Abstract
Background
Chronic inflammation has been proposed as a risk factor for ovarian cancer. Some data suggest that anti-inflammatory medications may be protective against ovarian cancer; however, results have been inconsistent.
Methods
We evaluated the risk of epithelial ovarian cancer with regular use of NSAIDs prospectively in the NIH-AARP Diet and Health Study, using Cox proportional hazard models. We also examined the risk of common subtypes of epithelial ovarian cancer (serous, mucinous, endometrioid, clear cell and other epithelial) with regular use of NSAIDs. In addition, we performed meta-analyses summarizing the risk of ovarian cancer with “regular use” of NSAIDs in previously published studies.
Results
We did not observe a significant association between regular use of NSAIDs with ovarian cancer risk in the AARP cohort (aspirin: RR 1.06, 95% CI 0.87–1.29; non-aspirin NSAIDs: RR 0.93, 95% CI 0.74–1.15); however summary estimates from prospective cohort studies demonstrated that use of non-aspirin NSAIDs may reduce the risk of ovarian cancer (RR 0.88, 95% CI 0.77–1.01). Although not significant, we found that mucinous tumors were inversely associated with non-aspirin NSAID use (RR 0.69, 95% CI 0.23–2.10) in the AARP cohort, which was supported by the meta-analysis (RR 0.69, CI 0.50–0.94.)
Conclusion
Although results from the NIH-AARP cohort study were not statistically significant, our meta-analysis suggests that non-aspirin NSAIDs may be protective against ovarian cancer. Additional analyses, focusing on dose, duration, and frequency of NSAID use and accounting for ovarian cancer heterogeneity are necessary to further elucidate the association between NSAID use and ovarian cancer risk.
Keywords: Aspirin, Non-steroidal anti-inflammatory drugs, Inflammation, Ovarian cancer, Prospective study
Introduction
Ovarian cancer is the tenth leading incident cancer in women in the United States. It has a higher annual mortality rate than any other gynecologic malignancy, accounting for more than 15,000 deaths per year [1]. Possible mechanisms of carcinogenesis which have been proposed include the incessant ovulation, gonadotropin, hormonal, and inflammation hypotheses; however, the etiology of ovarian cancer is poorly understood [2] and prevention strategies are limited. In 1999, Ness and Cottreau hypothesized that inflammation of the ovarian epithelium induced by ovulatory events and other ovarian cancer risk factors is a potential causal factor for ovarian tumors, whereby repeated inflammation events resulting in cell damage and oxidative stress can trigger mutagenesis [3].
Tzonou, et al [4] analyzed the role of unspecified analgesics, among other risk factors, as an ovarian cancer risk factor in a hospital-based case-control study in Greece over two decades ago. The findings from this analysis suggested that analgesics were inversely associated with ovarian cancer. Cramer, et al [5] revisited this topic in a case-control study based in Massachusetts and New Hampshire five years later and did not identify an association between ibuprofen use and ovarian cancer risk; however, this analysis revealed a non-significant inverse association with aspirin use, and a strong inverse association with acetaminophen use. Since then, the association between ovarian cancer and non-steroidal anti-inflammatory drug (NSAID) use and acetaminophen use has been studied in many case-control [6–15] and cohort [16–21] studies with inconclusive results.
In 2005, Bonovas, et al [22] provided a meta-analytic summary describing the association between NSAID use and ovarian cancer risk based on 10 studies; following this, in 2006, Bonovas, et al [23] published a similar meta-analytic summary describing the association between acetaminophen use and ovarian cancer risk based on 8 studies. These analyses did not show a significant association between the use of NSAIDs and ovarian cancer; however, relevant findings from at least five additional case-control studies [10,12,13,15,14], two cohort studies [20,21] and one clinical trial [24] have been published, with heterogeneous results.
To further assess the association between NSAID use and ovarian cancer risk, we examined the associations of regular aspirin and non-aspirin NSAID use with ovarian cancer risk in the large prospective NIH-AARP Diet and Health Study, including a large case series with detailed histologic classification of tumors allowing for examination of associations within histologic subtypes. To our knowledge, our study includes the second largest case series in a prospective analysis on this topic; furthermore, ours is the second study and the first prospective analysis to examine associations by tumor subtype. In addition, we conducted a systematic review and meta-analysis which summarizes results among studies published through December 2011 that assessed the relationship between regular use of aspirin or non-aspirin NSAID and the risk of epithelial ovarian cancer.
Material and methods
Study population
The NIH-AARP Diet and Health Study was established in 1995–1996 when a baseline questionnaire which gathered information about diet and lifestyle was mailed to 3.5 million AARP (formerly known as the "American Association of Retired Persons") members ages 50–71 years located in the state of California, Florida, Louisiana, New Jersey, North Carolina, or Pennsylvania, or in the metropolis of Atlanta, Georgia or Detroit, Michigan. In total 617,119 self-administered questionnaires were returned and 566,399 of these were satisfactorily completed and non-duplicate. Within 6 months from the return of a baseline questionnaire, a second questionnaire, the risk factor questionnaire, which gathered information about more detailed lifestyle and behavioral characteristics (including NSAID use), was mailed to participants. In total, 334,906 subjects returned the risk factor questionnaire and had no history of self-reported cancer in the breast, prostate or colon. Further details of the design and methodology have been described previously [25]. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute.
In our primary analysis, we included study participants who returned both baseline and risk factor questionnaires. Of these 334,906 individuals, we excluded study participants for which one or both of their questionnaires were completed by a proxy (n=10,383); men (n=188,116); women diagnosed with cancer before study entry (n=9,036); and those who had a history of oophorectomy or unknown oophorectomy status at baseline (n=29,725). Of the remaining 97,646 female participants we made further exclusions specific to case and non-case groups. We identified 96,272 women who were not diagnosed with ovarian cancer, who had no indication of death due to ovarian cancer by death reports and who did not have a record of ovarian cancer in the cancer registries, as non-cases. Cases were defined as 477 women diagnosed with ovarian tumors as their first primary registry confirmed neoplasm. The remaining 897 participants were reported to have died from cancer, but did not have registry confirmation and were excluded from the analysis group. In addition, we excluded subjects with missing diagnoses and those with borderline or non-epithelial tumors (n=39). The resulting cohort consisted of 96,710 women (438 cases and 96,272 non-cases) contributing 864,888 person years of follow-up (2,062 person years and 857,807 person years, respectively).
