Abstract
Rationale
Love has long been referred to as an addiction in literature and poetry. Scientists have often made comparisons between social attachment processes and drug addiction, and it has been suggested that the two may share a common neurobiological mechanism. Brain systems that evolved to govern attachments between parents and children, and between monogamous partners, may be the targets of drugs of abuse and serve as the basis for addiction processes.
Objectives
Here, we review research on drug addiction in parallel with research on social attachments, including parent-offspring attachments and social bonds between mating partners. This review focuses on the brain regions and neurochemicals with the greatest overlap between addiction and attachment, and in particular the mesolimbic dopamine pathway.
Results
Significant overlap exists between these two behavioral processes. In addition to conceptual overlap in symptomatology, there is a strong commonality between the two domains regarding the roles and sites of action of dopamine, opioids, and corticotrophin-releasing factor (CRF). The neuropeptides oxytocin and vasopressin are hypothesized to integrate social information into attachment processes that is not present in drug addiction.
Conclusions
Social attachment may be understood as a behavioral addiction, whereby the subject becomes addicted to another individual and the cues that predict social reward. Understandings from both fields may enlighten future research on addiction and attachment processes.
Keywords: social attachment, loved, addiction, substance dependence, dopamine, opioids, CRF, oxytocin, vasopressin, pair bond
INTRODUCTION
At first, each encounter was accompanied by a rush of euphoria – new experiences, new pleasures, each more exciting than the last. Every detail became associated with those intense feelings: places, times, objects, faces. Other interests suddenly became less important, as more time was spent pursuing the next joyful encounter. Gradually, the euphoria during these encounters waned, replaced imperceptibly by feelings of contentment, calm, and happiness. The moments between encounters seemed to grow longer, even as they stayed the same; and separation came to be filled with painful longing and desire. When everything was brought to an abrupt end, desperation and grief followed, leading slowly into depression.
Is this story describing falling in love, or becoming addicted to a drug? Love is often described as an addiction, a subtle and poetic metaphor that contains seeds of truth. When we are in love, we are inundated with sensations of our beloved: the face, the eyes, the sound of the voice, the smell of cologne or perfume, and the feel of the skin. These sensations are coupled with powerful experiences of social and sexual reward, and this conditioning leads them to adopt a strong positive valence. The pleasurable memories we form drive us to seek out more experiences with the beloved, and eventually, to be willing to perform incredible acts of romance and self-sacrifice.
Like those who fall in love, those who are exposed to drugs of abuse also experience powerful feelings of reward and euphoria that lead to reinforcement of drug-taking behavior. This reinforcement drives drug users to seek out more experiences with drugs, which can lead to strong addictions. Addicts are also willing to sacrifice in order to obtain and consume drugs; however, those exact same self-sacrificing behaviors that we see as romantic and laudable in the context of parental or romantic love, are seen as dangerous and self-destructive in the context of drug addiction.
These two behaviors share more than just psychological similarities. A deep and systematic concordance exists between the brain regions and neurochemicals involved in both addiction and social attachment. In this review we will address the hypothesis that love is a behavioral addiction. To do so, we will discuss the concordance and discordance between addiction and social attachment in psychiatry and in neurobiology, with a focus on those brain regions and neurochemical systems with the greatest overlap between the two. This includes primarily dopamine (DA), opioids, CRF, oxytocin (OT), and arginine vasopressin (AVP) within the mesolimbic DA pathway, a series of brain regions that govern many aspects of reward, reinforcement, and attachment. We will consider research performed largely in animal models, and in particular, extensive research conducted over decades on drug self-administration and substance dependence in rodents. Animal research will be presented alongside parallel findings in humans that demonstrate the conserved role of each neurochemical system discussed in governing human behavior.
In exploring attachment, we will discuss several animal models of social attachment that have been developed, including maternal attachment in sheep and other mammals, and the formation of selective monogamous pair bonds between mating partners in the prairie vole. Our extrapolation from animal studies of maternal attachment and pair bonding to human love relies on the supposition that these behaviors are the evolutionary antecedents of human social attachments, which we refer to as love. While this may be debated, it is likely that these behaviors in humans and animals share common underlying neurobiological mechanisms.
WHAT IS ADDICTION?
Addiction in humans
On June 18, 2009, then-Governor of South Carolina Mark Sanford, after having been dealt a major legislative loss, disappeared for 6 days (Brown and Dewan 2009). Rumors of his whereabouts abounded during this time, and the Lieutenant Governor admitted that neither he nor the governor’s family knew where the governor was. Finally, on June 24, Mark Sanford gave a rambling press conference where he admitted to having been in Argentina, having an affair with a woman named Maria.
Over the following week, the complete story slowly came out (Rutenberg 2009). He began the affair with Maria one year before, during which time he had made several secret trips to meet her. His wife had found out 5 months before, and forbid him to see her; but despite the risks to his family, his career, and multiple failed attempts to break off their relationship, he persisted in seeking her out. He was finally caught when he seemed to lose track of the amount of time he was spending with her. Mark Sanford later described the events as “a love story; a forbidden one, a tragic one.”
This story illustrates a subtle metaphor that is often used for love: that it is an addiction. Indeed, Mark Sanford seems to have displayed many characteristic addictive behaviors: stress-induced relapse, lack of regard for consequences, being unable to quit, and losing track of time. Addiction can be an incredibly powerful drive, seeming to rob individuals of their ability to make rational choices about the personal risks and rewards of their own behavior. Love, it seems, can do the same.
The DSM-IV TR defines addiction as “a maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following [criteria], occurring at any time in the same 12-month period” (American Psychiatric Association 2000). The seven DSM-IV criteria for addiction are listed in Table 1. This definition focuses entirely on drugs of abuse. Nonetheless, there is growing interest in the classification of some behavioral disorders as addictive, and commonalities between compulsive disorders and substance addiction have been identified in terms of symptomatology, neurochemistry, and adaptations in brain function (Shaffer 1999; Holden 2001; Potenza 2006; Leeman and Potenza 2012). These issues are being addressed in the development of DSM-V, where it is proposed to place compulsive gambling and internet addiction in the same category as substance addiction (American Psychiatric Association 2012).
Table 1.
DSM-IV criteria and other characteristics of substance dependence as compared to attachment.
| Substance Dependence Criteria | Analog to Social Attachment |
|---|---|
| Great deal of time spent in activities necessary to obtain, use, or recover from use |
Dating; parenting |
| Substance is taken in larger amounts or over a longer period than intended |
Sensation of “time flying” when with the partner |
| Important social, occupational, or recreational activities are given up or reduced |
Loss of time with friends |
| Tolerance | Transition from early euphoria to contentment |
| Withdrawal | Grief (from loss); separation anxiety when apart |
| Unsuccessful efforts to cut down or control use | Sensation of not being able to stay away from the partner; failed attempt(s) to break up |
| Continued use despite knowledge of a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by use |
Physically or emotionally abusive relationships; staying with someone who “isn’t right for you” |
| Related Behaviors | |
| Stress-induced reinstatement | Consolation-seeking |
| Dependence-induced increase in drug consumption |
Increase in time spent with the romantic partner as the relationship grows |
| Withdrawal-induced anhedonia and depression | Anhedonia and depression induced by loss or separation |
Romantic attachment is rarely thought to be a pathological disorder (but see Plato and Rowe 1986; Bédier and Belloc 2004). However, when the diagnostic criteria for substance dependence are looked at side-by-side with related phenomena observable in normal human relationships, striking parallels emerge. For instance, the DSM-IV TR defines “tolerance” as “either of the following: a need for markedly increased amounts of the substance to achieve intoxication or desired effect; or markedly diminished effect with continued use of the same amount of the substance.” In substance abuse, this manifests in increasing usage of the drug as the euphoric “high” slowly wanes and is replaced by the relief of negative affect. In romantic relationships, escalation of “dosage” has a natural upper limit, and therefore the primary mechanism of tolerance is through the gradual transition from the initial euphoric passion of an early relationship to later feelings of contentment and the relief from separation anxiety. Virtually everyone has experienced the significant dedication of time and resources necessary for dating; the sensation of losing track of time when with someone special; and the tradeoff of time with friends, family, or other activities and responsibilities that comes with a new romantic pursuit. Most people have also felt the acute pain that comes with the loss of a loved one, or with the end of a romantic relationship. The strong overlap between characteristics of substance dependence and characteristics of social attachment suggests that attachment behavior may draw on the same psychological constructs, and perhaps the same biological substrates, as dependence on drugs of abuse.
Addiction in animal research
While complete animal models of addiction are considered by some to be impossible (but see Wolffgramm and Heyne 1995; Spanagel and Holter 1999), a wide range of paradigms have been developed that model individual features of addiction behavior (Sanchis-Segura and Spanagel 2006). Many of these paradigms are referenced in this review, and we will discuss some of them briefly here.
Addiction as a behavioral phenomenon is complex and has several components or phases, including drug consumption, reinforcement learning, drug seeking, relapse, tolerance, and withdrawal (Sanchis-Segura and Spanagel 2006). The study of drug consumption or “drug-taking” behavior is almost exclusively performed using self-administration paradigms, which can be either operant (where some behavioral response leads to automatic delivery of a drug) or non-operant (as with oral consumption). This is related (but not equivalent) to the positive reinforcement provided by consumption of the drug, which is measured using a broad range of tests, including conditioned place preference; conditioned approach; and, more recently, drug-induced memory enhancement.
Drug-seeking behavior refers generally to any behavior an animal is willing to perform in order to acquire or obtain access to a drug; while this is complicated to separate from drug-taking, several paradigms exist to do this, and the most common is reinstatement of drug-seeking after extinction (de Wit and Stewart 1981). Reinstatement can occur as a result of stress, cues that predict the drug, or a dose of the drug itself, and is widely considered to be a valid model for drug-seeking and for relapse in general. More recently, paradigms have been developed to model compulsive drug-seeking despite negative consequences, which pair drug delivery with various aversive stimuli such as electric shock (Deroche-Gamonet et al 2004), bitter taste (Wolffgramm and Heyne 1995), or fear-conditioned cues (Vanderschuren and Everitt 2004). Other paradigms for testing drug-seeking include second-order self-administration, where subjects must perform a behavior in order to gain access to a device that delivers the drug (Everitt and Robbins 2000; Schindler et al 2002); or dependence-induced increases in self-administration (Rimondini et al 2002).
Tolerance refers to both physiological and behavioral adaptations that reduce the responses to drugs of abuse over the course of repeated exposure, and numerous tests exist to measure tolerance on a wide range of variables (Miller et al 1987). Conversely, withdrawal (or physical dependence) refers to maladaptations to prolonged drug use that result in negative affect or aversive responses during abstinence. It is important to note, however, that while many studies using tolerance and withdrawal as measures are cited in this review, not all researchers agree that tolerance and withdrawal represent addiction-related phenomena, despite their presence in the DSM-IV criteria for substance abuse in humans (Miller et al 1987; Volkow and Li 2005). Thus, the reader should exercise caution in interpreting the expression of tolerance or withdrawal in animals as indicative of addiction. (For a complete review of behavioral models relevant to addiction, see Sanchis-Segura and Spanagel 2006.)
DOPAMINE
DA has five different receptors in two classes. The DA D1-like receptors (including D1R and D5R) are excitatory and have a low affinity for DA, meaning they respond primarily to high DA concentrations occurring during phasic firing of dopaminergic neurons (Sibley et al 1993; Missale et al 1998; Dreyer et al 2010). The DA D2-like receptors (including D2R, D3R, and D4R) are inhibitory and have a high affinity for DA, allowing them to respond to low DA concentrations present during tonic firing. These two classes of receptors are generally expressed on separate neurons, and in the striatum, these neurons are associated with different output pathways.
Dopamine in Addiction
DA has a well-validated role in addiction processes. All known drugs of abuse cause DA release in the nucleus accumbens (NAC), preferentially in the nucleus accumbens shell region (NACs), and also more broadly throughout the mesolimbic DA pathway (Swanson 1982; Di Chiara and Imperato 1988; Koob and Bloom 1988; Pontieri et al 1995; Pontieri et al 1996; Tanda et al 1997; for a review, see Di Chiara et al 2004). DA release in the NAC is particularly important, and is correlated with human subjective ratings of drug reward and drug craving for many drugs of abuse (Volkow et al 1996a; Drevets et al 2001). However, the role of the mesolimbic DA pathway goes far beyond drug addiction – animal studies have shown that DA is released in this pathway in response to a wide variety of rewards, including sex, food, water, and intracranial self-stimulation (Cooper and Breese 1975; Hernandez and Hoebel 1988; Damsma et al 1992; Yoshida et al 1992; Young et al 1992). Though theories abound, the mesolimbic DA pathway is generally thought to be involved in both the motivation to act or work for rewards, and the salience of incentives (Mogenson 1987; Blackburn et al 1992; Ikemoto and Panksepp 1999; for a complete discussion of the many competing theories regarding the role of the mesolimbic DA pathway, see Wise 2004; Berridge 2007). These observations provide evidence for the theory that drugs of abuse activate brain systems that evolved to process natural motivation and the salience of reward-related cues (Kelley and Berridge 2002). Recent experiments also show that acute stressors are encoded by DA-releasing neurons in the VTA, cause DA sensitization in the NAC, and facilitate drug taking; demonstrating that mesolimbic DA release also encodes negative motivational states and stress responses (Miczek et al 2011; Wang and Tsien 2011).
These two classes of DA receptor also seem to have distinct roles in the response to drugs of abuse. Under normal conditions, a synergistic balance of D1R- and D2R-like activation exists in striatal regions (Walters et al 1987; LaHoste et al 1993; Gerfen et al 1995; Wise et al 1996; Hu and White 1997). Cocaine primarily activates D1R-containing neurons, which are necessary for reward-related learning and maintenance of reward-related behaviors (Nakajima 1986; Nakajima and Mckenzie 1986; Beninger et al 1987; Bertran-Gonzalez et al 2008). Chronic exposure to cocaine is associated with an increase in phasic D1R signaling, and these increases are involved in reward prediction, sensitized responses to reward, and in dampening further reinforcing effects of drugs of abuse (Ljungberg et al 1992; Henry and White 1995; Self et al 1996; Anderson et al 2008; Bertran-Gonzalez et al 2008; Zweifel et al 2009). Nonetheless, rodents will not self-administer selective D1R agonists, which, by themselves, induce conditioned place aversion (Woolverton et al 1984; Hoffman and Beninger 1988). Furthermore, if cocaine is administered with D2R-like antagonists, it loses its reinforcing effects (Woolverton and Virus 1989; Bachtell et al 2005; Claytor et al 2006; Peng et al 2009).
D2R is also necessary for reward and incentive learning, as well as maintenance of reward- and incentive-related responding, and rats will readily self-administer drugs that activate these receptors (Woolverton et al 1984; Sanger 1986; Woolverton 1986; Hoffman et al 1988; for an early review of the roles of D1R and D2R in reward and reinforcement, see Beninger et al 1989). The same chronic cocaine exposure is associated with decreases in tonic D2R signaling, and these decreases are involved in drug withdrawal, compulsive intake, and reinstatement of drug-seeking behavior (Nestler et al 1990; Self et al 1996; Perez et al 2011; Grieder et al 2012). PET studies show that D2R availability is decreased after long-term exposure to many different drugs of abuse (Volkow et al 1996b; Volkow et al 1997; Wang et al 1997a; Volkow et al 2001; Fehr et al 2008; reviewed in Volkow et al 2009). This same reduction in D2R is also seen in obesity, internet addiction, and trait impulsivity (Wang et al 2001; Buckholtz et al 2010; Kim et al 2011). This body of evidence suggests that a disruption in the balance of D1R- and D2R-like pathways in favor of D1R may underlie the behavioral changes seen in addiction and other impulse control disorders. Nonetheless, recent studies have shown that rats exposed to a wide range of drugs of abuse have large increases in the density of a high-affinity form of D2R, even when overall D2R availability is decreased (Seeman et al 2004; Seeman et al 2007; Briand et al 2008; Novak et al 2010), and so the functional consequence of these plastic changes in D2R remain incompletely understood. (For a review of drug-induced changes in dopamine systems, see Anderson and Pierce 2005; for a general review of addiction circuitry, see Volkow et al 2012.)
