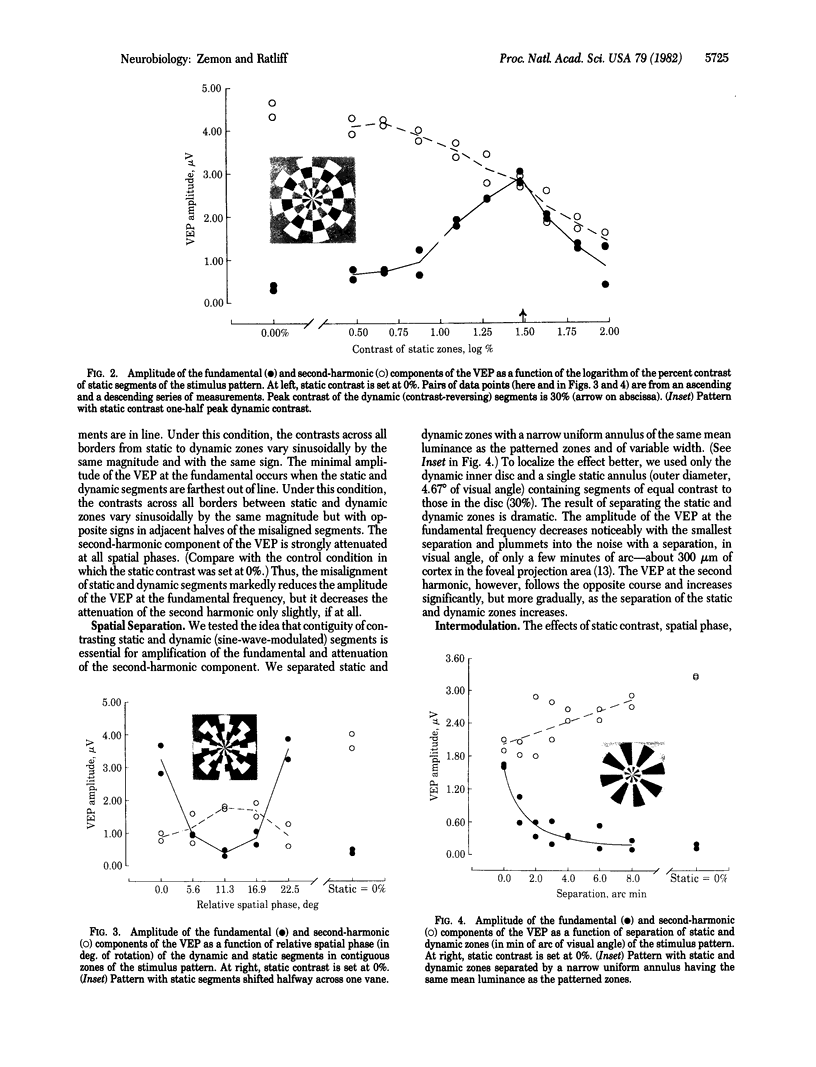
Electrical potentials evoked in the human brain by visual stimulation can easily be recorded by using electrodes attached to the scalp. It is difficult, however, to relate these visual evoked potentials (VEPs) to specific neural processes: scalp electrodes, far removed from the brain, sum potentials from large areas of cortex. We improved identification and localization of lateral interactions by differentially modulating small neighboring parts of a "windmill-dartboard" stimulus pattern-a central disc surrounded by three contiguous annuli, all radially divided into light and dark segments. With temporal contrast reversal of all segments in the pattern, the major component of the VEP is at the second harmonic of the frequency of modulation--as expected. Temporal contrast reversal of the segments in the central disc and second annulus, with contrast of segments held constant in the first and third annuli, unexpectedly amplifies the VEP at the fundamental frequency of modulation and attenuates it at the second harmonic. Slight spatial separation of static and dynamic zones reduces both the amplification of the fundamental and the attenuation of the second harmonic. Thus, both phenomena appear to result from strong lateral interactions over relatively short distances. Nevertheless, different neural mechanisms must be involved; fundamental and second-harmonic components of the VEP are different functions of spatial separation and relative contrast of the segments in contiguous static and dynamic zones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Visual resolution and receptive field size: examination of two kinds of cat retinal ganglion cell. Science. 1979 Sep 7;205(4410):1015–1017. doi: 10.1126/science.472720. [DOI] [PubMed] [Google Scholar]
- Cowey A., Rolls E. T. Human cortical magnification factor and its relation to visual acuity. Exp Brain Res. 1974;21(5):447–454. doi: 10.1007/BF00237163. [DOI] [PubMed] [Google Scholar]
- Harter M. R. Evoked cortical responses to checkerboard patterns: effect of check-size as a function of retinal eccentricity. Vision Res. 1970 Dec;10(12):1365–1376. doi: 10.1016/0042-6989(70)90088-x. [DOI] [PubMed] [Google Scholar]
- Ochs A. L., Aminoff M. J. Visual evoked potentials elicited by circular grating. Arch Neurol. 1980 May;37(5):308–309. doi: 10.1001/archneur.1980.00500540086014. [DOI] [PubMed] [Google Scholar]
- Ransom-Hogg A., Spillmann L. Perceptive field size in fovea and periphery of the light- and dark-adapted retina. Vision Res. 1980;20(3):221–228. doi: 10.1016/0042-6989(80)90106-6. [DOI] [PubMed] [Google Scholar]
- Ratliff F. Radial spatial patterns and multifrequency temporal patterns: possible clinical applications. Ann N Y Acad Sci. 1982;388:651–656. doi: 10.1111/j.1749-6632.1982.tb50830.x. [DOI] [PubMed] [Google Scholar]
- Ratliff F., Zemon V. Some new methods for the analysis of lateral interactions that influence the visual evoked potential. Ann N Y Acad Sci. 1982;388:113–124. doi: 10.1111/j.1749-6632.1982.tb50787.x. [DOI] [PubMed] [Google Scholar]
- Zemon V., Kaplan E., Ratliff F. Bicuculline enhances a negative component and diminishes a positive component of the visual evoked cortical potential in the cat. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7476–7478. doi: 10.1073/pnas.77.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]