Exposure assessment
In our primary analysis, information about exposure was ascertained through the risk factor questionnaire. Participants were asked if they had taken any aspirin products (including generic aspirin, Bayer, Bufferin, Anacin, Ecotrin or Excedrin) in the past twelve months (yes/no); and if yes, how often did they usually take them: less than 2 times per month, 2–3 times per month, 1–2 times per week, 3–4 times per week, 5–6 times per week, 1 time per day, 2 or more times per day. In a second question, participants were asked if they had taken any of the following non-aspirin NSAIDs in the past twelve months (yes/no): generic ibuprophen, Advil, Nupren, Mortin, Aleve, Orudis, Ketoprophen, Naprosyn, Anaprox, Feldene, Piroxicam, Clinoril, Sulindac, Indocin, Indomethacin, Relafen, Nalfon, Nambumetone, or Fenoprene. Participants were asked not to consider Tylenol, acetaminophen or other pain relievers when answering this question. If they had used any of the listed medications in the past twelve months, they were asked how often they usually took them. Based on their answers to these questions participants were classified in one of three use categories for (1) aspirin and (2) non-aspirin NSAID: unknown, regular user (taking one or more pills per week) or non-regular user (taking less than one pill per week). Individuals who answered “no” to any use, but identified themselves as regular users in the second question were classified as having unknown use patterns (N=208, aspirin; N=136, non-aspirin NSAIDs); similarly, individuals who answered “yes” to any use and who did not answer the second question were classified as having unknown use patterns (N=109, aspirin; N=204, non-aspirin NSAIDs). Individuals who did not answer “yes” or “no” to any use were classified into a use pattern category solely by their answer to the second question (N=13,807, aspirin; N=13,331, non-aspirin NSAIDs). Of the 96,710 study participants, 47,735 (49.4%) were not regular users of aspirin and non-aspirin NSAIDs, 20,913 (21.6%) were regular aspirin users only, 15,592 (16.1%) were regular non-aspirin NSAID users only, 10,045 (10.4%) were regular users of aspirin and non-aspirin NSAIDs, 649 (0.7%) had unknown patterns of aspirin use only, 890 (0.9%) had unknown patterns of non-aspirin NSAID use only and 886 (0.9%) had unknown patterns of aspirin and non-aspirin NSAID use.
Covariate assessment
Covariate data was collected from the baseline or the risk factor questionnaire; this included information about age at entry, gynecologic surgeries, demographics, reproductive history, menopausal status, anthropometric measures, lifestyle factors, and basic information (ever/never use and duration of use) about oral contraceptives (OC) and postmenopausal hormones (PMH). All covariates were modeled in categorical form, except for age at study entry, which was modeled as a continuous variable. The associations between these risk factors and ovarian cancer in the AARP cohort have been described previously [26].
Ascertainment of ovarian cancer
All cohort members were followed through the U.S. Postal Service national database of address changes; updated vital status was tracked through the U.S. Social Security Administration Death Master File and the National Death Index Plus. Incident ovarian cancers were identified using linkage with cancer registries in the original recruitment areas and in three common states of relocation (Arizona, Texas and Nevada). Identification of cases by cancer registry linkage had an estimated sensitivity of approximately 90% and specificity of 99.5%; completeness of case ascertainment has been reported previously [27]. Follow-up time was defined as time from study entry (the date at which a participant’s risk factor questionnaire was collected) until diagnosis of any cancer, date of death, the date a participant moved out of a registry ascertainment area, or date of last follow-up (December 31, 2006).
Only first primary incident ovarian cancers were included in our case set; participants with a history of any cancer at the study entry or those who had died from cancer during follow-up but that did not appear in the cancer registry data were excluded. Registry confirmed ovarian cancer diagnosis was defined using International Classification of Disease for Oncology, Third Edition (ICD-O-3) site and histology codes. Epithelial ovarian cancer cases were classified into five histologic subtypes: serous (8441, 8460-62), endometrioid (8380-81, 8560, 8570), mucinous (8470-71, 8480-81), clear cell (8310, 8313) and other epithelial types (8010, 8020-21, 8046, 8070, 8120, 8140, 8255, 8323, 8440, 8490, 8562, 8240, 8246, 8050, 8260, 8450); borderline and non-epithelial ovarian cancer cases were excluded from our analysis.
Statistical analysis
Cox proportional hazards models were used to estimate hazard rate ratios (RR) and 95% confidence intervals (CI) of all epithelial ovarian cancers combined and of histologic subtypes. Models examining all epithelial ovarian cancers compared all cases to all non-cases. To examine associations for serous, endometrioid, mucinous, clear cell and other epithelial tumor types, we constructed five separate models, stratifying the analysis by histologic subtype and comparing each case subtype to all non-cases.
All models considered the two exposures, aspirin and non-aspirin NSAID use, simultaneously; time on study (in year) was used as the time metric in all models; and well established risk factors for ovarian cancer, including entry age (continuous), race/ethnicity (white, non-white, unknown), age of menarche (<13 years, 13–14 years, 15+ years, unknown), parity (nulliparous, one, two, three+, unknown), age of (natural or surgical) menopause (premenopausal, <45 years, 45–49 years, 50–54 years, 55+ years, unknown), hysterectomy status (ever, never, unknown), PMH use (never, estrogen only <10 years, estrogen only 10+ years, estrogen and progesterone <10 years, estrogen and progesterone 10+ years, unknown), OC use (<1 year, 1 year to less than 10 years, 10+ years, unknown) and first and second degree family history of ovarian cancer (no, yes, unknown) were identified a priori and adjusted for in our analyses.