Dopamine in maternal behavior
All mammalian species exhibit maternal care of the young, and various animal models have been established to study maternal behavior and maternal attachment. Historically, rats and mice are the most widely studied model animals for maternal behavior. Most rodents are not spontaneously maternal, and naïve females will often attack or ignore pups (Wiesner and Sheard 1933). Shortly before giving birth, a strong change in motivational state occurs and females become highly interested in pups, displaying pup retrieval, licking/grooming, arched-back nursing, nesting, and maternal defense as archetypical maternal behaviors. Like humans, rodents are highly motivated to care for and protect offspring. Rat mothers will press a lever repeatedly to gain access to pups, and will cross an electrified grid in order to retrieve them (Nissen 1930; Wilsoncroft 1969). Rat mothers even prefer young pups to cocaine, indicating the power of their motivation toward maternal care (Mattson et al 2001). Nonetheless, rats and mice do not appear to be selective in their maternal care, as experienced mothers will direct maternal behavior toward any pups they encounter (Wiesner and Sheard 1933).
DA in the mesolimbic pathway also has a role in maternal behavior. DA is released naturally in the NAC of maternal rats during interactions with pups (Hansen et al 1993). This DA release in the NAC, and the subsequent activation of D1R, is both necessary for the normal expression of maternal behavior and sufficient to induce maternal behavior under conditions where it would otherwise not occur (Gaffori and Le Moal 1979; Hansen et al 1991a; Hansen et al 1991b; Keer and Stern 1999; Numan et al 2005; Stolzenberg et al 2007; Stolzenberg et al 2010; for a review, see Stolzenberg and Numan 2011). D2R also seems to be necessary for maternal behavior, though the site of action has not been determined (Silva et al 2001). Similarly, lesions that disrupt the release of DA from the VTA into the NAC, or simultaneous antagonism of D1R and D2R in the NAC, all disrupt motivated maternal behaviors such as retrieval without disrupting passive maternal behaviors such as nursing (Gaffori and Le Moal 1979; Hansen et al 1991a; Hansen et al 1991b; Keer and Stern 1999). This suggests that, similar to drugs of abuse, one role of the mesolimbic DA pathway and of accumbal DA in particular is to generate the powerful motivational states that produce such dramatic maternal behaviors (reviewed in Numan 2007; for a comprehensive treatment of the subject, see Numan and Insel 2003).
Dopamine in pair bonding
Adult attachments between mating partners are relatively rare in mammals, occurring in only 3-5% of mammalian species (Orians 1969; Kleiman 1977). This is in strong contrast to birds, where up to 90% of species are monogamous. This has been attributed, in part, to the fact that in birds, both parents can contribute equally to care and feeding of the young; while in mammals, lactation in the mother is the primary source of nutrition. Therefore, these attachments between mating partners in mammals are advantageous primarily only in harsh environments where low food availability and high predation rates make biparental care more beneficial to survival of the young. Nonetheless, monogamy has evolved independently in multiple orders of the class Mammalia, suggesting that the evolutionary precursor for monogamous bonding is present in most if not all mammals; and some have proposed that maternal attachment is that precursor (Getz and Hofmann 1986; Ross and Young 2009; for a review of the evolution of monogamy, see Freeman and Young, in press).
Because of the relative rarity of monogamy in mammals, research on these adult attachments, referred to as “pair bonds,” has focused on a very small number of species, with the preponderance of the research being performed in prairie voles (Microtus ochrogaster). Prairie voles are socially monogamous rodents indigenous to most of the Midwest United States and Canada (Tamarin 1985). In this species, mating partners form highly selective pair bonds, share a nest, coordinate care of offspring, and display high levels of affiliative behavior toward each other and their young (Thomas and Birney 1979; Getz et al 1981; Ahern et al 2011). They are also spontaneously parental, with offspring often assisting in the care of siblings (Carter and Roberts 1997). Pair bonding in prairie voles is operationally defined based on two observable behaviors: preference for the partner over the stranger in a choice test (or “partner preference”); and intense aggression toward prairie voles other than the mate (or “selective aggression”) (Williams et al 1992; Winslow et al 1993; Insel et al 1995; Wang et al 1997b; Ahern et al 2009). These two observable behavioral measures describe different dimensions of the pair bond: partner preference measures the motivational force bringing the partners together, while selective aggression measures mate-guarding and the rejection of new, potential partners (Carter et al 1995). Although mating is not required for pair bonding, prairie voles will reliably form a pair bond with a partner after 24 hours of mating, but not after 6 hours of cohabitation where mating is prevented (Wang et al 1999). These two conditions have been used extensively in the prairie vole to test pharmacological and neurological manipulations given only during the cohabitation period that either prevent or enhance the subsequent formation of pair bonds (for an early review see Carter et al 1995; more recently see Young and Wang 2004; McGraw and Young 2010).
Like with drugs of abuse, mesolimbic DA is a major contributor to the formation of pair bonds in prairie voles, and particularly in the NAC shell region. Mating has been shown to cause DA release in the NAC in rodents (Damsma et al 1992; Gingrich et al 2000). In prairie voles, pair bonding between mating partners is prevented if DA receptors in the NAC shell are non-specifically blocked (Wang et al 1999; Aragona et al 2003). Furthermore, non-specific activation of DA receptors in the NAC shell is sufficient to induce pair bonding, even if no mating occurs.
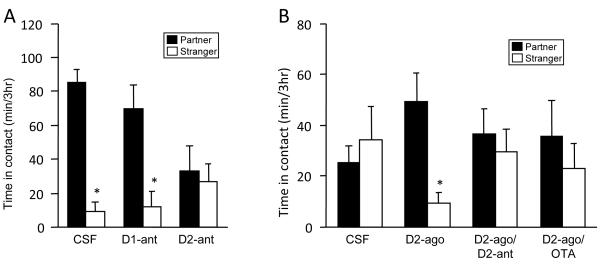
However, despite these non-specific effects, the two DA receptor types seem to play opposite roles in pair bonding (Fig. 1). Mating-induced pair bonding is prevented by selective blockade of D2R in the NAC shell, while activation of these receptors induces bonding in the absence of mating (Wang et al 1999; Gingrich et al 2000; Aragona et al 2006). Conversely, pharmacological activation of D1R prevents pair bond formation, with or without concurrent activation of D2R (Aragona et al 2006). Blockade of D1R neither enhances nor prevents pair bonding (Wang et al 1999; Aragona et al 2006; Curtis et al 2006). Furthermore, the mixed D1R and D2R agonist apomorphine enhances pair bonding when injected into the NAC shell in low doses, where it would be expected to bind preferentially to D2R; but fails to enhance pair bonding at high doses where it would be expected to bind to D1R as well (Aragona et al 2003). Finally, amphetamine injected directly into the NAC shell creates a 20-fold increase in local extracellular dopamine, which enhances pair bonding only if a D1R antagonist is also given (Curtis and Wang 2007). These results suggest that D2R activation in the NAC shell enhances, while D1R activation inhibits, pair bond formation. (For a review see Aragona and Wang 2009.) These data are consistent with human genetic association studies showing links between attachment style and polymorphisms in DA-related genes (Lakatos et al 2000 [but see Bakermans-Kranenburg and van Ijzendoorn 2007]; Gillath et al 2008; Luijk et al 2011).
Fig. 1.
The role of DA in pair bonding. (A) Prairie voles that mate with a partner during a 24-hour cohabitation will spend more time with the partner rather than a stranger during a subsequent partner preference test, which is the operational definition of a pair bond. Prairie voles injected with either saline or a DA D1R antagonist prior to this preference test form a pair bond normally, while injection of a D2R antagonist prior to cohabitation prevents pair bonding. These antagonist effects were shown in subsequent experiments to occur in the NAC shell. (B) Prairie voles that spend 6 hours with a partner without mating do not form a pair bond with the partner. Microinjection of a D2R agonist induces the formation of a pair bond during this 6-hour cohabitation, an effect that is blocked by a D2R antagonist. An OT antagonist also blocks agonist-induced pair bonding, suggesting that concurrent activation of D2R and OTR is necessary for pair bonding. Figure adapted from Young and Wang 2004.
There is strong overlap between these findings and the literature on drug addiction. In the context of pair bonding, the aversion induced by pure pharmacological D1R activation may associate with social cues, leading to a “conditioned partner aversion” that could explain the disruption of pair bonding. Selective activation of D1R also disrupts several kinds of reward and incentive learning (Beninger et al 1989) and therefore may disrupt the learning of associations between the social reward provided by the partner, and the partner’s specific identity cues. Furthermore, cocaine seeking behavior is inhibited by pharmacological D1R activation (Self et al 1996), suggesting that this experimental activation of D1R may reduce the drive to seek out rewards. Meanwhile, activation of D2R in the NAC shell is rewarding and enhances reward-based learning (Beninger et al 1989), which aligns well with the role of D2R in pair bonding.
DA also plays a prominent role in pair bond maintenance. Sexually naïve male prairie voles are highly social and show very little aggressive behavior toward novel conspecifics (Insel et al 1995). However, when males have cohabitated with females for two weeks, they develop intense selective aggression toward male and female strangers and not toward their female partners (Insel et al 1995; Wang et al 1997b). Since this selective aggression serves, in part, to reject potential new partners, it represents an ongoing mechanism of maintenance of the pair bond (Carter et al 1995).
Over the course of 2 weeks of cohabitation, while selective aggression behavior is forming, the mesolimbic DA system in male prairie voles undergoes plastic changes (Aragona et al 2006). Expression of D1R in the NAC is 60% higher in males that cohabitate with a female than it is in males that cohabitate with a same-sex sibling; while D1R in dorsal striatum, and D2R in both areas, remains unchanged. When D1R is blocked with antagonist in the NAC shell of pair-bonded males, selective aggression toward an unfamiliar female is greatly reduced and affiliative behaviors are increased (Aragona et al 2006). These data suggest that the plastic change in D1R that occurs during pair bonding is causative in the development of selective aggression behavior, which helps to prevent the formation of a new pair bond with a new potential mate. (These topics are reviewed in Aragona and Wang 2009.) This presents an interesting contrast with literature on other types of aggression in rodents, where both D1R and D2R in the NAC play a role in aggressive behavior and in the motivation to aggress (Tidey and Miczek 1992a; Tidey and Miczek 1992b; Rodriguez-Arias et al 1998; Couppis and Kennedy 2008; reviewed in Siegel et al 1999, Miczek et al 2002); and to the treatment of aggression in schizophrenia and other psychiatric disorders in humans, where D2 antagonists have long been used despite the nonspecific effects on behavior in general (Yudofsky et al 1987; Buckley 1999).
There is an exceptionally strong parallel between these plastic changes from pair bonding, and the plastic changes seen in drug addiction. As D1R is upregulated during pair bonding and D2R is stable, this plastic change represents an alteration in the balance of D1R/D2R signaling in the striatum in favor of D1R, similar to what is seen in human PET studies of drug addiction (Volkow et al 2009). More directly, administering amphetamines to prairie voles daily for 3 days causes an upregulation of D1R in the NAC that mimics the upregulation seen in pair bonding (Liu et al 2010). Sexually naïve male prairie voles that receive this treatment reject potential female partners and fail to bond with them, exactly as is observed in pair-bonded males (Liu et al 2010). These same males are able to form a pair bond if D1R in the NAC shell is blocked with an antagonist, a manipulation that prevents the expression of selective aggression in drug-naïve, pair bonded males (Aragona et al 2006; Liu et al 2010). Furthermore, the changes in D1R signaling that occur during pair bonding act directly to decrease the reinforcing effects of amphetamines (Liu et al 2011). Taken together, these studies demonstrate a mechanism of cross-tolerance between pair bonding and amphetamines, which provides strong evidence that the mechanisms that govern both maintenance of social bonds (via selective aggression) and addiction to drugs of abuse (such as amphetamine) are anatomically and functionally overlapping (for a review of drugs of abuse and social behavior, see Young et al 2011b; for a comprehensive review of this section, see Young et al 2011a).
OPIOIDS
The endogenous opiate system is well-known to modulate, in part, the rewarding and reinforcing effects of food, water, sex, intracranial self-stimulation, and other rewards (Broekkamp and Phillips 1979; Turkish and Cooper 1983; West et al 1983; Agmo and Berenfeld 1990; Yeomans and Gray 1996; Gerrits et al 2003). This system is comprised of three types of opioid receptors: μ (MOR), δ (DOR), and κ (KOR); and their endogenous ligands, endorphin, enkephalin and dynorphin (Hughes et al 1975; Birdsall and Hulme 1976; Goldstein et al 1979; Evans et al 1992; Kieffer et al 1992; Chen et al 1993; Yasuda et al 1993). All three receptor types are GI protein-coupled receptors that are inhibitory when activated (Burns et al 1983; Tsunoo et al 1986; North et al 1987; for a review see Knapp et al 1995). Nonetheless, the three receptor types have different behavioral domains, with MOR and DOR mediating generally positive motivation and affect, while KOR mediates generally negative motivation and aversion (Pfeiffer et al 1986; Shippenberg et al 1987; Pecina and Berridge 2000; McLaughlin et al 2003; for reviews see Van Ree et al 2000; Le Merrer et al 2009). These three receptor types have distinct patterns of expression throughout the brain, and these patterns have significant inter-species variability (Khachaturian et al 1985; Robson et al 1985; Mansour et al 1987; Mansour et al 1988; Mansour et al 1994a; Mansour et al 1994b; Curran and Watson 1995; Resendez et al 2012).
Opioids in addiction
While opiate drugs are themselves strongly addictive, the role of the endogenous opioid system in addiction is not limited to these, as this system plays a strong role in the development of addiction to every class of drug (Altshuler et al 1980; Karras and Kane 1980; De Vry et al 1989; Kuzmin et al 1997; Ismayilova and Shoaib 2010; for a review see van Ree et al, 1999). Opioids are involved in every stage of the addiction process, including initiation, maintenance, withdrawal, and relapse (Gerrits et al 2003). Opioids also interact with other addiction-related neurochemical systems, including DA and glutamate, though these interactions do not entirely explain the role of opioids in addiction (Dichiara and Imperato 1988; Johnson and North 1992; Scavone et al 2011).
Based on animal studies, the role of opioids in the positive reinforcing effects of natural rewards and drugs of abuse has largely been attributed to MOR in the NAC, VP, and VTA, though some part is also played by DOR (van Ree and de Wied 1980; Shippenberg et al 1987; Hubner and Koob 1990; Olmstead and Franklin 1997a; Corrigall et al 2000; Pecina and Berridge 2000; Van Ree et al 2000; Gerrits et al 2003; Smith and Berridge 2005). Activation of MOR in the VTA, and DOR in the NAC, is directly rewarding in rats, producing conditioned place preferences (Goeders et al 1984; Olmstead and Franklin 1997b). Similarly, the rewarding or reinforcing value of many drugs of abuse can be blocked by MOR antagonists injected into the NAC, the VP, or the VTA (Hiroi and White 1993; Skoubis and Maidment 2003; Soderman and Unterwald 2008). Mice that lack the MOR gene do not develop place preferences or withdrawal symptoms in response to morphine (Matthes et al 1996). (For a complete review of this topic, see Le Merrer et al 2009.)
Human studies have largely corroborated the link between MOR and positive reinforcement from natural rewards and drugs of abuse. Opioid antagonists effectively reverse the effects of opiate drugs in humans (Bradberry and Raebel 1981). Furthermore, opioid antagonists are known to reduce cravings, and are now being used to treat an increasing variety of disorders including alcoholism, opioid dependence, and obesity (O’Malley et al 1992; Volpicelli et al 1992; Greenway et al 2010; Minozzi et al 2011). This link is also verified by human studies on a genetic variant of the MOR gene, A118G. The A118G variant has enhanced binding and signaling properties, but low expression of mRNA (Zhang et al 2005; Kroslak et al 2007).