To assess differences in effect estimates across histologic subtypes we utilized a method which accounts for competing risks [28]. We have assumed that a diagnosis of one histological subtype of ovarian cancer prohibited a case from being diagnosed with a different histological subtype of ovarian cancer. In order to assess heterogeneity using the latter method, we created five duplicate data sets with one record for each subtype; four of the five outcomes were entered as a non-event in each record. The probability of histology-specific failure risk was estimated by censoring each subtype at the time when it was diagnosed. We then compared this model to a model with a single estimate for all cases using a likelihood ratio test to assess statistical significance of heterogeneity.
Evidence for effect modification by parity status (parous, nulliparous), PMH use (ever, never), oral contraceptive use (ever, never) and first and/or second degree history of ovarian cancer (yes, no) was evaluated. Individuals with unknown status for the potential effect modifier were dropped from this analysis. We assessed effect modification by including cross-product terms between the exposure of interest (dichotomous) and the potential effect modifier (dichotomous) in multivariate models and evaluating the p-value associated with the cross-product term.
Sensitivity analyses were performed to assess whether simultaneously derived estimates (aspirin and non-aspirin NSAID use modeled together) were similar to separately derived estimates (aspirin or non-aspirin NSAID use only), in addition to assessing ovarian cancer risk with monthly, weekly or daily use of aspirin and non-aspirin NSAIDs (less than 4 times monthly, 1 to 6 times weekly, or 1 or more times daily, respectively), compared to never use, in multivariate models assessing aspirin and non-aspirin NSAID use simultaneously and separately; risk in heavy aspirin and non-aspirin NSAID users (5 or more times weekly), compared to never users, was also assessed simultaneously and separately in multivariate models. In addition, risk among “either” users (meaning users of aspirin, non-aspirin NSAIDs or both) compared to “neither” users (meaning, users of neither analgesic type or individuals whose use patterns of both analgesic type fell into the comparison group) was assessed in regular compared to non-regular users, monthly, weekly or daily compared to never users, and heavy compared to never users. We also conducted sensitivity analyses examining the effect of removing cases with short follow-up (less than one or less than two years) from our analysis on effect estimates. Exploratory analyses were also performed to evaluate whether stratification by clear cell and endometrioid tumor types combined were similar to clear cell or endometrioid tumor types alone. Likewise, pooled estimates of serous and other epithelial tumor types were examined and compared to serous or other epithelial tumor types alone.
In all analyses, p ≤ 0.05 was considered statistically significant, using two-sided tests. All analyses were performed using SAS software release version 9.3 (SAS Institute, Cary, NC).
Meta-Analysis
All articles examining the use of aspirin, non-aspirin anti-inflammatory drugs and/or acetaminophen and ovarian cancer risk published through December 31, 2011 in the English language on the PubMed electronic database were identified by the systematic keyword searches: ("anti-inflammatory agents, non-steroidal" [MeSH Terms] OR ("anti-inflammatory" [All Fields] AND "agents" [All Fields] AND "non-steroidal" [All Fields]) OR "non-steroidal anti-inflammatory agents" [All Fields] OR "nsaids" [All Fields] OR "anti-inflammatory agents, non-steroidal" [Pharmacological Action]) AND ("ovarian neoplasms" [MeSH Terms] OR ("ovarian" [All Fields] AND "neoplasms" [All Fields]) OR "ovarian neoplasms" [All Fields] OR ("ovarian" [All Fields] AND "cancer" [All Fields]) OR "ovarian cancer" [All Fields]). Additional studies were identified through review of references in articles identified through the above described search. A total of 247 studies were identified. Only original articles contributing information about cases and non-cases that used aspirin or non-aspirin NSAIDs were selected for inclusion. A total of 19 articles met these selection criteria; of these, 12 were case-control studies, 6 were cohort studies (not including the study presented here) and one was a randomized clinical trial. First author, date of publication, study sample size, type, frequency and duration of use, effect estimate (RR) and 95% CI, histology information, and details about the study population were extracted for all of these studies. Only studies which examined use patterns that could be translated into a similar metric were included; regular and non-regular use was defined by number of pills taken per week on average. In addition, the randomized clinical trial was not included in our analysis because we wanted to capture true use patterns; we were concerned about compliance with assigned use. A total of 13 studies examining the association between regular aspirin use and ovarian cancer were included in our analysis; a total of 6 studies examining the association between regular non-aspirin NSAID use and ovarian cancer were included in our analysis. We also included our data estimates from the current analysis of the AARP cohort study. Details of all 19 articles which met our initial selection criteria, including reasons for inclusion or exclusion in our analyses are included in Supplemental Table SI.
Sensitivity analyses were performed to determine whether removal of any of the included studies affected our estimates; in addition, inclusion of studies examining ibuprofen use alone was assessed to determine whether this would alter our estimates for regular use of non-aspirin NSAIDs. Estimates were also stratified by study design (case-control studies compared to cohort studies) to assess possible effects of recall bias and selection bias in case-control studies.
Summary estimates and 95% CIs were calculated using random effects models; heterogeneity among studies was assessed using Cochran’s Q tests, for which p ≤ 0.05 was considered statistically significant; I2 estimates were calculated to determine the degree of heterogeneity between tests, which was interpreted using a previously described scale: high heterogeneity was observed in estimates with I2≥75%, moderate heterogeneity was observed in estimates with 50%≤I2<75% and low heterogeneity was observed in estimates with I2<50% [29]. All statistical analyses were performed using STATA software release version 11 (STATA Corporation, College Station, TX); forest plots and I2 estimates were generated using R software package version 2.12.2 (http://cran.r-project.org/).
RESULTS
NIH-AARP Diet and Health Study Cohort
The study population of 96,710 women comprised 438 cases and 96,272 non-cases, ranging in age from 50 to 71 years old and including 47,212 subjects who used aspirin and/or non-aspirin NSAIDs at a regular frequency. Detailed characteristics of the study population by aspirin and non-aspirin NSAID use patterns are described in Table I.
Table I.
Distribution of demographics, lifestyle and risk factor characteristics within the AARP study population contributing to categories of aspirin and non-aspirin NSAID use patterns
| Aspirin Use | Non-Aspirin NSAIDs Use | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Regular | Regular | Non-Regular | Regular | ||||||
| N | % | N | % | N | % | N | % | ||
| N | 63,864 | 31,311 | 68,988 | 25,946 | |||||
| Average age at entry (yr) | 61.6 | 62.4 | 62.0 | 61.5 | |||||
| Follow-up (person-yr) | 569,795 | 276,635 | 614,179 | 230,111 | |||||
| Race/Ethnicity | |||||||||
| White Non-Hispanic | 57,915 | 90.7 | 29,071 | 92.8 | 62,878 | 91.1 | 23,911 | 92.2 | |
| Other | 5,225 | 8.2 | 1,883 | 6.0 | 5,340 | 7.7 | 1,730 | 6.7 | |
| Unknown | 724 | 1.1 | 357 | 1.1 | 770 | 1.1 | 305 | 1.2 | |
| Education | |||||||||
| High school diploma or less | 24,698 | 38.7 | 12,663 | 40.4 | 26,912 | 39.0 | 10,353 | 39.9 | |
| Post-secondary school | 37,601 | 58.9 | 17,788 | 56.8 | 40,396 | 58.6 | 14,879 | 57.3 | |
| Unknown | 1,565 | 2.5 | 860 | 2.7 | 1,680 | 2.4 | 714 | 2.8 | |
| Body Mass Index | |||||||||
| <25 kg/m2 | 29,625 | 46.4 | 13,475 | 43.0 | 33,102 | 48.0 | 9,906 | 38.2 | |
| 25–30 kg/m2 | 19,444 | 30.4 | 10,089 | 32.2 | 21,168 | 30.7 | 8,268 | 31.9 | |
| 30+ kg/m2 | 13,050 | 20.4 | 6,947 | 22.2 | 12,803 | 18.6 | 7,169 | 27.6 | |
| Unknown | 1,745 | 2.7 | 800 | 2.6 | 1,915 | 2.8 | 603 | 2.3 | |
| Smoking | |||||||||
| Never | 29,063 | 45.5 | 13,493 | 43.1 | 31,293 | 45.4 | 11,166 | 43.0 | |
| Former | 24,938 | 39.0 | 12,561 | 40.1 | 26,602 | 38.6 | 10,832 | 41.7 | |
| Current | 8,301 | 13.0 | 4,403 | 14.1 | 9,351 | 13.6 | 3,293 | 12.7 | |
| Unknown | 1,562 | 2.4 | 854 | 2.7 | 1,742 | 2.5 | 655 | 2.5 | |
| Alcohol Consumption | |||||||||
| <1 drink/day | 54,513 | 85.4 | 26,438 | 84.4 | 58,629 | 85.0 | 22,105 | 85.2 | |
| 1–3 drinks/day | 7,376 | 11.5 | 3,762 | 12.0 | 8,127 | 11.8 | 2,989 | 11.5 | |
| 3+ drinks/day | 1,975 | 3.1 | 1,111 | 3.5 | 2,232 | 3.2 | 852 | 3.3 | |
| Age at menarche | |||||||||
| <13 yrs | 30,673 | 48.0 | 15,270 | 48.8 | 32,653 | 47.3 | 13,181 | 50.8 | |
| 13–14 yrs | 27,169 | 42.5 | 13,134 | 41.9 | 29,752 | 43.1 | 10,452 | 40.3 | |
| 15+ yrs | 5,841 | 9.1 | 2,830 | 9.0 | 6,389 | 9.3 | 2,253 | 8.7 | |
| Unknown | 181 | 0.3 | 77 | 0.2 | 194 | 0.3 | 60 | 0.2 | |
| Parity | |||||||||
| Nulliparous | 10,117 | 15.8 | 4,683 | 15.0 | 11,235 | 16.3 | 3,541 | 13.6 | |
| One | 6,463 | 10.1 | 3,097 | 9.9 | 7,143 | 10.4 | 2,390 | 9.2 | |
| Two | 16,723 | 26.2 | 7,897 | 25.2 | 17,888 | 25.9 | 6,660 | 25.7 | |
| Three+ | 30,358 | 47.5 | 15,520 | 49.6 | 32,500 | 47.1 | 13,265 | 51.1 | |
| Unknown | 203 | 0.3 | 114 | 0.4 | 222 | 0.3 | 90 | 0.3 | |
| Age First Birth | |||||||||
| Nulliparous | 9,665 | 15.1 | 4,459 | 14.2 | 10,705 | 15.5 | 3,392 | 13.1 | |
| <20 yrs | 9,695 | 15.2 | 5,031 | 16.1 | 10,040 | 14.6 | 4,624 | 17.8 | |
| 20–24 yrs | 27,462 | 43.0 | 13,962 | 44.6 | 29,578 | 42.9 | 11,765 | 45.3 | |
| 25–29 yrs | 12,269 | 19.2 | 5,727 | 18.3 | 13,405 | 19.4 | 4,540 | 17.5 | |
| 30+ yrs | 4,276 | 6.7 | 1,872 | 6.0 | 4,686 | 6.8 | 1,442 | 5.6 | |
| Unknown | 497 | 0.8 | 260 | 0.8 | 574 | 0.8 | 183 | 0.7 | |
| Age at Menopause | |||||||||
| Premenopausal | 4,006 | 6.3 | 1,637 | 5.2 | 3,876 | 5.6 | 1,759 | 6.8 | |
| <45 yrs | 16,163 | 25.3 | 8,725 | 27.9 | 17,223 | 25.0 | 7,592 | 29.3 | |