Human subjects possessing this variant of the MOR gene have altered reinforcement learning and increased risk for alcohol dependence (Lee et al 2011; Koller et al 2012). Rhesus macaques also have a functionally similar variant of the MOR gene, C77G, which also results in increased alcohol intake (Barr et al 2007). Interestingly, both human A118G subjects and Rhesus C77G subjects are more likely to respond positively to opioid antagonist therapy for alcoholism, which suggests that the differences in receptor properties may be a direct physiological mechanism for the increased risk for alcoholism (Oslin et al 2003).
KOR is also involved in addiction-related processes. Drugs of abuse cause dynorphin release and up-regulation of dynorphin in the dorsal and ventral striatum, which inhibits DA release by acting on KOR receptors and is therefore thought to be compensatory (Hanson et al 1988; Sivam 1989; Spanagel et al 1990; Hurd et al 1992; Daunais et al 1993; El Daly et al 2000; Isola et al 2009). During withdrawal, this up-regulation and subsequent activation of KOR may contribute to negative affect and thus promote relapse (Przewlocka et al 1997; Simonin et al 1998; Walker and Koob 2008). As such, KOR may be a mechanism for maintenance of drug use (for a review of KOR in addiction, see Bruijnzeel 2009).
Opioids in maternal behavior
Opioids have also been implicated in the neurobiology of social reward in animals, largely by early work by Jaak Panksepp. In puppies, guinea pigs, and chicks, separation from the mother is highly stressful, and the offspring use separation-induced distress vocalizations to call to the mother. Distress vocalizations are effectively blocked by low, non-sedative doses of opioid agonists, and, in most cases, induced by opioid antagonists (Panksepp et al 1978; Warnick et al 2005). Similarly, social solicitation for attention in puppies is increased with opioid antagonists, although this same treatment reduces the comfort received from subsequent social contact (Panksepp et al 1980). Low-dose morphine also reduces social contact in guinea pigs and rats (Herman and Panksepp 1978; Panksepp et al 1979). These and other observations led to the opioid hypothesis of social attachment, which posits that opioid receptors mediate both social reward and social motivation (Herman and Panksepp 1978). According to this hypothesis, high activation of opioid receptors signals a social reward state, and low activation induces a drive to seek social rewards. Thus, opioid antagonists induce a social drive while simultaneously blocking the rewarding effects of social contact. (For a review of this literature, see Panksepp et al 1980.) This hypothesis has subsequently been supported by work on social motivation in rats and Rhesus macaques (Panksepp et al 1985; Martel et al 1993; Martel et al 1995).
Opioids also mediate many aspects of maternal behavior, and in fact, the opiate system was the first brain system to be implicated in social attachment in animals (Panksepp et al 1978). In rats, guinea pigs, sheep, and Rhesus macaques, acute administration of opioid antagonists increases social need and the solicitation for care by offspring, while agonists decrease solicitation in rats and Rhesus macaques (Panksepp et al 1980; Kalin et al 1988; Martel et al 1993; Panksepp et al 1994; Martel et al 1995; Shayit et al 2003). Studies in Rhesus macaques show similar effects of acute administration of agonists and antagonists on maternal care (Kalin et al 1995). Conversely, long-term exposure to opioid antagonists decreases maternal competence and motivation, suggesting that mothers adapt their behavior based on reduced social reward (Martel et al 1993; Martel et al 1995). Opioid agonists potentiate the formation of mother-offspring bonds and subsequent maternal behavior in sheep, while opioid antagonists prevent both mother-offspring and offspring-mother bonding (Kendrick and Keverne 1989; Keverne and Kendrick 1994; Shayit et al 2003). Some evidence also suggests that these effects may be specific to the MOR, at least with respect to infant-mother attachment. For instance, in chicks, a reduction in DVs was only observed when a MOR-selective agonist was used (Warnick et al 2005). In MOR knockout mice, pups emit fewer DVs in response to maternal separation and show less selectivity for their mothers’ cues (Moles et al 2004). (For a comprehensive discussion of this subject, see Numan and Insel 2003.)
Additionally, the same polymorphisms of the MOR gene that are associated with increased risk for alcoholism in humans and Rhesus macaques are also related to maternal behavior in both species (Barr et al 2007; Barr et al 2008; Higham et al 2011; Troisi et al 2011b). Infant Rhesus macaques possessing the C77G polymorphism of the MOR gene show increased attachment to their mothers, while Rhesus mothers possessing the same polymorphism show enhanced maternal care and higher OT release during maternal behaviors (Barr et al 2008; Higham et al 2011). Humans with the analogous A118G polymorphism are susceptible to developing fearful attachment style in response to low maternal care (Troisi et al 2011b). These studies suggest that the role of MOR in maternal behavior is conserved in humans, and also show a direct overlap between mechanisms that encode for altered attachment style and risk of alcohol dependence. (For a concise overview of these topics, see Curley 2011.)
Opioids in pair bonding
The majority of mammalian species are promiscuous breeders that do not form selective partner preferences toward specific mating partners (Kleiman 1977). Instead, when rats mate, they associate the reward from ejaculation with non-social cues and can form preferences for the location or for non-social odors (Miller and Baum 1987; Mehrara and Baum 1990; Ismail et al 2009). The formation of these non-social preferences is prevented by peripheral administration of non-selective opioid antagonists.
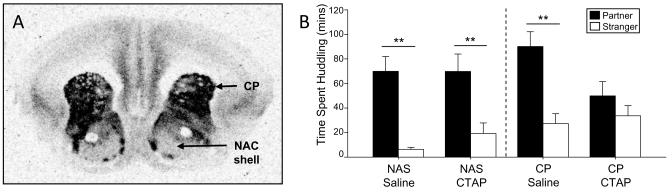
Investigations into the role of the opiate system in the formation and maintenance of pair bonds have only recently been conducted using prairie voles (Fig. 2) (Burkett et al 2011 [see also Furay and Neumaier 2011]; Resendez et al 2012). In this species, a peripherally administered, non-selective opioid antagonist prevents pair bond formation between prairie voles, but also reduces mating (Burkett et al 2011). However, a MOR-selective antagonist administered into the caudate-putamen (CP), but not the NAC shell, prevents pair bonding without affecting sexual behavior. These experiments demonstrate that MOR and the CP are necessary for pair bond formation in this species, and the roles of both seem to be conserved in humans. Individuals possessing the A118G polymorphism of the MOR gene show increased likelihood to engage in affectionate relationships, increased sensitivity to social rejection, and increased brain activity in a rejection task, possibly signaling an altered attachment style (Way et al 2009; Troisi et al 2011a). Additionally, the CP is activated in humans when viewing the faces of loved ones, and this activation is correlated with romantic love and passion scores (Bartels and Zeki 2000; Aron et al 2005; Acevedo et al 2012).
Fig. 2.
MOR and pair bonding. (A) Receptor autoradiography showing ligand binding to MOR in prairie vole brain. MOR density in the NAC shell is moderate, but much lower than MOR density in the CP. (B) After 24 hours of cohabitation with a partner, prairie voles receiving saline to the NAC shell or CP formed pair bonds as normal. MOR antagonist injected into the NAS shell did not affect pair bonding, while MOR antagonist in the CP prevented the formation of a pair bond. These data show that MOR in the CP is necessary for pair bond formation. Figure adapted from Burkett et al 2011.
A second recent study investigated the role of KOR in the maintenance of pair bonds in prairie voles (Resendez et al 2012). The authors first showed that a peripherally administered KOR antagonist, but not a MOR-preferential antagonist, prevents the expression of selective aggression in voles that have already formed pair bonds. Additionally, they localized this effect to the NAC shell, where a KOR antagonist (but not a MOR antagonist) abolished selective aggression.
These pair bonding studies reveal an interesting overlap between the opioid and DA systems. In the striatum, DA D2R is expressed in striatal neurons containing enkephalin, the endogenous ligand for MOR (Gerfen and Young 1988); both of which receptors are involved in pair bond formation but do not have a clear role in maintenance. Conversely, D1R is expressed in striatal neurons containing dynorphin, the endogenous ligand for KOR; and both of these receptors are necessary for pair bond maintenance, but not for formation. This suggests that the two receptor systems are acting in a coordinated fashion in the striatum to modulate different aspects of pair bonding (Resendez et al 2012). Furthermore, it is interesting to note that the relationships between MOR and reward/formation, and between KOR and maintenance, are the same as in drug addiction.
CRF
Corticotropin-releasing factor (CRF), sometimes called corticotropin-releasing hormone, is part of a family of proteins consisting of four endogenous ligands: CRF, urocortin-1, urocortin-2, and urocortin-3; and two receptors, CRF-R1 and CRF-R2 (reviewed in Bale and Vale 2004). This CRF system coordinates stress responding on several levels, including behavior, autonomic response, and the HPA axis. Administration of CRF into the brain of rats reproduces many different behavioral responses analogous to stress, while CRF antagonists reduce or prevent stress responses (Dunn and Berridge 1990; Heinrichs et al 1994; Menzaghi et al 1994). CRF-R1 activation mediates many of these stress effects in rats, while CRF-R2 activation is alternately the same as CRF-R1, opposing CRF-R1, or ineffective in modulating stress, depending on the assay and the brain region (Ho et al 2001; Takahashi et al 2001; Valdez et al 2004; Zhao et al 2007; for a review see Heinrichs and Koob, 2004).
CRF in addiction
In drug addiction, CRF is primarily involved in withdrawal from drugs of abuse (Koob and Kreek 2007; Koob 2008). CRF production in, and release from, the amygdala is greatly potentiated during withdrawal from a variety of drugs of abuse, as well as during chronic stress (Merlo Pich et al 1995; Rodriguez de Fonseca et al 1997; Richter and Weiss 1999; Stout et al 2000; Zorrilla et al 2001; Olive et al 2002; Funk et al 2006; George et al 2007). This CRF release produces a withdrawal-induced anxiety state that is reversed by CRF-R1 antagonists (Sarnyai et al 1995; Tucci et al 2003; Knapp et al 2004; Skelton et al 2007). In turn, increased stress and withdrawal symptoms increase drug craving, generating a powerful motivation to continue use or to relapse from abstinence (Hershon 1977; Cooney et al 1997; Sinha et al 2000).
Nonselective CRF antagonists, as well as CRF-R1 antagonists, selectively block excessive consumption of several drugs of abuse in dependent rats, but not in non-dependent rats (Valdez et al 2004; Funk et al 2006; Funk et al 2007). However, a CRF-R2 agonist also blocked excessive alcohol consumption in dependent rats, suggesting opposite roles for these two receptors (Valdez et al 2004). This research shows that CRF receptors, particularly in the amygdala, are important in mediating the motivational effects of withdrawal from drugs. These data also suggest that the ability of CRF-R1 antagonists to block excessive consumption is related to the ability of these drugs to block the aversive aspects of withdrawal (Koob and Zorrilla 2010).
Using stress-induced reinstatement of drug seeking, it has been demonstrated that stress causes CRF release into the VTA and NAC shell (Wang et al 2005; Chen et al 2012a), and this CRF release acts on CRF receptors to induce reinstatement (Ungless et al 2003; Wang et al 2005; Wang et al 2007; Blacktop et al 2011). Some of the stress-related effects of CRF in the NAC shell may be DA-dependent; recall that DA is also modulated by acute and chronic stressors (Miczek et al 2011; Wang and Tsien 2011; Chen et al 2012b). The sources of CRF-releasing projections into the VTA and NAC shell are currently unknown (Tagliaferro and Morales 2008). However, the research discussed above strongly implicates the extended amygdala as the source of CRF-containing neurons mediating both drug dependence and relapse. (For a complete review of this section, see Koob 2010.)
CRF in pair bonding
Being separated from loved ones for a prolonged period of time can be highly stressful in humans. We become preoccupied with thoughts of the beloved, recalling pleasant memories of when we were together or imagining the moment of reunion. We may become obsessed with ways to bring about a reunion, particularly if the separation is due to the end of a relationship. When this loss is permanent, these thoughts can be persistent and accompanied by powerful, prolonged grief and psychic pain (Prigerson et al 1995; Horowitz et al 1997). This permanent social loss can be traumatic and cause both deterioration of physical health and susceptibility to depression (Prigerson et al 1997; Ott 2003).
The prairie vole has served as an interesting model for examining the neurochemistry of social loss. In the wild, when one member of a mating pair is lost, the surviving member typically will not take on a new partner for the duration of his or her life (Getz et al 1981). Studies have shown that total social isolation in prairie voles leads to depressive-like behavior and an increase in CRF-immunoreactive neurons in the PVN (Grippo et al 2007a; Grippo et al 2007b). Of more direct relevance is the finding that 4 days of separation from a pair-bonded mate leads to increases in passive coping strategies, a type of depressive-like behavior, in male prairie voles, and that this increase does not occur in response to separation from a male sibling (Bosch et al 2009). Specifically, males separated from their partner display robust increases in immobility and hanging in the forced swim test and tail suspension tests, respectively. This depressive-like behavior is reversed by CRF-R1 or CRF-R2 antagonists injected into the cerebral ventricle. The study also showed that pair bonding induces an increase in CRF mRNA in the bed nucleus of the stria terminalis, a part of the extended amygdala; suggesting that this region may be the source of separation-induced CRF release, and is primed for such release during pair bond formation. These experiments provide good evidence for the theory that CRF mediates the symptoms of social loss and depression, and that this circuitry is subverted by drugs of abuse into inducing withdrawal and enhanced consumption in drug dependence. Furthermore, it may be that CRF release during separation from the partner creates an aversive, stressful state that motivates the prairie vole to return to the partner, which in turn maintains the pair bond (Bosch et al 2009).
While it is not known where in the brain CRF acts to mediate depressive-like behaviors due to social loss, literature in other rodents allows us to form a hypothesis. The NAC shell is activated during stress in rats and mice, and this same activation in mice is blocked by CRF-R1 antagonist (Pliakas et al 2001; Kreibich et al 2009). Furthermore, CRF injected into in the NAC shell induces depressive-like behavior in rats, and this effect is reversed by a CRF-R1 antagonist (Chen et al 2012b). CRF in the NAC shell can also enhance the positive motivational value and salience of incentive cues (Pecina et al 2006; Kreibich et al 2009). Taken together, these findings indicate that CRF within the NAC shell may have a role in the stress-induced increase in incentive value of cues previously associated with reward, which may complement or create its more general effect on depression in that nucleus. This suggests that CRF may act in the NAC during social loss to increase the incentive value of partner-related cues, creating a positive incentive to return to the partner. Nonetheless, this hypothesis remains to be tested in future experiments.
CRF is also involved in the formation of pair bonds. Monogamous and non-monogamous species of voles differ greatly in the distribution of CRF-R1 and CRF-R2 in the brain, even while the distribution of CRF peptide is highly conserved (Lim et al 2005; Lim et al 2006). In male prairie voles, forced swim stress or peripheral corticosterone injections both enhance the subsequent formation of pair bonds, while these treatments inhibit pair bonding in females (DeVries et al 1996). Pair bonding in males is also enhanced by activation of CRF receptors in the brain, an effect which can be localized to the NAC shell (DeVries et al 2002; Lim et al 2007). This literature also suggests that the role of CRF in pair bonding is principally focused on the NAC shell. The facilitative effects of acute stressors on male pair bonding are directly analogous to the facilitative effects of acute stressors on drug-taking behavior, a study which, interestingly, was also performed in male rodents (Miczek et al 2011).
NEUROPEPTIDES AND SOCIAL INFORMATION
DA, opioids, and CRF all mediate the processing of different aspects of reward, reinforcement, and motivated behavior. However, since attachments are normally formed to conspecifics and not to food or other natural non-social rewards, it is logical to hypothesize the existence of a mechanism or mechanisms whereby circuits that mediate both attachment and addiction integrate social information. Such mechanisms should be known to process social information, have a demonstrated role in attachment, and interact meaningfully with the reward and salience circuitry discussed previously.
OT and AVP represent two candidate systems. These two paralogous 9-amino-acid peptides are unique to mammals, though homologues exist in a wide variety of vertebrates and invertebrates (Archer 1974; van Kesteren et al 1992). Both OT and AVP are synthesized in the hypothalamus and released from the posterior pituitary into the peripheral circulation (Gainer and Wray 1994; Burbach et al 2005). In addition, both peptides are released in the brain and bind to receptors there to affect social behaviors (Buijs et al 1983; Alonso et al 1986; Loup et al 1991). OT has a single receptor (OTR) both in the periphery and in the brain. AVP has three receptors (V1aR, V1bR, and V2R); V1bR and V2R are expressed primarily in the periphery, and while V1aR and V1bR are present in the brain, V1aR is the principal receptor implicated in social processes (Gainer and Wray 1994; Burbach et al 2005; for a review of the AVP system and behavior see Caldwell et al 2008; for a review of the OT system and behavior see Ross and Young 2009).