| 45–49 yrs | 14,699 | 23.0 | 7,209 | 23.0 | 16,038 | 23.2 | 5,818 | 22.4 | |
| 50–54 yrs | 23,374 | 36.6 | 11,056 | 35.3 | 25,741 | 37.3 | 8,611 | 33.2 | |
| 55+ yrs | 5,312 | 8.3 | 2,532 | 8.1 | 5,780 | 8.4 | 2,043 | 7.9 | |
| Unknown | 310 | 0.5 | 152 | 0.5 | 330 | 0.5 | 123 | 0.5 | |
| Hysterectomy Status | |||||||||
| Never | 48,568 | 76.0 | 23,101 | 73.8 | 53,098 | 77.0 | 18,377 | 70.8 | |
| Ever | 15,014 | 23.5 | 8,085 | 25.8 | 15,584 | 22.6 | 7,473 | 28.8 | |
| Unknown | 282 | 0.4 | 125 | 0.4 | 306 | 0.4 | 96 | 0.4 | |
| Post-menopausal Hormone Use | |||||||||
| Never | 29,631 | 46.4 | 13,701 | 43.8 | 33,394 | 48.4 | 9,780 | 37.7 | |
| Estrogen only, <10 yr | 6,982 | 10.9 | 3,659 | 11.7 | 7,321 | 10.6 | 3,311 | 12.8 | |
| Estrogen only, 10 yr+ | 4,215 | 6.6 | 2,535 | 8.1 | 4,353 | 6.3 | 2,377 | 9.2 | |
| Estrogen + progesterone, <10 yr | 14,840 | 23.2 | 7,137 | 22.8 | 15,332 | 22.2 | 6,619 | 25.5 | |
| Estrogen + progesterone, 10 yr+ | 4,743 | 7.4 | 2,575 | 8.2 | 4,963 | 7.2 | 2,355 | 9.1 | |
| Unknown | 3,453 | 5.4 | 1,704 | 5.4 | 3,625 | 5.3 | 1,504 | 5.8 | |
| Oral Contraceptive Use | |||||||||
| <1 yr | 37,307 | 58.4 | 18,765 | 59.9 | 41,407 | 60.0 | 14,471 | 55.8 | |
| 1 yr to <10 yr | 19,632 | 30.7 | 9,290 | 29.7 | 20,355 | 29.5 | 8,524 | 32.9 | |
| 10 yr+ | 6,543 | 10.2 | 3,050 | 9.7 | 6,792 | 9.8 | 2,801 | 10.8 | |
| Unknown | 382 | 0.6 | 206 | 0.7 | 434 | 0.6 | 150 | 0.6 | |
| First Degree Relatives with Ovarian Cancer | |||||||||
| No | 48,488 | 75.9 | 23,480 | 75.0 | 52,186 | 75.6 | 19,627 | 75.6 | |
| Yes | 3,379 | 5.3 | 1,663 | 5.3 | 3,548 | 5.1 | 1,482 | 5.7 | |
| Unknown | 11,997 | 18.8 | 6,168 | 19.7 | 13,254 | 19.2 | 4,837 | 18.6 | |
| Second Degree Relatives with Ovarian Cancer | |||||||||
| No | 57,856 | 90.6 | 28,309 | 90.4 | 62,658 | 90.8 | 23,333 | 89.9 | |
| Yes | 3,379 | 5.3 | 1,698 | 5.4 | 3,517 | 5.1 | 1,539 | 5.9 | |
| Unknown | 2,629 | 4.1 | 1,304 | 4.2 | 2,813 | 4.1 | 1,074 | 4.1 | |
| First Degree Relatives with Breast Cancer | |||||||||
| No | 44,583 | 69.8 | 21,540 | 68.8 | 47,898 | 69.4 | 18,063 | 69.6 | |
| Yes | 8,529 | 13.4 | 4,142 | 13.2 | 9,173 | 13.3 | 3,495 | 13.5 | |
| Unknown | 10,752 | 16.8 | 5,629 | 18.0 | 11,917 | 17.3 | 4,388 | 16.9 | |
| Second Degree Relatives with Breast Cancer | |||||||||
| No | 50,074 | 78.4 | 24,675 | 78.8 | 54,450 | 78.9 | 20,123 | 77.6 | |
| Yes | 11,161 | 17.5 | 5,332 | 17.0 | 11,725 | 17.0 | 4,749 | 18.3 | |
| Unknown | 2,629 | 4.1 | 1,304 | 4.2 | 2,813 | 4.1 | 1,074 | 4.1 | |
The adjusted RR comparing the risk of ovarian cancer in regular aspirin users to non-regular aspirin users was 1.06, 95% CI 0.87–1.29. The RR comparing the risk of ovarian cancer in regular non-aspirin NSAID users to non-regular users was 0.93, 95% CI 0.74–1.15. Of the 438 epithelial ovarian cancers in this cohort, 237 were identified as serous, 20 as mucinous, 31 as endometrioid, 16 as clear cell and 134 as other epithelial tumor subtypes. Stratified analyses by serous (aspirin: RR 1.00, 95% CI 0.76–1.32; non-aspirin NSAIDs: RR 0.88, 95% CI 0.65–1.19), mucinous (aspirin: RR 1.57, 95% CI 0.63–3.93; non-aspirin NSAIDs: RR 0.69, 95% CI 0.23–2.10), endometrioid (aspirin: RR 1.37, 95% CI 0.66–2.84; non-aspirin NSAIDs: RR 0.91, 95% CI 0.40–2.06), clear cell (aspirin: RR 1.36, 95% CI 0.49–3.75; non-aspirin NSAIDs: RR 0.38, 95% CI 0.09–1.71), and other epithelial tumor types (aspirin: RR 0.99, 95% CI 0.69–1.42; non-aspirin NSAIDs: RR 1.13, 95% CI 0.77–1.65) comparing regular users to non-regular users did not show significant associations. These results are summarized in Table II. Heterogeneity across subtypes was also assessed; the effects of aspirin and non-aspirin NSAID use on the risk of ovarian cancer were both found to be homogeneous across subtypes (data not shown).
Table II.
Effect estimates for risk of ovarian cancer and ovarian cancer histologic subtypes in aspirin and non-aspirin NSAID users in the AARP study population
| All Cases (N=438) a | Serous (N=237) a | Mucinous (N=20) a | Endometrioid (N=31) a | Clear Cell (N=16) a | Other Epithelial (N=134) a | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Use Type and Frequency | N b | Follow-Up (person-yr) |
RR | 95% CI | N b | Follow-Up (person-yr) |
RR | 95% CI | N b | Follow-Up (person-yr) |
RR | 95% CI | N b | Follow-Up (person-yr) |
RR | 95% CI | N b | Follow-Up (person-yr) |
RR | 95% CI | N b | Follow-Up (person-yr) |
RR | 95% CI | |
| Aspirin | |||||||||||||||||||||||||
| Non-Regular Use c | 283 | 1,359 | ref | ref | 155 | 781 | ref | ref | 11 | 45 | ref | ref | 19 | 84 | ref | ref | 10 | 37 | ref | ref | 88 | 411 | ref | ref | |
| Regular Use d | 150 | 683 | 1.06 | 0.87, 1.29 | 78 | 367 | 1.00 | 0.76, 1.32 | 8 | 46 | 1.57 | 0.63, 3.93 | 12 | 50 | 1.37 | 0.66, 2.84 | 6 | 36 | 1.36 | 0.49, 3.75 | 46 | 185 | 0.99 | 0.69, 1.42 | |
| Non-Aspirin NSAIDs | |||||||||||||||||||||||||
| Non-Regular Use c | 319 | 1,510 | ref | ref | 172 | 845 | ref | ref | 16 | 80 | ref | ref | 23 | 102 | ref | ref | 14 | 67 | ref | ref | 94 | 415 | ref | ref | |
| Regular Use d | 111 | 489 | 0.93 | 0.74, 1.15 | 58 | 267 | 0.88 | 0.65, 1.19 | 4 | 12 | 0.69 | 0.23, 2.10 | 8 | 32 | 0.91 | 0.40, 2.06 | 2 | 6 | 0.38 | 0.09, 1.71 | 39 | 173 | 1.13 | 0.77, 1.65 | |
Multivariate analysis adjusting for entry age, race/ethnicity, age of menarche, parity, age of (natural or surgical) menopause, hysterectomy status, PMH use, oral contraceptive use and first and second degree family history of ovarian cancer.
The number of non-regular and regular users in the aspirin and the other NSAIDs group do not add to the total number of cases because there are missing values in each.
Non-regular use was defined as taking less than one pill per week on average
Regular use was defined as taking one or more pills per week
Evidence of effect modification by parity status, PMH use, oral contraceptive use or history of ovarian cancer was not observed (data not shown.)
Sensitivity analyses did not indicate that there was a difference in effect estimates when aspirin use and non-aspirin NSAID use were modeled separately compared to jointly. There was not an effect of monthly, weekly or daily use of aspirin or non-aspirin NSAIDs compared to never use on risk of ovarian cancer; furthermore, there were no associations between heavy use of aspirin or non-aspirin NSAIDs compared to never use. There were also no notable differences in estimates for pooled histologic subtypes compared to stratified estimates (clear cell and endometrioid tumors pooled compared to clear cell tumors only or endometrioid tumors only; serous and other epithelial tumors pooled compared to serous tumors only or other epithelial tumors only.) Further, we did not observe differences in effect estimates when removing cases with short follow-up (less than one or less than two years) from our analyses.
Summary Results for Published Studies Using Meta-Analytic Approach
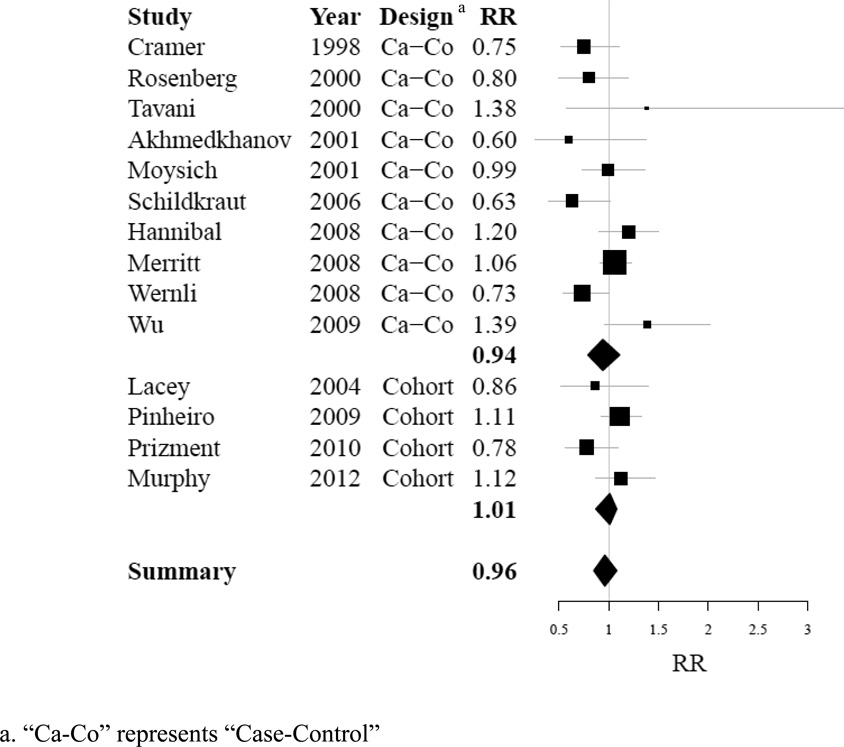
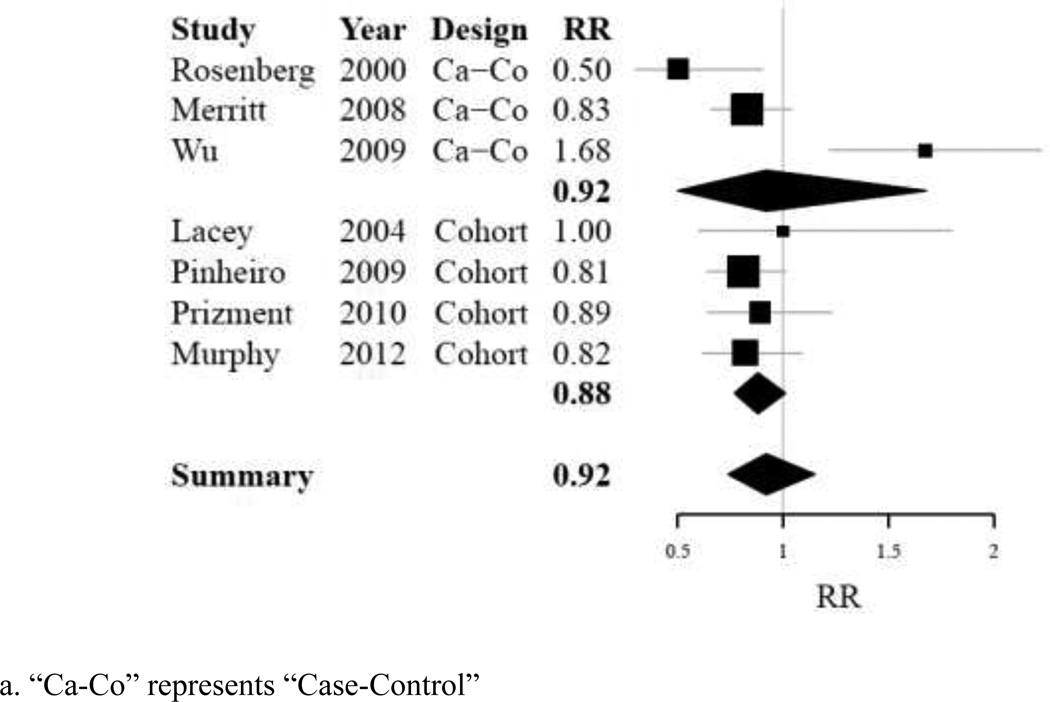