OT is released peripherally during labor and helps to evoke uterine contractions (Burbach et al 2005). The release of OT is also induced by vaginocervical stimulation, either from birth or from copulation. OT is also released peripherally in response to nipple stimulation, which, in the context of nursing, induces the letdown of milk from the mammary glands (Christensson et al 1989). AVP is released peripherally in response to an osmotic challenge and acts in the kidney to induce the reabsorption of water, concentrating the urine (Gainer and Wray 1994).
OT in addiction
With respect to drugs of abuse, OT is best known for being released by 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy), and is associated with the prosocial effects of this drug in both humans and rats (Wolff et al 2006; Thompson et al 2007; Dumont et al 2009; Thompson et al 2009). In general, OT appears to play a modulatory role in many aspects of drug addiction. Exogenous OT attenuates many of the immediate behavioral effects of drugs of abuse, including reducing drug consumption, and some of the attenuating effects of OT are localized to the NAC (Kovacs et al 1990; Sarnyai et al 1990; Sarnyai et al 1991; Qi et al 2009; Carson et al 2010a; Baracz et al 2012). This exogenous OT acts in the NAC to reduce both formation and expression of tolerance to some of the behavioral and physiological effects of drugs of abuse (Krivoy et al 1974; Van Ree and De Wied 1977; Kovacs et al 1981; Kovacs and Vanree 1985; Ibragimov et al 1987; Kovacs and Telegdy 1987; Szabo et al 1989; Sarnyai et al 1992). This may be through OT’s modulatory effects on multiple aspects of both normal and drug-induced DA neurotransmission in the NAC (Kovacs et al 1986; Szabo et al 1988; Kovacs et al 1990; Sarnyai et al 1990; Carson et al 2010b). OT also mitigates the withdrawal symptoms of morphine and alcohol, and reduces drug-primed reinstatement of responding for amphetamine (Kovacs et al 1981; Szabo et al 1988; Carson et al 2010a). (For a review of this literature, see Kovacs et al 1998; McGregor and Bowen 2012.)
In many of these studies, the effects of exogenously administered OT and OT agonists were reversed with OT antagonists in the brain, showing the specificity of the results to central OT. Nonetheless, there is a relative lack of studies showing that OT antagonists alone affect these processes (but see Kovacs et al 1987). This suggests the possibility that the baseline activity of the endogenous OT system in these paradigms is not sufficient to affect drug-related behaviors. The overlapping presence of OT receptors in regions responsible for addiction processes may have adapted to integrate a type of stimulus not present in these studies, such as social information.
OT/AVP and social information
Evidence for the involvement of OT and AVP in social information processing in the brain comes from studies on social memory. Mice exposed to a novel intruder will show robust investigation behavior that is decreased over repeated exposures in a short time, which is considered to be a result of social memory (Dantzer et al 1987). OT and AVP facilitate this kind of social memory, while OT and AVP antagonists interfere with it. Furthermore, studies in genetic knock-out (KO) mice demonstrate that these effects are specific to social memory. Mice lacking the OT gene have impaired social memory that can be recovered by OT treatment prior to the initial social encounter, demonstrating that the presence of OT at the time of the salient event is necessary and sufficient for social memory (Ferguson et al 2000; Ferguson et al 2001). Importantly, the OT KO mice display no deficits in spatial memory, non-social olfactory memory, and habituation, and can retain a social memory if the novel intruders are painted with a non-social odor (Ferguson et al 2000; author’s unpublished data). Mice with reduced AVP release in the LS, or that are lacking AVP V1aR entirely, have similarly selective deficits in social memory, and re-introduction of AVP or V1aR respectively in the LS results in rescue of social memory (Bielsky et al 2004; Bielsky et al 2005; Lukas et al 2011). Taken together, these data demonstrate a role for OT and AVP in the selective processing of social aspects of memory (for reviews, see Bielsky and Young 2004; Wacker and Ludwig 2012).
OT/AVP in social attachment
The role of OT in regulating peripheral responses critical to maternal behavior is mirrored in the brain. OT is released centrally and peripherally during birth, and centrally released OT is both necessary for the rapid onset of maternal behavior in virgin rats, and sufficient to induce it (Pedersen and Prange 1979; Fahrbach et al 1985; van Leengoed et al 1987; Borrow and Cameron 2012). Central OT is also sufficient to induce selective mother-offspring bonds in sheep (Kendrick et al 1987). The sites of action of OT in modulating maternal behavior also overlap with the mesolimbic DA pathway. The onset of maternal behavior can be prevented by injection of an OTR antagonist into the VTA (Pedersen et al 1994). Additionally, in prairie voles, the expression of spontaneous maternal behavior in virgin females is organized by developmental (and not adult) levels of OTR in the NAC shell, and is prevented by OTR antagonist injected into this region (Olazabal and Young 2006a; Ross et al 2009b; Keebaugh and Young 2011). Early expression of maternal behavior in virgin rodents is correlated with OTR density in the NAC shell, the lateral septum (LS) and parts of the extended amygdala, all regions implicated in pair bonding and in the regulation of motivated behaviors (Champagne et al 2001; Sheehan et al 2004; Olazabal and Young 2006b; Modi and Young 2011). However, OT does not appear to be necessary for the maintenance of maternal behavior in experienced females (Fahrbach et al 1985). The dual central and peripheral role for OT in the onset of maternal behavior, and particularly in the formation of mother-offspring attachments, suggests that coordinated peripheral and central OT release during labor and nursing act to induce bonding between mother and infant (Ross and Young 2009; Feldman 2012). This coordination may be, in part, through release of OT into the forebrain by axon collaterals from projections from the hypothalamus to the posterior pituitary, which may serve to synchronize central and peripheral release. This has contributed to the theory that the OT release induced by vaginocervical stimulation and nipple stimulation during human sex, acts analogous to labor and nursing, serves to induce bonding between sexual partners (Young et al 2005); and furthermore, that pair bonding is mediated by neural systems that were elaborated in evolution from circuits originally adapted for maternal behavior (Ross et al 2009a).
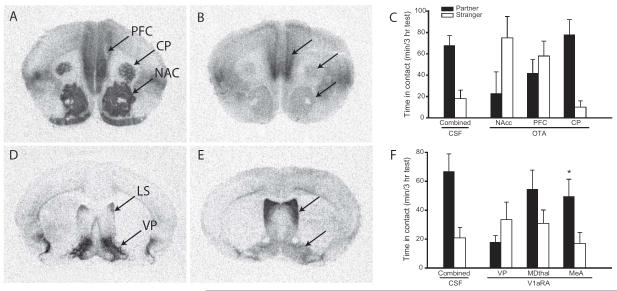
Evidence for this theory is provided by experiments demonstrating the role of OT in pair bonding between prairie voles (Fig. 3a-c). Monogamous prairie voles have significantly greater OTR density in the prefrontal cortex (the cortical component of the mesolimbic DA pathway), as CP, and NAS than do non-monogamous vole species (Insel and Shapiro 1992). OTR activation in the NAC and prefrontal cortex, but not the CP, is necessary for the formation of pair bonds in female prairie voles (Young et al 2001). In addition, OT injected into the NAC is sufficient to induce pair bonds in female prairie voles in the absence of mating, and interacts with DA D2R to do so (Liu and Wang 2003). These experiments show that OT within the mesolimbic DA pathway is essential for pair bonding in female prairie voles.
Fig. 3.
OT and AVP in pair bonding. (A) Monogamous prairie voles have high densities of OTR in the PFC, CP, and NAC. (B) By comparison, non-monogamous montane voles have relatively low densities of OTR in these regions. (C) Infusion of an OTR antagonist into the PFC and NAC of prairie voles, but not the CP, during a 24-hour cohabitation prevents the formation of pair bonds. (D) Male prairie voles have a higher density of AVP V1aR in the VP than do (E) montane voles. Infusion of a V1aR antagonist into the VP, but not into the mediodorsal thalamus (MDThal) or medial amygdala (MeA), of male prairie voles during a 24-hour cohabitation prevents the formation of pair bonds. This suggests that species differences in OTR and V1aR density in these regions may be a direct causal factor in species differences in social attachment. Figure adapted from Young and Wang 2004.
Human studies have shown the role of OT and OTR is largely conserved across species. In humans, a genetic variant of the OTR gene has been shown to correlate with pair bonding behavior in women (Walum et al 2012). OT is released in humans during hugging, touching, massage, nipple stimulation, and orgasm (Carmichael et al 1987; Christensson et al 1989; Turner et al 1999; Light et al 2000; Light et al 2005), and promotes increased eye gaze, trust, and attention to emotional cues (Kosfeld et al 2005; Domes et al 2007; Guastella et al 2008; Andari et al 2010). OT in humans is also higher during early romantic relationships, is correlated with couples’ interactive reciprocity, and predicts which couples will stay together after 6 months (Schneiderman et al 2012). These studies lend support to the hypothesis that OT release during human romantic activities serves to induce the formation of long-term bonds (Young et al 2005; Feldman 2012).
AVP also plays a role in paternal care and pair bonding in male prairie voles (Fig. 3d-f). AVP injected into the LS increases, while V1aR antagonist decreases, paternal behavior in male prairie voles (Wang et al 1994). AVP induces pair bonding in male prairie voles when injected into the LS, and V1aR antagonist in the LS or the ventral pallidum (VP) prevents pair bonding (Wang et al 1994; Lim and Young 2004). Comparative work between prairie and meadow voles has further shown that species differences in the pattern of V1aR expression are responsible for this species-typical male bonding behavior. Meadow voles are a closely related vole species that mates promiscuously without forming bonds between mating partners (Madison 1980). In male meadow voles, V1aR density in the VP is significantly lower than in prairie voles (Insel et al 1994; Young et al 1997). When these receptors are up-regulated in the VP of meadow voles to the “prairie vole-like” phenotype, these animals become capable of forming a partner preference toward a female mate (Lim et al 2004). This demonstrates that a change in the expression of a single gene in evolution can contribute significantly to species differences in highly complex social behaviors (Donaldson and Young 2008). Evidence for conservation of this role in humans was provided by a genetic study showing that males possessing one variant of the AVP V1aR gene were half as likely to be married to their partner, twice as likely to experience major relationship problems, and had partners who reported lower levels of relationship quality (Walum et al 2008).
Taken together, these data demonstrate that the OT and AVP systems have three characteristics necessary for a mechanism that integrates social information into attachment processes. Both AVP and OT are involved in social information processing and are thought to act to enhance the salience of social stimuli. Both peptides have a demonstrated role in modulating the formation of attachments. Finally, both peptides interact directly with the mesolimbic DA pathway to modulate behavior. Therefore, the OT and AVP systems are well positioned to provide social information to circuitry involved in attachment.
THE PARTNER ADDICTION HYPOTHESIS
The convergence of evidence from the fields of attachment and addiction reveals an inexorable series of parallels (see Fig. 4, Table 2). These parallels provide strong evidence for a link between attachment and addiction (MacLean 1990; Nelson and Panksepp 1998; Insel 2003; Fisher 2004; Reynaud et al 2010). In line with these previous authors, we propose that both attachment and addiction processes can be understood in relation to an object of addiction, whether that object is a partner (partner addiction) or a substance (substance addiction). Furthermore, the accumulation of neurochemical data now permits us to elaborate on this theory and propose a specific framework for understanding the roles of the neurochemical systems involved.
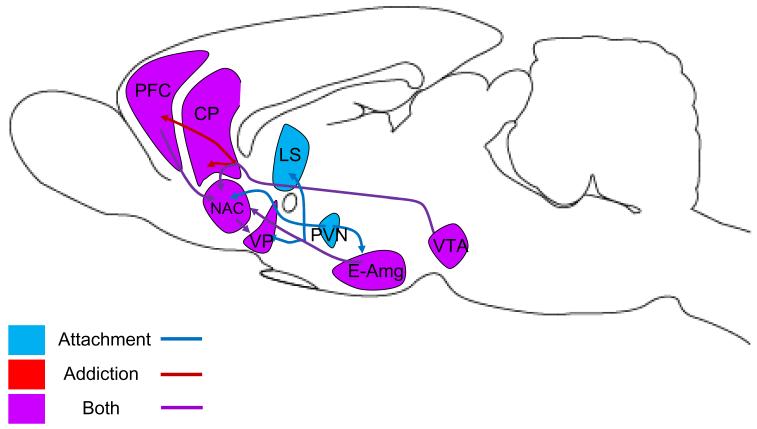
Fig. 4.
Overlapping circuits for attachment and addiction. The VTA sends dopaminergic projections to the NAC, prefrontal cortex (PFC), and CP; these projections are all implicated in addiction, while only projections to NAC are implicated in attachment. The extended amygdala (E-Amg) is the presumptive source of AVP to the VP and LS in attachment, and CRF and glutamate to the NAC in addiction and attachment. The PVN is the source of OT release in the E-Amg and NAC. Glutamatergic projections link the PFC with the NAC, and GABAergic projections link the NAC and VP.
Table 2.
Parallels between neurochemical systems involved in attachment and addiction, including DA, opioids (OP), CRF, OT, and AVP.
| Social Attachment | Maternal Attachment | Drug Addiction | |
|---|---|---|---|
| FORMATION | |||
| DA | Released during mating | Released by drugs of abuse | |
| D1R inhibits bonding | D1R inhibits some aspects of drug reward, is necessary for others |
||
| D2R promotes bonding | D2R promotes drug reward | ||
| OP | Released during mating | Released by drugs of abuse | |
| MOR promotes bonding | OP promotes bonding | MOR, DOR promote drug reward |
|
| CRF | Acute stress, CRF promote male bonding |
Acute stress promotes drug taking |
|
| Acute stress inhibits female bonding |
|||
| OT | Released during mating | Released during birth, nursing |
Released by some drugs |
| OTR promotes female bonding |
OTR promotes maternal bonding |
OTR inhibits drug taking, inhibits tolerance |
|
| OTR promotes onset of maternal behavior |
|||
| AVP | V1aR promotes male bonding |
||
| MAINTENANCE | |||
| DA | Released during maternal care |
||
| D1R promotes maintenance | D1R, D2R promote maintenance |
D1R, D2R promote maintenance |
|
| Plastic changes in striatal D1R promote maintenance |
Plastic changes in striatal D2R promote maintenance |
||
| OP | Acute OP blockade promotes maintenance |
Acute OP blockade promotes maintenance |
|
| Chronic OP blockade inhibits maintenance |
Chronic OP blockade inhibits maintenance |
||
| KOR promotes maintenance | KOR promotes maintenance | ||
| Plastic changes in KOR promote maintenance |
|||
| CRF | CRF promotes maintenance | CRF-R1 promotes maintenance |
|
| CRF-R2 may inhibit maintenance |
|||
| Plastic changes in CRF promote maintenance |
Plastic changes in CRF promote maintenance |
||
| OT | OT is not necessary for maintenance |
OT is not necessary for maintenance |
OTR inhibits maintenance |
In the nascent phase of addiction, large amounts of sensory information are gathered about the object of addiction. In substance addiction, this applies to the sensory modalities appropriate for the drug: the taste and smell; the particular experience unique to the drug; and the context in which the drug is taken. With partner addiction, this information is primarily social: looks, touches, words, scents, the shape of the body and face, and possibly sexual experiences. When these early interactions with the object of addiction produce rewarding outcomes, DA is released in the NAC shell, which acts to increase the salience of incentive cues that predict the reward. Concurrent activation of D1R and D2R may represent a balance of positive and negative behavioral responses – D1R enhancing the positive incentive value of active or aggressive responses, and D2R enhancing the positive incentive value of passive, reward-related, or pro-social responses. In these addictive processes, activation of opioid receptors, and in particular MOR, occurs concurrently with experienced reward, either due to the direct effects of the substance or due to sexual contact with the partner. The opiate system interacts with the DA and OT systems to coordinate a positive response. These neurochemical systems cooperate to create a positive feedback loop where stimuli and responses coincide with reward from DA and opioids, behavior and predictive cues are positively reinforced, and positive associations accumulate.