Of the 13 published studies identified as suitable for use in a summary analysis, all contributed data to assess the risk of ovarian cancer with regular use of aspirin compared to non-regular aspirin use; 6 contributed data to assess the risk of ovarian cancer with regular use of non-aspirin NSAIDs compared to non-regular use of non-aspirin NSAIDs. None of the individual studies showed a significant association between regular aspirin use and ovarian cancer risk. Two studies [16,7] showed a significant inverse association between regular non-aspirin NSAID use and ovarian cancer risk. Summary effects for each type of analgesic were null when including risk estimates from the AARP cohort data in our analysis (aspirin: RR 0.96, 95% CI 0.85–1.08, p-heterogeneity=0.06, I2 = 40.7%; non-aspirin NSAIDs: RR 0.92, 95% CI 0.74–1.15, p-heterogeneity= 0.00, I2 = 73.1%). Detailed information about these summary results is compiled in Table III and Table IV. Sensitivity analyses did not indicate that removal of any one study from our analysis would alter our effect estimate for regular aspirin use; however, removal of one case-control study (Wu [14]) altered our effect estimate for non-aspirin NSAID use substantially (with exclusion of study: RR 0.85, 95% CI 0.75–0.95, p-heterogeneity=0.44, I2 = 0.0%). Furthermore, sensitivity analyses did not indicate that inclusion of studies examining ibuprofen use only (Cramer [5]; Wernli [15]) would alter effect estimates for regular use of non-aspirin NSAIDs (data not shown). Stratification did not demonstrate significant differences in effect estimates between case-control and cohort study designs for either analgesic type; however the estimate obtained for non-aspirin NSAIDs among cohort studies was borderline statistically significant association (non-aspirin NSAIDs, cohort studies: RR 0.88, 95% CI 0.77–1.01, p-heterogeneity= 0.81, I2 = 0.0%). Summary results are displayed in Table V. Forest plots demonstrating distribution of results by study are shown in Figure IA, for aspirin use, and Figure IB, for non-aspirin NSAIDs use.
Table III.
Data from fourteen studies contributing to summary effect estimates for ovarian cancer risk in aspirin users [5,8,20,7,6,9,10,12,13,15,14,19,21]
| Author | Year | Cases | "Regular Use" Definition | RR (95% CI) | |
|---|---|---|---|---|---|
| Case-Control | |||||
| Cramer | 1998 | 563 | ≥ 1×/wk | 0.75 (0.52–1.10) | |
| Rosenberg | 2000 | 780 | ≥ 4×/wk | 0.80 (0.50–1.20) | |
| Tavani | 2000 | 749 | ≥ 1×/wk | 1.38 (0.57–3.36) | |
| Akhmedkhanov | 2001 | 68 | ≥ 3×/wk | 0.60 (0.26–1.38) | |
| Moysich | 2001 | 547 | ≥ 1×/wk | 0.99 (0.73–1.36) | |
| Schildkraut | 2006 | 586 | ≥ 2×/wk | 0.63 (0.39–1.02) | |
| Hannibal | 2008 | 812 | > 1×/wk | 1.20 (0.90–1.50) | |
| Merritt | 2008 | 1,576 | ≥ 2×/wk | 1.06 (0.80–1.41) | |
| Wernli | 2008 | 487 | ≥ 2×/wk | 0.73 (0.54–1.00) | |
| Wu a | 2009 | 609 | ≥ 1×/wk | 1.39 (0.96–2.02) | |
| Cohort Studies | |||||
| Lacey | 2004 | 116 | ≥ 1×/wk | 0.86 (0.52–1.40) | |
| Pinheiro | 2009 | 666 | ≥ 2×/wk | 1.11 (0.92–1.33) | |
| Prizment | 2010 | 167 | ≥ 1×/wk | 0.78 (0.56–1.09) | |
| AARP | 2012 | 438 | ≥ 1×/wk | 1.06 (0.87–1.29) | |
Estimate associated with "regular use" definition was determined by pooling two or more estimates provided in the manuscript.