Unlike drugs of abuse, with partner addiction, every encounter has a strong social component. Social encounters cause OT and AVP release, converging with DA in the mesolimbic DA pathway to increase the salience of social cues and information. This draws the attention of the subject to the sights, sounds, odors, unique behaviors, and other characteristics that identify the specific partner. The OT system, as an evolutionary elaboration of maternal circuitry, may promote nurturing behaviors and the identification of the partner as an object of care. The AVP system, as an evolutionary elaboration of circuitry for aggression and territoriality, may promote protective behaviors and the identification of the partner as an extension of territory (Young and Alexander 2012). Furthermore, OT may act in both types of addiction to mitigate some of the aversive or maladaptive effects of tolerance. The combined effect of these receptor systems in partner addiction is to ensure that social information and social cues become the substrates for the positive reinforcement and conditioning that occurs as a result of DA and opioids.
As the positive feedback loop continues and positive associations accumulate, adaptation occurs within the circuit in both partner and substance addiction that primes the circuitry for maintenance. The balance of DA signaling is altered in favor of D1R, leading to a progressive decrease in reward and an increase in negative affect or aggressive responses. In partner addiction, this has three principal effects. First, the early, euphoric excitement that comes with new relationships subsides, and this euphoria is gradually replaced by a more subdued sense of contentment. This first effect can be understood as tolerance to the addictive partner; tolerance to opioids may contribute to this effect as well. Second, encounters with the partner become more frequent, and the relationship may continue despite negative emotions or consequences; this can be understood as a dependence-induced escalation of consumption of the object of addiction. Opioids may also contribute here to the transition from more reward-oriented behavior toward compulsion. Finally, encounters with new potential mates continue to cause novelty-induced DA release, but now a predominance of D1R signaling promotes rejection, aggressive responses to defend the territory (including the mate), and a decrease in the probability that a second pair bond will form. In substance addiction, analogous physiological adaptations lead to drug tolerance, diminished reward, compulsive and escalating abuse, and the transition from euphoria to the relief of negative affect.
Simultaneously, CRF stress circuitry is primed for maintenance through the up-regulation of CRF peptide in the extended amygdala. This potentiated system is strongly activated during drug withdrawal and separation anxiety. This activation results in a positive motivational state driving the subject toward the object of addiction. In the case of partner addiction, this is referred to as a reunion, which can be understood as a relapse process. Upregulation of dynorphin and subsequent activation of KOR during withdrawal promotes negative affect and drives maintenance behavior. OT released during the withdrawal period may act to mitigate withdrawal symptoms and decrease the probability of relapse, which could explain both consolation-seeking behavior during break-ups, and the strong ability of social support to promote positive outcomes in drug addiction (Wills and Cleary 1996; Measelle et al 2006). When relapse is impossible, either due to loss of the partner or to continued abstinence from drug taking, the persistent anxiety state can result in prolonged negative affect and depressive-like behaviors.
Thus, addiction is created by positive reinforcement and incentive salience from DA; by reward from opioids; and, in the case of partner addiction, by enhanced salience of social cues by OT and AVP. Once the addiction is formed, it is maintained by altered DA signaling and by withdrawal-related changes in CRF and KOR signaling.
CONCLUSION
Human love is the most powerful of all emotions. When we fall in love, we experience an exquisite euphoria, loss of control, loss of time, and a powerful motivation to seek out the partner. Everything about the partner attracts us, drawing us further into an irreversible addiction. The psychology of human love and drug addiction share powerful overlaps at virtually every level of the addictive process, from initial encounters to withdrawal. A preponderance of evidence from human studies and animal models now demonstrates that these overlaps extend to the level of neurobiology as well, where virtually every neurochemical system implicated in addiction also participates in social attachment processes. These observations suggest that treatments used in one domain may be effective in the other; for instance, treatments used to reduce drug cravings may be effective in treating grief from loss of a loved one or a bad break-up (O’Malley et al 1992; Volpicelli et al 1992; Koob and Zorrilla 2010; Minozzi et al 2011). These data also provide evidence for the theory that social attachment systems governing maternal bonding and pair bonding to a mating partner are subverted by drugs of abuse to create addictions that are just as powerful as natural attachments. In a very real sense, we may be addicted to the ones we love.
Footnotes
Funding: We acknowledge funding from MH64692 to LJY, NIH RR00165 to YNPRC, and the Emory Scholars Program in Interdisciplinary Neuroscience Research to JPB.
Conflicts of Interest: The authors declare no conflicts of interest.
Bibliography
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural Correlates of Long-Term Intense Romantic Love. Soc Cogn Affect Neurosci. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmo A, Berenfeld R. Reinforcing Properties of Ejaculation in the Male Rat: Role of Opioids and Dopamine. Behav Neurosci. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Ahern TH, Hammock EAD, Young LJ. Parental Division of Labor, Coordination, and the Effects of Family Structure on Parenting in Monogamous Prairie Voles (Microtus Ochrogaster) Dev Psychobiol. 2011;53:118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of Two Automated Metrics for Analyzing Partner Preference Tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Szafarczyk A, Assenmacher I. Radioautographic Evidence That Axons from the Area of Supraoptic Nuclei in the Rat Project to Extrahypothalamic Brain Regions. Neurosci Lett. 1986;66:251–256. doi: 10.1016/0304-3940(86)90027-3. [DOI] [PubMed] [Google Scholar]
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of Ethanol Self-Administration by Naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting Social Behavior with Oxytocin in High-Functioning Autism Spectrum Disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. Camkii: A Biochemical Bridge Linking Accumbens Dopamine and Glutamate Systems in Cocaine Seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-Induced Alterations in Dopamine Receptor Signaling: Implications for Reinforcement and Reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A Critical Role for Nucleus Accumbens Dopamine in Partner-Preference Formation in Male Prairie Voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus Accumbens Dopamine Differentially Mediates the Formation and Maintenance of Monogamous Pair Bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine Regulation of Social Choice in a Monogamous Rodent Species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer R. Chemistry of the Neurohypophyseal Hormones: An Example of Molecular Evolution. In: Knobil E, Sawyer W, editors. Handbook of Physiology. American Physiological Society; Washington DC: 1974. pp. 119–130. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, Motivation, and Emotion Systems Associated with Early-Stage Intense Romantic Love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders : Dsm-Iv-Tr. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Association AP. American Psychiatric Association DSM-5 Development. American Psychiatric Association; [Accessed June 6, 2012]. 2012. Substance Use and Addictive Disorders. Available via www.dsm5.org. [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of Intra-Nucleus Accumbens Shell Administration of Dopamine Agonists and Antagonists on Cocaine-Taking and Cocaine-Seeking Behaviors in the Rat. Psychopharmacol (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Research Review: Genetic Vulnerability or Differential Susceptibility in Child Development: The Case of Attachment. J Child Psychol Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Crf and Crf Receptors: Role in Stress Responsivity and Other Behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin Directly Administered into the Nucleus Accumbens Core or Subthalamic Nucleus Attenuates Methamphetamine-Induced Conditioned Place Preference. Behav Brain Res. 2012;228:185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a Functional Polymorphism in the Mu-Opioid Receptor Gene with Alcohol Response and Consumption in Male Rhesus Macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the Mu-Opioid Receptor Gene (Oprm1) Influences Attachment Behavior in Infant Primates. Proc Natl Acad Sci U S A. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The Neural Basis of Romantic Love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bédier J, Belloc H. The Romance of Tristan and Iseult. Dover Publications; Mineola, NY: 2004. [Google Scholar]
- Beninger RJ, Cheng M, Hahn BL, Hoffman DC, Mazurski EJ, Morency MA, Ramm P, Stewart RJ. Effects of Extinction, Pimozide, Sch 23390, and Metoclopramide on Food-Rewarded Operant Responding of Rats. Psychopharmacol (Berl) 1987;92:343–349. doi: 10.1007/BF00210842. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Hoffman DC, Mazurski EJ. Receptor Subtype-Specific Dopaminergic Agents and Conditioned Behavior. Neurosci Biobehav Rev. 1989;13:113–122. doi: 10.1016/s0149-7634(89)80019-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The Debate over Dopamine’s Role in Reward: The Case for Incentive Salience. Psychopharmacol (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing Patterns of Signaling Activation in Dopamine D1 and D2 Receptor-Expressing Striatal Neurons in Response to Cocaine and Haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a Vasopressin Receptor Is Necessary and Sufficient for Normal Social Recognition: A Gene Replacement Study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacol. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, Vasopressin, and Social Recognition in Mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Birdsall NJ, Hulme EC. C Fragment of Lipotropin Has a High Affinity for Brain Opiate Receptors. Nature. 1976;260:793–795. doi: 10.1038/260793a0. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine Functions in Appetitive and Defensive Behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. AugmentedCocaine Seeking in Response to Stress or Crf Delivered into the Ventral Tegmental Area Following Long-Access Self-Administration Is Mediated by Crf Receptor Type 1 but Not Crf Receptor Type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow AP, Cameron NM. The Role of Oxytocin in Mating and Pregnancy. Horm Behav. 2012;61:266–276. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The Crf System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacol. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry JC, Raebel MA. Continuous Infusion of Naloxone in the Treatment of Narcotic Overdose. Drug Intell Clin Pharm. 1981;15:945–950. doi: 10.1177/106002808101501205. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine Self-Administration Produces a Persistent Increase in Dopamine D2 High Receptors. Eur Neuropsychopharmacol. 2008;18:551–556. doi: 10.1016/j.euroneuro.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Phillips AG. Facilitation of Self-Stimulation Behavior Following Intracerebral Microinjections of Opioids into the Ventral Tegmental Area. Pharmacol Biochem Behav. 1979;11:289–295. doi: 10.1016/0091-3057(79)90137-0. [DOI] [PubMed] [Google Scholar]
- Brown R, Dewan S. The New York Times. Mysteries Remain after Governor Admits an Affair. 2009 [Google Scholar]
- Bruijnzeel AW. Kappa-Opioid Receptor Signaling and Brain Reward Function. Brain Research Reviews. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic Network Differences in Human Impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF. The Role of Typical and Atypical Antipsychotic Medications in the Management of Agitation and Aggression. Journal of Clinical Psychiatry. 1999;60:52–60. [PubMed] [Google Scholar]
- Buijs RM, De Vries GJ, Van Leeuwen FW, Swaab DF. Vasopressin and Oxytocin: Distribution and Putative Functions in the Brain. Prog Brain Res. 1983;60:115–122. doi: 10.1016/S0079-6123(08)64379-4. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Young LJ, Russell J. Oxytocin: Synthesis, Secretion, and Reproductive Functions. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Academic Press; New York: 2005. pp. 3055–3127. [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of Mu-Opioid Receptors in the Dorsal Striatum Is Necessary for Adult Social Attachment in Monogamous Prairie Voles. Neuropsychopharmacol. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DL, Hewlett EL, Moss J, Vaughan M. Pertussis Toxin Inhibits Enkephalin Stimulation of Gtpase of Ng108-15 Cells. J Biol Chem. 1983;258:1435–1438. [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral Roles of an "Original" Neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma Oxytocin Increases in the Human Sexual-Response. Journal of Clinical Endocrinology & Metabolism. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin Decreases Methamphetamine Self-Ad Ministration, Methamphetamine Hyperactivity, and Relapse to Methamphetamine-Seeking Behaviour in Rats. Neuropharmacology. 2010a;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically Administered Oxytocin Decreases Methamphetamine Activation of the Subthalamic Nucleus and Accumbens Core and Stimulates Oxytocinergic Neurons in the Hypothalamus. Addict Biol. 2010b;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological Substrates of Mammalian Monogamy: The Prairie Vole Model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Roberts RL. In: Cooperative Breeding in Mammals. Solomon NG, French JA, editors. Cambridge University Press; Cambridge, U.K. ; New York, NY, USA: 1997. [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally Occurring Variations in Maternal Behavior in the Rat Are Associated with Differences in Estrogen-Inducible Central Oxytocin Receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular Cloning and Functional Expression of a Mu-Opioid Receptor from Rat Brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-Releasing Factor in the Nucleus Accumbens Shell Induces Swim Depression, Anxiety, and Anhedonia Along with Changes in Local Dopamine/Acetylcholine Balance. Neuroscience. 2012a doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-Releasing Factor in the Nucleus Accumbens Shell Induces Swim Depression, Anxiety, and Anhedonia Along with Changes in Local Dopamine/Acetylcholine Balance. Neuroscience. 2012b;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Christensson K, Nilsson BA, Stock S, Matthiesen AS, Uvnasmoberg K. Effect of Nipple Stimulation on Uterine Activity and on Plasma-Levels of Oxytocin in Full Term, Healthy, Pregnant-Women. Acta Obstetricia Et Gynecologica Scandinavica. 1989;68:205–210. doi: 10.3109/00016348909020990. [DOI] [PubMed] [Google Scholar]
- Claytor R, Lile JA, Nader MA. The Effects of Eticlopride and the Selective D3-Antagonist Pnu 99194-a on Food- and Cocaine-Maintained Responding in Rhesus Monkeys. Pharmacol Biochem Behav. 2006;83:456–464. doi: 10.1016/j.pbb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol Cue Reactivity, Negative-Mood Reactivity, and Relapse in Treated Alcoholic Men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Breese GR. A Role for Dopamine in the Psychopharmacology of Electrical Self-Stimulation of the Lateral Hypothalamus, Substantia Nigra, and Locus Coeruleus. Psychopharmacol Bull. 1975;11:30–31. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. (2000) Response of Nicotine Self-Administration in the Rat to Manipulations of Mu-Opioid and Gamma-Aminobutyric Acid Receptors in the Ventral Tegmental Area. Psychopharmacol (Berl) 149;107:114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH. The Rewarding Effect of Aggression Is Reduced by Nucleus Accumbens Dopamine Receptor Antagonism in Mice. Psychopharmacology (Berl) 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Curley JP. The Mu-Opioid Receptor and the Evolution of Mother-Infant Attachment: Theoretical Comment on Higham Et Al. (2011) Behav Neurosci. 2011;125:273–278. doi: 10.1037/a0022939. [DOI] [PubMed] [Google Scholar]
- Curran EJ, Watson SJ., Jr. Dopamine Receptor Mrna Expression Patterns by Opioid Peptide Cells in the Nucleus Accumbens of the Rat: A Double in Situ Hybridization Study. J Comp Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and Monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Amphetamine Effects in Microtine Rodents: A Comparative Study Using Monogamous and Promiscuous Vole Species. Neuroscience. 2007;148:857–866. doi: 10.1016/j.neuroscience.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual Behavior Increases Dopamine Transmission in the Nucleus Accumbens and Striatum of Male Rats: Comparison with Novelty and Locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of Social Memory in Male Rats by Neurohypophyseal Peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine Self-Administration Increases Preprodynorphin, but Not C-Fos, Mrna in Rat Striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, Van Ree JM. Food Deprivation and Acquisition of Intravenous Cocaine Self-Administration in Rats: Effect of Naltrexone and Haloperidol. J Pharmacol Exp Ther. 1989;251:735–740. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of Cocaine-Reinforced Responding in the Rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-Like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The Effects of Stress on Social Preferences Are Sexually Dimorphic in Prairie Voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-Releasing Factor Induces Social Preferences in Male Prairie Voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and Drug Addiction: The Nucleus Accumbens Shell Connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichiara G, Imperato A. Opposite Effects of Mu-Opiate and Kappa-Opiate Agonists on Dopamine Release in the Nucleus Accumbens and in the Dorsal Caudate of Freely Moving Rats. Journal of Pharmacology and Experimental Therapeutics. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin Improves "Mind-Reading" in Humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-Induced Dopamine Release in Human Ventral Striatum Correlates with Euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of Phasic and Tonic Dopamine Release on Receptor Activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, van Gerven JM, Buitelaar JK, Verkes RJ. Increased Oxytocin Concentrations and Prosocial Feelings in Humans after Ecstasy (3,4-Methylenedioxymethamphetamine) Administration. Soc Neurosci. 2009;4:359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and Behavioral Responses to Corticotropin-Releasing Factor Administration: Is Crf a Mediator of Anxiety or Stress Responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- El Daly E, Chefer V, Sandill S, Shippenberg TS. Modulation of the Neurotoxic Effects of Methamphetamine by the Selective Kappa-Opioid Receptor Agonist U69593. J Neurochem. 2000;74:1553–1562. doi: 10.1046/j.1471-4159.2000.0741553.x. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr., Morrison H, Magendzo K, Edwards RH. Cloning of a Delta Opioid Receptor by Functional Expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-Order Schedules of Drug Reinforcement in Rats and Monkeys: Measurement of Reinforcing Efficacy and Drug-Seeking Behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Possible Role for Endogenous Oxytocin in Estrogen-Facilitated Maternal Behavior in Rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of Low Striatal Dopamine D2 Receptor Availability with Nicotine Dependence Similar to That Seen with Other Drugs of Abuse. Am J Psychiatry. 2008;165:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and Social Affiliation in Humans. Horm Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the Medial Amygdala Is Essential for Social Recognition in the Mouse. Journal of Neuroscience. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social Amnesia in Mice Lacking the Oxytocin Gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Fisher HE. We Love : The Nature and Chemistry of Romantic Love. H. Holt; New York: 2004. [Google Scholar]
- Freeman SM, Young LJ. Oxytocin,Vasopressin, and the Evolution of Monogamy in Mammals. In: Choleris E, Pfaff DW, editors. Oxytocin and Vasopressin. Cambridge Press; in press. [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-Releasing Factor within the Central Nucleus of the Amygdala Mediates Enhanced Ethanol Self-Administration in Withdrawn, Ethanol-Dependent Rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-Releasing Factor 1 Antagonists Selectively Reduce Ethanol Self-Administration in Ethanol-Dependent Rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furay AR, Neumaier JF. Opioid Receptors: Binding That Ties. Neuropsychopharmacol. 2011;36:2157–2158. doi: 10.1038/npp.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffori O, Le Moal M. Disruption of Maternal Behavior and Appearance of Cannibalism after Ventral Mesencephalic Tegmentum Lesions. Physiol Behav. 1979;23:317–323. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- Gainer H, Wray S. Cellular and Molecular Biology of Oxytocin and Vasopressin. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 1099–1129. [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. Crf-Crf1 System Activation Mediates Withdrawal-Induced Increases in Nicotine Self-Administration in Nicotine-Dependent Rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 Dopamine Receptor Function in the Striatum: Coactivation of D1- and D2-Dopamine Receptors on Separate Populations of Neurons Results in Potentiated Immediate Early Gene Response in D1-Containing Neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of Striatonigral and Striatopallidal Peptidergic Neurons in Both Patch and Matrix Compartments: An in Situ Hybridization Histochemistry and Fluorescent Retrograde Tracing Study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Lesscher HB, van Ree JM. Drug Dependence and the Endogenous Opioid System. Eur Neuropsychopharmacol. 2003;13:424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The Mating System of the Prairie Vole, Microtus-Ochrogaster - Field and Laboratory Evidence for Pair-Bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz LL, Hofmann JE. Social-Organization in Free-Living Prairie Voles, Microtus-Ochrogaster. Behavioral Ecology and Sociobiology. 1986;18:275–282. [Google Scholar]
- Gillath O, Shaver PR, Baek JM, Chun DS. Genetic Correlates of Adult Attachment Style. Pers Soc Psychol Bull. 2008;34:1396–1405. doi: 10.1177/0146167208321484. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 Receptors in the Nucleus Accumbens Are Important for Social Attachment in Female Prairie Voles (Microtus Ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Lane JD, Smith JE. Self-Administration of Methionine Enkephalin into the Nucleus Accumbens. Pharmacol Biochem Behav. 1984;20:451–455. doi: 10.1016/0091-3057(84)90284-3. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an Extraordinarily Potent Opioid Peptide. Proc Natl Acad Sci U S A. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of Naltrexone Plus Bupropion on Weight Loss in Overweight and Obese Adults (Cor-I): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Grieder TE, George O, Tan H, George SR, Le Foll B, Laviolette SR, van der Kooy D. Phasic D1 and Tonic D2 Dopamine Receptor Signaling Double Dissociate the Motivational Effects of Acute Nicotine and Chronic Nicotine Withdrawal. Proc Natl Acad Sci U S A. 2012;109:3101–3106. doi: 10.1073/pnas.1114422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-Like Behavior and Stressor-Induced Neuroendocrine Activation in Female Prairie Voles Exposed to Chronic Social Isolation. Psychosom Med. 2007a;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social Isolation Induces Behavioral and Neuroendocrine Disturbances Relevant to Depression in Female and Male Prairie Voles. Psychoneuroendocrinol. 2007b;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin Increases Gaze to the Eye Region of Human Faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with Pups Enhances Dopamine Release in the Ventral Striatum of Maternal Rats: A Microdialysis Study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The Effects of 6-Ohda-Induced Dopamine Depletions in the Ventral or Dorsal Striatum on Maternal and Sexual Behavior in the Female Rat. Pharmacol Biochem Behav. 1991a;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. Mesotelencephalic Dopamine System and Reproductive Behavior in the Female Rat: Effects of Ventral Tegmental 6-Hydroxydopamine Lesions on Maternal and Sexual Responsiveness. Behav Neurosci. 1991b;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of Methamphetamine Effects on the Striatal-Nigral Dynorphin System. European Journal of Pharmacology. 1988;155:11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-Releasing Factor in Brain: A Role in Activation, Arousal, and Affect Regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-Stress Action of a Corticotropin-Releasing Factor Antagonist on Behavioral Reactivity to Stressors of Varying Type and Intensity. Neuropsychopharmacology. 1994;11:179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The Persistence of Behavioral Sensitization to Cocaine Parallels Enhanced Inhibition of Nucleus Accumbens Neurons. J Neurosci. 1995;15:6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of Morphine and Naloxone on Separation Distress and Approach Attachment: Evidence for Opiate Mediation of Social Affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food Reward and Cocaine Increase Extracellular Dopamine in the Nucleus Accumbens as Measured by Microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hershon HI. Alcohol Withdrawal Symptoms and Drinking Behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-Opioid Receptor (Oprm1) Variation, Oxytocin Levels and Maternal Attachment in Free-Ranging Rhesus Macaques Macaca Mulatta. Behav Neurosci. 2011;125:131–136. doi: 10.1037/a0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. The Ventral Pallidum Area Is Involved in the Acquisition but Not Expression of the Amphetamine Conditioned Place Preference. Neurosci Lett. 1993;156:9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of Fear Conditioning by Antisense Inhibition of Brain Corticotropin Releasing Factor-2 Receptor. Brain Res Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Beninger RJ. Selective D1 and D2 Dopamine Agonists Produce Opposing Effects in Place Conditioning but Not in Conditioned Taste Aversion Learning. Pharmacol Biochem Behav. 1988;31:1–8. doi: 10.1016/0091-3057(88)90302-4. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Dickson PR, Beninger RJ. The Dopamine D2 Receptor Agonists, Quinpirole and Bromocriptine Produce Conditioned Place Preferences. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:315–322. doi: 10.1016/0278-5846(88)90050-4. [DOI] [PubMed] [Google Scholar]
- Holden C. Compulsive Behaviors: “Behavioral’ Addictions: Do They Exist? Science. 2001;294:980–982. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ, Siegel B, Holen A, Bonanno GA, Milbrath C, Stinson CH. Diagnostic Criteria for Complicated Grief Disorder. Am J Psychiatry. 1997;154:904–910. doi: 10.1176/ajp.154.7.904. [DOI] [PubMed] [Google Scholar]
- Hu XT, White FJ. Dopamine Enhances Glutamate-Induced Excitation of Rat Striatal Neurons by Cooperative Activation of D1 and D2 Class Receptors. Neurosci Lett. 1997;224:61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Koob GF. The Ventral Pallidum Plays a Role in Mediating Cocaine and Heroin Self-Administration in the Rat. Brain Res. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of Two Related Pentapeptides from the Brain with Potent Opiate Agonist Activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine Self-Administration Differentially Alters Mrna Expression of Striatal Peptides. Brain Res Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Ibragimov R, Kovacs GL, Szabo G, Telegdy G. Microinjection of Oxytocin into Limbic-Mesolimbic Brain Structures Disrupts Heroin Self-Administration Behavior - a Receptor-Mediated Event. Life Sci. 1987;41:1265–1271. doi: 10.1016/0024-3205(87)90205-0. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The Role of Nucleus Accumbens Dopamine in Motivated Behavior: A Unifying Interpretation with Special Reference to Reward-Seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is Social Attachment an Addictive Disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the Monogamous Male: Behavioral Consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin Receptor Distribution Reflects Social-Organization in Monogamous and Polygamous Voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of Brain Vasopressin Receptor Distribution Associated with Social Organization in Microtine Rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Girard-Beriault F, Nakanishi S, Pfaus JG. Naloxone, but Not Flupenthixol, Disrupts the Development of Conditioned Ejaculatory Preference in the Male Rat. Behav Neurosci. 2009;123:992–999. doi: 10.1037/a0017096. [DOI] [PubMed] [Google Scholar]
- Ismayilova N, Shoaib M. Alteration of Intravenous Nicotine Self-Administration by Opioid Receptor Agonist and Antagonists in Rats. Psychopharmacol (Berl) 2010;210:211–220. doi: 10.1007/s00213-010-1845-4. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Acute Nicotine Changes Dynorphin and Prodynorphin Mrna in the Striatum. Psychopharmacology (Berl) 2009;201:507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids Excite Dopamine Neurons by Hyperpolarization of Local Interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate Modulation of Separation-Induced Distress in Non-Human Primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE. Opiate Systems in Mother and Infant Primates Coordinate Intimate Contact During Reunion. Psychoneuroendocrinol. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Karras A, Kane JM. Naloxone Reduces Cigarette Smoking. Life Sci. 1980;27:1541–1545. doi: 10.1016/0024-3205(80)90562-7. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing Oxytocin Receptor Expression in the Nucleus Accumbens of Pre-Pubertal Female Prairie Voles Enhances Alloparental Responsiveness and Partner Preference Formation as Adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine Receptor Blockade in the Nucleus Accumbens Inhibits Maternal Retrieval and Licking, but Enhances Nursing Behavior in Lactating Rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The Neuroscience of Natural Rewards: Relevance to Addictive Drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB. Effects of Intracerebroventricular Infusions of Naltrexone and Phentolamine on Central and Peripheral Oxytocin Release and on Maternal Behaviour Induced by Vaginocervical Stimulation in the Ewe. Brain Res. 1989;505:329–332. doi: 10.1016/0006-8993(89)91462-5. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular Oxytocin Stimulates Maternal Behaviour in the Sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Kendrick KM. Maternal Behaviour in Sheep and Its Neuroendocrine Regulation. Acta Paediatr Suppl. 1994;397:47–56. doi: 10.1111/j.1651-2227.1994.tb13265.x. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Schafer MKH, Watson SJ. Anatomy of the Cns Opioid Systems. Trends Neurosci. 1985;8:111. doi: 10.1016/0166-2236(88)90093-8. &. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The Delta-Opioid Receptor: Isolation of a Cdna by Expression Cloning and Pharmacological Characterization. Proc Natl Acad Sci U S A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Baik SH, Park CS, Kim SJ, Choi SW, Kim SE. Reduced Striatal Dopamine D2 Receptors in People with Internet Addiction. Neuroreport. 2011;22:407–411. doi: 10.1097/WNR.0b013e328346e16e. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in Mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. Sb242084, Flumazenil, and Cra1000 Block Ethanol Withdrawal-Induced Anxiety in Rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, Roeske WR, Yamamura HI. Molecular Biology and Pharmacology of Cloned Opioid Receptors. FASEB J. 1995;9:516–525. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- Koller G, Zill P, Rujescu D, Ridinger M, Pogarell O, Fehr C, Wodarz N, Bondy B, Soyka M, Preuss UW. Possible Association between Oprm1 Genetic Variance at the 118 Locus and Alcohol Dependence in a Large Treatment Sample: Relationship to Alcohol Dependence Symptoms. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A Role for Brain Stress Systems in Addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The Role of Crf and Crf-Related Peptides in the Dark Side of Addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and Molecular Mechanisms of Drug Dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological Mechanisms of Addiction: Focus on Corticotropin-Releasing Factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin Increases Trust in Humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Faludi M, Falkay G, Telegdy G. Peripheral Oxytocin Treatment Modulates Central Dopamine Transmission in the Mouse Limbic Structures. Neurochemistry International. 1986;9:481–485. doi: 10.1016/0197-0186(86)90138-5. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Babarczi E, Szabo G, Telegdy G. The Role of Oxytocin Dopamine Interactions in Cocaine-Induced Locomotor Hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Izbeki F, Szabo G, Telegdy G, Barth T, Jost K, Brtnik F. Effects of Oxytocin-Related Peptides on Acute Morphine Tolerance: Opposite Actions by Oxytocin and Its Receptor Antagonists. J Pharmacol Exp Ther. 1987;241:569–574. [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Szabo G. Oxytocin and Addiction: A Review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Szontagh L, Balaspiri L, Hodi K, Bohus P, Telegdy G. On the Mode of Action of an Oxytocin Derivative (Z-Pro-D-Leu) on Morphine-Dependence in Mice. Neuropharmacology. 1981;20:647–651. doi: 10.1016/0028-3908(81)90111-8. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Telegdy G. Beta-Endorphin Tolerance Is Inhibited by Oxytocin. Pharmacology Biochemistry and Behavior. 1987;26:57–60. doi: 10.1016/0091-3057(87)90533-8. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Vanree JM. Behaviorally Active Oxytocin Fragments Simultaneously Attenuate Heroin Self-Administration and Tolerance in Rats. Life Sci. 1985;37:1895–1900. doi: 10.1016/0024-3205(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-Induced Potentiation of Cocaine Reward: A Role for Crf R1 and Creb. Neuropsychopharmacology. 2009;34:2609–2617. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoy WA, Zimmerma E, Lande S. Facilitation of Development of Resistance to Morphine Analgesia by Desglycinamide9-Lysine Vasopressin. Proc Natl Acad Sci U S A. 1974;71:1852–1856. doi: 10.1073/pnas.71.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The Single Nucleotide Polymorphism A118g Alters Functional Properties of the Human Mu Opioid Receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin AV, Gerrits MA, van Ree JM, Zvartau EE. Naloxone Inhibits the Reinforcing and Motivational Aspects of Cocaine Addiction in Mice. Life Sci. 1997;60:PL-257–264. doi: 10.1016/s0024-3205(97)00130-6. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Yu J, Marshall JF. Striatal Fos Expression Is Indicative of Dopamine D1/D2 Synergism and Receptor Supersensitivity. Proc Natl Acad Sci U S A. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Ney K, Sasvari-Szekely M, Gervai J. Dopamine D4 Receptor (Drd4) Gene Polymorphism Is Associated with Attachment Disorganization in Infants. Mol Psychiatry. 2000;5:633–637. doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward Processing by the Opioid System in the Brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Gallen CL, Zhang X, Hodgkinson CA, Goldman D, Stein EA, Barr CS. Functional Polymorphism of the Mu-Opioid Receptor Gene (Oprm1) Influences Reinforcement Learning in Humans. PLoS One. 2011;6:e24203. doi: 10.1371/journal.pone.0024203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. Similarities and Differences between Pathological Gambling and Substance Use Disorders: A Focus on Impulsivity and Compulsivity. Psychopharmacol (Berl) 2012;219:469–490. doi: 10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More Frequent Partner Hugs and Higher Oxytocin Levels Are Linked to Lower Blood Pressure and Heart Rate in Premenopausal Women. Biol Psychol. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin Responsivity in Mothers of Infants: A Preliminary Study of Relationships with Blood Pressure During Laboratory Stress and Normal Ambulatory Activity. Health Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. Crf Receptors in the Nucleus Accumbens Modulate Partner Preference in Prairie Voles. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and Sex Differences in Brain Distribution of Corticotropin-Releasing Factor Receptor Subtypes 1 and 2 in Monogamous and Promiscuous Vole Species. J Comp Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. Distribution of Corticotropin-Releasing Factor and Urocortin 1 in the Vole Brain. Brain Behav Evol. 2006;68:229–240. doi: 10.1159/000094360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced Partner Preference in a Promiscuous Species by Manipulating the Expression of a Single Gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-Dependent Neural Circuits Underlying Pair Bond Formation in the Monogamous Prairie Vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus Accumbens Dopamine Mediates Amphetamine-Induced Impairment of Social Bonding in a Monogamous Rodent Species. Proc Natl Acad Sci U S A. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus Accumbens Oxytocin and Dopamine Interact to Regulate Pair Bond Formation in Female Prairie Voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social Bonding Decreases the Rewarding Properties of Amphetamine through a Dopamine D1 Receptor-Mediated Mechanism. J Neurosci. 2011;31:7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of Monkey Dopamine Neurons During Learning of Behavioral Reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of High-Affinity Binding Sites for Oxytocin and Vasopressin in the Human Brain. An Autoradiographic Study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Luijk MP, Roisman GI, Haltigan JD, Tiemeier H, Booth-Laforce C, van Ijzendoorn MH, Belsky J, Uitterlinden AG, Jaddoe VW, Hofman A, Verhulst FC, Tharner A, Bakermans-Kranenburg MJ. Dopaminergic, Serotonergic, and Oxytonergic Candidate Genes Associated with Infant Attachment Security and Disorganization? In Search of Main and Interaction Effects. J Child Psychol Psychiatry. 2011;52:1295–1307. doi: 10.1111/j.1469-7610.2011.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early Life Stress Impairs Social Recognition Due to a Blunted Response of Vasopressin Release within the Septum of Adult Male Rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The Triune Brain in Evolution : Role in Paleocerebral Functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Madison DM. Space Use and Social Structure in Meadow Voles, Microtus Pennsylvanicus. Behav Ecol Sociobiol. 1980;7:65–71. [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 Receptor Mrna Distribution in the Rat Cns: Comparison to Kappa Receptor Binding and Prodynorphin Mrna. Mol Cell Neurosci. 1994a;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. Mu-Opioid Receptor Mrna Expression in the Rat Cns: Comparison to Mu-Receptor Binding. Brain Res. 1994b;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic Differentiation of Mu, Delta, and Kappa Opioid Receptors in the Rat Forebrain and Midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of Cns Opioid Receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Rayment FD, Simpson MJ, Keverne EB. Opioid Receptor Blockade Reduces Maternal Affect and Social Grooming in Rhesus Monkeys. Psychoneuroendocrinology. 1993;18:307–321. doi: 10.1016/0306-4530(93)90027-i. [DOI] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of Opioid Receptor Blockade on the Social Behavior of Rhesus Monkeys Living in Large Family Groups. Dev Psychobiol. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of Morphine-Induced Analgesia, Reward Effect and Withdrawal Symptoms in Mice Lacking the Mu-Opioid-Receptor Gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of Two Positive Reinforcing Stimuli: Pups and Cocaine Throughout the Postpartum Period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The Prairie Vole: An Emerging Model Organism for Understanding the Social Brain. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the Loop: Oxytocin as a Potential Treatment for Drug Addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa Opioid Receptor Antagonism and Prodynorphin Gene Disruption Block Stress-Induced Behavioral Responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measelle JR, Stice E, Springer DW. A Prospective Test of the Negative Affect Model of Substance Abuse: Moderating Effects of Social Support. Psychol Addict Behav. 2006;20:225–233. doi: 10.1037/0893-164X.20.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrara BJ, Baum MJ. Naloxone Disrupts the Expression but Not the Acquisition by Male Rats of a Conditioned Place Preference Response for an Oestrous Female. Psychopharmacol (Berl) 1990;101:118–125. doi: 10.1007/BF02253728. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a Novel and Potent Corticotropin-Releasing Factor Antagonist in Rats. J Pharmacol Exp Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of Extracellular Corticotropin-Releasing Factor-Like Immunoreactivity Levels in the Amygdala of Awake Rats During Restraint Stress and Ethanol Withdrawal as Measured by Microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and Neural Determinants of Aggressive Behavior: Pharmacotherapeutic Targets at Serotonin, Dopamine and Gamma-Aminobutyric Acid Systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or Suppressed Cocaine Reward, Tegmental Bdnf, and Accumbal Dopamine Caused by Episodic Versus Continuous Social Stress in Rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Dackis CA, Gold MS. The Relationship of Addiction, Tolerance, and Dependence to Alcohol and Drugs - a Neurochemical Approach. Journal of Substance Abuse Treatment. 1987;4:197–207. doi: 10.1016/s0740-5472(87)80014-4. [DOI] [PubMed] [Google Scholar]
- Miller RL, Baum MJ. Naloxone Inhibits Mating and Conditioned Place Preference for an Estrous Female in Male Rats Soon after Castration. Pharmacol Biochem Behav. 1987;26:781–789. doi: 10.1016/0091-3057(87)90611-3. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral Naltrexone Maintenance Treatment for Opioid Dependence. Cochrane Database Syst Rev. 2011:CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. D-Cycloserine Facilitates Socially Reinforced Learning in an Animal Model Relevant to Autism Spectrum Disorders. Biol Psychiatry. 2011;70:298–304. doi: 10.1016/j.biopsych.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ. Limbic-Motor Integration. In: Epstein AN, editor. Progress in Psychobiology and Physiological Psychology. Academic; New York: 1987. pp. 117–170. [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR, editors. Deficit in Attachment Behavior in Mice Lacking the Mu-Opioid Receptor Gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Nakajima S, editor. Suppression of Operant Responding in the Rat by Dopamine D1-Receptor Blockade with Sch-23390. Physiol Psychol. 1986;14:111–114. [Google Scholar]
- Nakajima S, Mckenzie GM, editors. Reduction of the Rewarding Effect of Brain-Stimulation by a Blockade of Dopamine D1 Receptor with Sch-23390. Pharmacol Biochem Be. 1986;24:919–923. doi: 10.1016/0091-3057(86)90437-5. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J, editors. Brain Substrates of Infant-Mother Attachment: Contributions of Opioids, Oxytocin, and Norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS, editors. Chronic Cocaine Treatment Decreases Levels of the G Protein Subunits Gi Alpha and Go Alpha in Discrete Regions of Rat Brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Nissen HW, editor. A Study of Maternal Behavior in the White Rat by Means of the Obstruction Method. Pegagog Semin J Gen. 1930;37:377–393. [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ, editors. Mu and Delta Receptors Belong to a Family of Receptors That Are Coupled to Potassium Channels. Proc Natl Acad Sci U S A. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Seeman P, Le Foll B, editors. Exposure to Nicotine Produces an Increase in Dopamine D2(High) Receptors: A Possible Mechanism for Dopamine Hypersensitivity. Int J Neurosci. 2010;120:691–697. doi: 10.3109/00207454.2010.513462. [DOI] [PubMed] [Google Scholar]
- Numan M, editor. Motivational Systems and the Neural Circuitry of Maternal Behavior in the Rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR, editors. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD, editors. The Effects of D1 or D2 Dopamine Receptor Antagonism in the Medial Preoptic Area,Ventral Pallidum, or Nucleus Accumbens on the Maternal Retrieval Response and Other Aspects of Maternal Behavior in Rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B, editors. Naltrexone and Coping Skills Therapy for Alcohol Dependence. A Controlled Study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ, editors. Oxytocin Receptors in the Nucleus Accumbens Facilitate "Spontaneous" Maternal Behavior in Adult Female Prairie Voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ, editors. Species and Individual Differences in Juvenile Female Alloparental Care Are Associated with Oxytocin Receptor Density in the Striatum and the Lateral Septum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW, editors. Elevated Extracellular Crf Levels in the Bed Nucleus of the Stria Terminalis During Ethanol Withdrawal and Reduction by Subsequent Ethanol Intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB, editors. The Development of a Conditioned Place Preference toMorphine: Effects of Lesions of Various Cns Sites. Behav Neurosci. 1997a;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB, editors. The Development of a Conditioned Place Preference to Morphine: Effects of Microinjections into Various Cns Sites. Behav Neurosci. 1997b;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Orians GH, editor. On Evolution of Mating Systems in Birds and Mammals. American Naturalist. 1969;103:589. &. [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP, editors. A Functional Polymorphism of the Mu-Opioid Receptor Gene Is Associated with Naltrexone Response in Alcohol-Dependent Patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Ott CH, editor. The Impact of Complicated Grief on Mental and Physical Health at Various Points in the Bereavement Process. Death Stud. 2003;27:249–272. doi: 10.1080/07481180302887. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP, editors. The Biology of Social Attachments: Opiates Alleviate Separation Distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG, editors. Endogenous Opioids and Social Behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P, editors. Opiates and Play Dominance in Juvenile Rats. Behav Neurosci. 1985;99:441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Najam N, Soares F, editors. Morphine Reduces Social Cohesion in Rats. Pharmacol Biochem Behav. 1979;11:131–134. doi: 10.1016/0091-3057(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S, editors. Brain Opioids and Mother-Infant Social Motivation. Acta Paediatr Suppl. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC, editors. Opioid Site in Nucleus Accumbens Shell Mediates Eating and Hedonic ‘Liking’ for Food: Map Based on Microinjection Fos Plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC, editors. Nucleus Accumbens Corticotropin-Releasing Factor Increases Cue-Triggered Motivation for Sucrose Reward: Paradoxical Positive Incentive Effects in Stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA, editors. Oxytocin Activates the Postpartum Onset of Rat Maternal Behavior in the Ventral Tegmental and Medial Preoptic Areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ Jr., editors. Induction of Maternal Behavior in Virgin Rats after Intracerebroventricular Administration of Oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR Jr., Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX, editors. The Preferential Dopamine D3 Receptor Antagonist S33138 Inhibits Cocaine Reward and Cocaine-Triggered Relapse to Drug-Seeking Behavior in Rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, Ford KA, Goussakov I, Stutzmann GE, Hu XT, editors. Repeated Cocaine Exposure Decreases Dopamine D-Like Receptor Modulation of Ca(2+) Homeostasis in Rat Nucleus Accumbens Neurons. Synapse. 2011;65:168–180. doi: 10.1002/syn.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM, editors. Psychotomimesis Mediated by Kappa Opiate Receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Plato, Rowe CJ, editors. Phaedrus. Aris & Phillips; Warminster, Wiltshire, England Atlantic Highlands, N.J.: 1986. Distributed in the U.S.A. and Canada by Humanities Press. [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr., editors. Altered Responsiveness to Cocaine and Increased Immobility in the Forced Swim Test Associated with Elevated Camp Response Element-Binding Protein Expression in Nucleus Accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G, editors. Intravenous Cocaine, Morphine, and Amphetamine Preferentially Increase Extracellular Dopamine in the "Shell" as Compared with the "Core" of the Rat Nucleus Accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G, editors. Effects of Nicotine on the Nucleus Accumbens and Similarity to Those of Addictive Drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Potenza MN, editor. Should Addictive Disorders Include Non-Substance-Related Conditions? Addiction. 2006;101:142–151. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF 3rd, Shear MK, Day N, Beery LC, Newsom JT, Jacobs S, editors. Traumatic Grief as a Risk Factor for Mental and Physical Morbidity. Am J Psychiatry. 1997;154:616–623. doi: 10.1176/ajp.154.5.616. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Reynolds CF 3rd, Bierhals AJ, Newsom JT, Fasiczka A, Frank E, Doman J, Miller M, editors. Inventory of Complicated Grief: A Scale to Measure Maladaptive Symptoms of Loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R, editors. Ethanol Withdrawal Enhances the Prodynorphin System Activity in the Rat Nucleus Accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF, editors. Effects of Oxytocin on Methamphetamine-Induced Conditioned Place Preference and the Possible Role of Glutamatergic Neurotransmission in the Medial Prefrontal Cortex of Mice in Reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ, editors. Kappa-Opioid Receptors within the Nucleus Accumbens Shell Mediate Pair Bond Maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud M, Karila L, Blecha L, Benyamina A, editors. Is Love Passion an Addictive Disorder? Am J Drug Alcohol Ab. 2010;36:261–267. doi: 10.3109/00952990.2010.495183. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F, editors. In Vivo Crf Release in Rat Amygdala Is Increased During Cocaine Withdrawal in Self-Administering Rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M, editors. Long-Lasting Increase in Voluntary Ethanol Consumption and Transcriptional Regulation in the Rat Brain after Intermittent Exposure to Alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Robson LE, Gillan MG, Kosterlitz HW, editors. Species Differences in the Concentrations and Distributions of Opioid Binding Sites. European Journal of Pharmacology. 1985;112:65–71. doi: 10.1016/0014-2999(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Minarro J, Aguilar MA, Pinazo J, Simon VM, editors. Effects of Risperidone and Sch 23390 on Isolation-Induced Aggression in Male Mice. Eur Neuropsychopharmacol. 1998;8:95–103. doi: 10.1016/s0924-977x(97)00051-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F., editors. Activation of Corticotropin-Releasing Factor in the Limbic System During Cannabinoid Withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ, editors. Characterization of the Oxytocin System Regulating Affiliative Behavior in Female Prairie Voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren XH, Terwilliger EF, Young LJ, editors. Variation in Oxytocin Receptor Density in the Nucleus Accumbens Has Differential Effects on Affiliative Behaviors in Monogamous and Polygamous Voles. Journal of Neuroscience. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ, editors. Oxytocin and the Neural Mechanisms Regulating Social Cognition and Affiliative Behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenberg J, editor. The New York Times. Sanford Acknowledges Other Flirtations. 2009 [Google Scholar]
- Sanchis-Segura C, Spanagel R, editors. Behavioural Assessment of Drug Reinforcement and Addictive Features in Rodents: An Overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, editor. Response Decrement Patterns after Neuroleptic and Non-Neuroleptic Drugs. Psychopharmacol (Berl) 1986;89:98–104. doi: 10.1007/BF00175198. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G, editors. Selective Attenuation of Cocaine-Induced Stereotyped Behaviour by Oxytocin: Putative Role of Basal Forebrain Target Sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Babarczy E, Vecsernyes M, Laczi F, Szabo G, Krivan M, Kovacs GL, Telegdy G, editors. Oxytocin Modulates Behavioural Adaptation to Repeated Treatment with Cocaine in Rats. Neuropharmacology. 1992;31:593–598. doi: 10.1016/0028-3908(92)90192-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G, editors. Brain Corticotropin-Releasing Factor Mediates ‘Anxiety-Like’ Behavior Induced by Cocaine Withdrawal in Rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabo G, Kovacs GL, Telegdy G, editors. Oxytocin Attenuates the Cocaine-Induced Exploratory Hyperactivity in Mice. Neuroreport. 1990;1:200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Asan E, Van Bockstaele EJ, editors. Unraveling Glutamate-Opioid Receptor Interactions Using High-Resolution Electron Microscopy: Implications for Addiction-Related Processes. Exp Neurol. 2011;229:207–213. doi: 10.1016/j.expneurol.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR, editors. Second-Order Schedules of Drug Self-Administration in Animals. Psychopharmacology (Berl) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R, editors. Oxytocin During the Initial Stages of Romantic Attachment: Relations to Couples’ Interactive Reciprocity. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, McCormick PN, Kapur S, editors. Increased Dopamine D2(High) Receptors in Amphetamine-Sensitized Rats, Measured by the Agonist [(3)H](+)Phno. Synapse. 2007;61:263–267. doi: 10.1002/syn.20367. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F, editors. Alcohol-Withdrawn Animals Have a Prolonged Increase in Dopamine D2high Receptors, Reversed by General Anesthesia: Relation to Relapse? Synapse. 2004;52:77–83. doi: 10.1002/syn.20005. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ, editors. Opposite Modulation of Cocaine-Seeking Behavior by D1- and D2-Like Dopamine Receptor Agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, editor. Strange Bedfellows: A Critical View of Pathological Gambling and Addiction. Addiction. 1999;94:1445–1448. doi: 10.1046/j.1360-0443.1999.941014451.x. [DOI] [PubMed] [Google Scholar]
- Shayit M, Nowak R, Keller M, Weller A, editors. Establishment of a Preference by the Newborn Lamb for Its Mother: The Role of Opioids. Behav Neurosci. 2003;117:446–454. doi: 10.1037/0735-7044.117.3.446. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS, editors. Regulation of Affect by the Lateral Septum: Implications for Neuropsychiatry. Brain Research Reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A, editors. Motivational Properties of Opioids: Evidence That an Activation of Delta-Receptors Mediates Reinforcement Processes. Brain Res. 1987;436:234–239. doi: 10.1016/0006-8993(87)91667-2. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ Jr., Shen Y, editors. Molecular Neurobiology of Dopaminergic Receptors. Int Rev Neurobiol. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR, editors. Neuropharmacology of Brain-Stimulation-Evoked Aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Felicio LF, editors. Effects of Dopamine Receptor Antagonists on Ongoing Maternal Behavior in Rats. Pharmacol Biochem Behav. 2001;68:461–468. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL, editors. Disruption of the Kappa-Opioid Receptor Gene in Mice Enhances Sensitivity to Chemical Visceral Pain, Impairs Pharmacological Actions of the Selective Kappa-Agonist U-50,488h and Attenuates Morphine Withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS, editors. Psychological Stress, Drug-Related Cues and Cocaine Craving. Psychopharmacol (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sivam SP, editor. Cocaine Selectively Increases Striatonigral Dynorphin Levels by a Dopaminergic Mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Skelton KH, Gutman DA, Thrivikraman KV, Nemeroff CB, Owens MJ, editors. The Crf1 Receptor Antagonist R121919 Attenuates the Neuroendocrine and Behavioral Effects of Precipitated Lorazepam Withdrawal. Psychopharmacol (Berl) 2007;192:385–396. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Maidment NT, editors. Blockade of Ventral Pallidal Opioid Receptors Induces a Conditioned Place Aversion and Attenuates Acquisition of Cocaine Place Preference in the Rat. Neuroscience. 2003;119:241–249. doi: 10.1016/s0306-4522(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, editors. The Ventral Pallidum and Hedonic Reward: Neurochemical Maps of Sucrose "Liking" and Food Intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM, editors. Cocaine Reward and Hyperactivity in the Rat: Sites of Mu Opioid Receptor Modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS, editors. The Effects of Opioid Peptides on Dopamine Release in the Nucleus Accumbens: An in Vivo Microdialysis Study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, editors. Long-Term Alcohol Self-Administration with Repeated Alcohol Deprivation Phases: An Animal Model of Alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M, editors. Dopamine D1 Receptor Stimulation of the Nucleus Accumbens or the Medial Preoptic Area Promotes the Onset of Maternal Behavior in Pregnancy-Terminated Rats. Behav Neurosci. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M, editors. Hypothalamic Interaction with the Mesolimbic Da System in the Control of the Maternal and Sexual Behaviors in Rats. Neurosci Biobehav Rev. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Bress J, Numan M, editors. Dopamine D(1) Receptor Activation of Adenylyl Cyclase, Not Phospholipase C, in the Nucleus Accumbens Promotes Maternal Behavior Onset in Rats. Horm Behav. 2010;57:96–104. doi: 10.1016/j.yhbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J, editors. Increased Corticotropin-Releasing Factor Concentrations in the Bed Nucleus of the Stria Terminalis of Anhedonic Rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, editor. The Projections of the Ventral Tegmental Area and Adjacent Regions: A Combined Fluorescent Retrograde Tracer and Immunofluorescence Study in the Rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, Telegdy G, editors. Brain Monoamines Are Involved in Mediating the Action of Neurohypophyseal Peptide Hormones on Ethanol Tolerance. Acta Physiol Hung. 1988;71:459–466. [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, Telegdy G, editors. Intraventricular Administration of Neurohypophyseal Hormones Interferes with the Development of Tolerance to Ethanol. Acta Physiol Hung. 1989;73:97–103. [PubMed] [Google Scholar]
- Tagliaferro P, Morales M, editors. Synapses between Corticotropin-Releasing Factor-Containing Axon Terminals and Dopaminergic Neurons in the Ventral Tegmental Area Are Predominantly Glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP, editors. Antagonism of Crf(2) Receptors Produces Anxiolytic Behavior in Animal Models of Anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Tamarin RH, editor. Biology of New World Microtus. American Society of Mammologists; 1985. [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G, editors. Cannabinoid and Heroin Activation of Mesolimbic Dopamine Transmission by a Common Mu1 Opioid Receptor Mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Birney EC, editors. Parental Care and Mating System of the Prairie Vole, Microtus-Ochrogaster. Behav Ecol Sociobiol. 1979;5:171–186. [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS, editors. A Role for Oxytocin and 5-Ht(1a) Receptors in the Prosocial Effects of 3,4 Methylenedioxymethamphetamine ("Ecstasy") Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS, editors. Neural Correlates of Mdma ("Ecstasy")-Induced Social Interaction in Rats. Soc Neurosci. 2009;4:60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA, editors. Effects of Skf 38393 and Quinpirole on Aggressive, Motor and Schedule-Controlled Behaviors in Mice. Behav Pharmacol. 1992a;3:553–565. [PubMed] [Google Scholar]
- Tidey JW, Miczek KA, editors. Morphine Withdrawal Aggression: Modification with D1 and D2 Receptor Agonists. Psychopharmacology (Berl) 1992b;108:177–184. doi: 10.1007/BF02245304. [DOI] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, Moles A, Siracusano A, Gross C, editors. Social Hedonic Capacity Is Associated with the A118g Polymorphism of the Mu-Opioid Receptor Gene (Oprm1) in Adult Healthy Volunteers and Psychiatric Patients. Soc Neurosci. 2011a;6:88–97. doi: 10.1080/17470919.2010.482786. [DOI] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, Siracusano A, Gross C, editors. Variation in the {Micro}-Opioid Receptor Gene (Oprm1) Moderates the Influence of Early Maternal Care on Fearful Attachment. Soc Cogn Affect Neurosci. 2011b doi: 10.1093/scan/nsr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoo A, Yoshii M, Narahashi T, editors. Block of Calcium Channels by Enkephalin and Somatostatin in Neuroblastoma-Glioma Hybrid Ng108-15 Cells. Proc Natl Acad Sci U S A. 1986;83:9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci S, Cheeta S, Seth P, File SE, editors. Corticotropin Releasing Factor Antagonist, Alpha-Helical Crf(9-41), Reverses Nicotine-Induced Conditioned, but Not Unconditioned, Anxiety. Psychopharmacology (Berl) 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- Turkish S, Cooper SJ, editors. Fluid Consumption in Water-Deprived Rats after Administration of Naloxone or Quaternary Naloxone. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:835–839. doi: 10.1016/0278-5846(83)90078-7. [DOI] [PubMed] [Google Scholar]
- Turner RA, Altemus M, Enos T, Cooper B, McGuinness T, editors. Preliminary Research on Plasma Oxytocin in Normal Cycling Women: Investigating Emotion and Interpersonal Distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A, editors. Corticotropin-Releasing Factor Requires Crf Binding Protein to Potentiate Nmda Receptors Via Crf Receptor 2 in Dopamine Neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF, editors. Increased Anxiety-Like Behavior and Ethanol Self-Administration in Dependent Rats: Reversal Via Corticotropin-Releasing Factor-2 Receptor Activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- van Kesteren RE, Smit AB, Dirks RW, de With ND, Geraerts WP, Joosse J, editors. Evolution of the Vasopressin/Oxytocin Superfamily: Characterization of a Cdna Encoding a Vasopressin-Related Precursor, Preproconopressin, from the Mollusc Lymnaea Stagnalis. Proc Natl Acad Sci U S A. 1992;89:4593–4597. doi: 10.1073/pnas.89.10.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leengoed E, Kerker E, Swanson HH, editors. Inhibition of Post-Partum Maternal Behaviour in the Rat by Injecting an Oxytocin Antagonist into the Cerebral Ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, De Wied D, editors. Effect of Neurohypophyseal Hormones on Morphine-Dependence. Psychoneuroendocrinology. 1977;2:35–41. doi: 10.1016/0306-4530(77)90029-4. [DOI] [PubMed] [Google Scholar]
- van Ree JM, de Wied D, editors. Involvement of Neurohypophyseal Peptides in Drug-Mediated Adaptive Responses. Pharmacol Biochem Behav. 1980;13(Suppl 1):257–263. doi: 10.1016/s0091-3057(80)80039-6. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Gerrits MA, Vanderschuren LJ, editors. Opioids, Reward and Addiction: An Encounter of Biology, Psychology, and Medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL, editors. Endogenous Opioids and Reward. Eur J Pharmacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ, editors. Drug Seeking Becomes Compulsive after Prolonged Cocaine Self-Administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK, editors. The Neuroscience of Addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N, editors. Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association with Metabolism in the Orbitofrontal Cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F, editors. Imaging Dopamine’s Role in Drug Abuse and Addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R, Lieberman J, editors. Relationship between Psychostimulant-Induced "High" and Dopamine Transporter Occupancy. Proc Natl Acad Sci U S A. 1996a;93:10388–10392. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N, editors. Decreased Striatal Dopaminergic Responsiveness in Detoxified Cocaine-Dependent Subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K, editors. Decreases in Dopamine Receptors but Not in Dopamine Transporters in Alcoholics. Alcohol Clin Exp Res. 1996b;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, editors. Addiction Circuitry in the Human Brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP, editors. Naltrexone in the Treatment of Alcohol Dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M, editors. Vasopressin, Oxytocin, and Social Odor Recognition. Horm Behav. 2012;61:259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF, editors. Pharmacological Evidence for a Motivational Role of Kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR, editors. D1 Dopamine Receptor Activation Required for Postsynaptic Expression of D2 Agonist Effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsater H, Larsson H, Westberg L, editors. Variation in the Oxytocin Receptor Gene Is Associated with Pair-Bonding and Social Behavior. Biol Psychiatry. 2012;71:419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P, editors. Genetic Variation in the Vasopressin Receptor 1a Gene (Avpr1a) Associates with Pair-Bonding Behavior in Humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB, editors. Cocaine Experience Establishes Control of Midbrain Glutamate and Dopamine by Corticotropin-Releasing Factor: A Role in Stress-Induced Relapse to Drug Seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA, editors. Stress-Induced Relapse to Cocaine Seeking: Roles for the Crf(2) Receptor and Crf-Binding Protein in the Ventral Tegmental Area of the Rat. Psychopharmacol (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ, editors. Convergent Processing of Both Positive and Negative Motivational Signals by the Vta Dopamine Neuronal Populations. PLoS One. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K, editors. Dopamine D2 Receptor Availability in Opiate-Dependent Subjects before and after Naloxone-Precipitated Withdrawal. Neuropsychopharmacology. 1997a;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS, editors. Brain Dopamine and Obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ, editors. Role of Septal Vasopressin Innervation in Paternal Behavior in Prairie Voles (Microtus Ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR, editors. Dopamine D2 Receptor-Mediated Regulation of Partner Preferences in Female Prairie Voles (Microtus Ochrogaster): A Mechanism for Pair Bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Hulihan TJ, Insel TR, editors. Sexual and Social Experience Is Associated with Different Patterns of Behavior and Neural Activation in Male Prairie Voles. Brain Res. 1997b;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Warnick JE, McCurdy CR, Sufka KJ, editors. Opioid Receptor Function in Social Attachment in Young Domestic Fowl. Behav Brain Res. 2005;160:277–285. doi: 10.1016/j.bbr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI, editors. Variation in the Mu-Opioid Receptor Gene (Oprm1) Is Associated with Dispositional and Neural Sensitivity to Social Rejection. Proc Natl Acad Sci U S A. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Schaefer GJ, Michael RP, editors. Increasing the Work Requirements Lowers the Threshold of Naloxone for Reducing Self-Stimulation in the Midbrain of Rats. Pharmacol Biochem Behav. 1983;18:705–710. doi: 10.1016/0091-3057(83)90010-2. [DOI] [PubMed] [Google Scholar]
- Wiesner BP, Sheard NM, editors. Maternal Behavior in the Rat. Oliver & Boyd; Edinburgh: 1933. [Google Scholar]
- Williams JR, Catania KC, Carter CS, editors. Development of Partner Preferences in Female Prairie Voles (Microtus Ochrogaster): The Role of Social and Sexual Experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Wills TA, Cleary SD, editors. How Are Social Support Effects Mediated? A Test with Parental Support and Adolescent Substance Use. Journal of Personality and Social Psychology. 1996;71:937–952. doi: 10.1037//0022-3514.71.5.937. [DOI] [PubMed] [Google Scholar]
- Wilsoncroft WE, editor. Babies by Bar-Press - Maternal Behavior in Rat. Behav Res Meth Inst. 1969;1:229. &. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, editors. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wise RA, editor. Dopamine, Learning and Motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR, editors. The Frontal Cortex-Basal Ganglia System in Primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, Aitchison KJ, editors. Vasopressin and Oxytocin Secretion in Response to the Consumption of Ecstasy in a Clubbing Population. J Psychopharmacol. 2006;20:400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A, editors. From Controlled Drug Intake to Loss of Control: The Irreversible Development of Drug Addiction in the Rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, editor. Effects of a D1 and a D2 Dopamine Antagonist on the Self-Administration of Cocaine and Piribedil by Rhesus Monkeys. Pharmacol Biochem Behav. 1986;24:531–535. doi: 10.1016/0091-3057(86)90553-8. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ, editors. Intravenous Self-Administration of Dopamine Receptor Agonists by Rhesus Monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]
- Woolverton WL, Virus RM, editors. The Effects of a D1 and a D2 Dopamine Antagonist on Behavior Maintained by Cocaine or Food. Pharmacol Biochem Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI, editors. Cloning and Functional Comparison of Kappa and Delta Opioid Receptors from Mouse Brain. Proc Natl Acad Sci U S A. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW, editors. Selective Effects of Naltrexone on Food Pleasantness and Intake. Physiol Behav. 1996;60:439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M, editors. Eating and Drinking Cause Increased Dopamine Release in the Nucleus Accumbens and Ventral Tegmental Area in the Rat: Measurement by in Vivo Microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA, editors. Increased Dopamine Release in Vivo in Nucleus Accumbens and Caudate Nucleus of the Rat During Drinking: A Microdialysis Study. Neuroscience. 1992;48:871–876. doi: 10.1016/0306-4522(92)90275-7. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z, editors. The Neurobiology of Pair Bonding: Insights from a Socially Monogamous Rodent. Front Neuroendocrinol. 2011a;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang Z, editors. The Role of Mesocorticolimbic Dopamine in Regulating Interactions between Drugs of Abuse and Social Behavior. Neurosci Biobehav Rev. 2011b;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Alexander BR, editors. The Chemistry between Us: Love, Sex, and the Science of Attraction. Current Hardcover. 2012 in press. [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR, editors. Cellular Mechanisms of Social Attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA, editors. Anatomy and Neurochemistry of the Pair Bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Young LJ, editor. The Neurobiology of Pair Bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR, editors. Species Differences in V1a Receptor Gene Expression in Monogamous and Nonmonogamous Voles: Behavioral Consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Yudofsky SC, Silver JM, Schneider SE, editors. Pharmacological Treatment of Aggression. Psychiatric Annals. 1987;17:397. &. [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W, editors. Allelic Expression Imbalance of Human Mu Opioid Receptor (Oprm1) Caused by Variant A118g. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP, editors. Subtype-Selective Corticotropin-Releasing Factor Receptor Agonists Exert Contrasting, but Not Opposite, Effects on Anxiety-Related Behavior in Rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F, editors. Changes in Levels of Regional Crf-Like-Immunoreactivity and Plasma Corticosterone During Protracted Drug Withdrawal in Dependent Rats. Psychopharmacol (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD, editors. Disruption of Nmdar-Dependent Burst Firing by Dopamine Neurons Provides Selective Assessment of Phasic Dopamine-Dependent Behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]