Table IV.
Data from seven studies contributing to summary effect estimates for ovarian cancer risk in non-aspirin NSAID users [7,13,14,19–21]
| Author | Year | Cases | "Regular Use" Definition | RR (95% CI) | |
|---|---|---|---|---|---|
| Case-Control | |||||
| Rosenberg | 2000 | 780 | ≥ 4×/wk | 0.50 (0.30–0.90) | |
| Merritt | 2008 | 1,576 | ≥ 2×/wk | 0.83 (0.66–1.04) | |
| Wu a | 2009 | 609 | ≥ 1×/wk | 1.68 (1.23–2.23) | |
| Cohort Studies | |||||
| Lacey | 2004 | 116 | ≥ 1×/wk | 1.00 (0.60–1.80) | |
| Pinheiro | 2009 | 666 | ≥ 2×/wk | 0.81 (0.64–1.01) | |
| Prizment | 2010 | 167 | ≥ 1×/wk | 0.89 (0.64–1.23) | |
| AARP | 2012 | 438 | ≥ 1×/wk | 0.93 (0.74–1.15) | |
Estimate associated with "regular use" definition was determined by pooling two or more estimates provided in the manuscript.
Table V.
Effect estimates for ovarian cancer risk in regular aspirin and non-aspirin NSAID users overall and stratified by type of study design
| Use Type and Frequency | Cases | RR (95% CI) | p-heterogeneity | I2 | |
|---|---|---|---|---|---|
| Aspirin | |||||
| Case-Control Studies | 6,777 | 0.94 (0.79–1.11) | 0.04 | 48.4% | |
| Cohort Studies | 1,387 | 1.01 (0.87–1.17) | 0.27 | 0.0% | |
| Summary Estimate | 8,164 | 0.96 (0.85–1.08) | 0.06 | 40.7% | |
| Non-Aspirin NSAIDs | |||||
| Case-Control Studies | 2,965 | 0.92 (0.50–1.68) | 0.00 | 90.1% | |
| Cohort Studies | 1,387 | 0.88 (0.77–1.01) | 0.81 | 0.0% | |
| Summary Estimate | 4,352 | 0.92 (0.74–1.15) | 0.00 | 73.1% | |
Figure I.
A: Forest plot summarizing individual effect estimates from fourteen studies contributing to pooled effect estimates for ovarian cancer risk in aspirin users [5,8,20,7,6,9,10,12,13,15,14,19,21]
B: Forest plot summarizing individual effect estimates from seven studies [7,13,14,19–21] contributing to pooled effect estimates for ovarian cancer risk in non-aspirin NSAID users
DISCUSSION
Our analysis within a large prospective cohort including 438 incident ovarian cancer cases did not find an association between aspirin or non-aspirin NSAID use with ovarian cancer risk. In a meta-analysis summarizing data from available studies including our study, we found that the regular use of aspirin was not associated with ovarian cancer risk; and the regular use of non-aspirin NSAIDs was not significantly associated with ovarian cancer risk, but statistically significant heterogeneity among studies was observed. Overall, our data suggest no association (RR 0.92, 95% CI 0.74–1.15) between non-aspirin NSAID use and ovarian cancer risk while data from only prospective cohort studies demonstrated that use of non-aspirin NSAIDs may reduce the risk of ovarian cancer (RR 0.88, 95% CI 0.77–1.01).
We noted through sensitivity analyses that exclusion of one case-control study [14] from our analysis exploring the relationship between use of non-aspirin NSAIDs and ovarian cancer risk altered our summary estimates substantially (RR 0.85, 95% CI 0.75–0.95), providing more support for a potential inverse association between non-aspirin NSAIDs and ovarian cancer. Interestingly, this study was the single case-control study with the most lenient definition for regular use (≥ 1×/wk) incorporated into our analysis. We note that two studies (one included in our meta-analysis and one excluded due to overlap with an included study) with regular use patterns defined as ≥4×/wk [7,16] demonstrated strong statistically significant inverse associations with ovarian cancer (RR 0.50, 95% CI 0.30–0.90; RR 0.59, 95% CI 0.35–0.98), indicating that more frequent use may be relevant. Furthermore, we found that prospective cohort studies in our meta-analysis of non-aspirin use and ovarian cancer provided homogenous results overall, but case-control studies showed more heterogeneous effect estimates than cohort studies. We attribute much of this heterogeneity to the vast differences in the definition of “regular use” across studies (varying from ≥1×/wk to ≥4×/wk,)
Recent studies have demonstrated that risk factors for ovarian cancer differ by histologic subtype [26,30]. Although our dataset contained a comparably large number of cases, there were limited numbers of the less common epithelial ovarian cancer subtypes. We observed point estimates which suggested that non-aspirin NSAIDs were inversely associated with mucinous and clear cell tumors, for example; however, these results were not significant (RR 0.69, 95% CI 0.23–2.10 and RR 0.38, 95% CI 0.09–1.71, respectively.) Furthermore, tests for heterogeneity demonstrated that there was no difference between estimates for histologic subtypes (aspirin: p-heterogeneity = 0.792; non-aspirin NSAIDs: p-heterogeneity = 0.594). However, the analysis was limited by low numbers of less common subtypes.
Only one other study examined the association between aspirin or non-aspirin NSAID use and ovarian cancer subtypes, including 994 serous, 191 mucinous, 141 endometriod and 88 clear cell tumors [13]; this study demonstrated a significant inverse association between non-aspirin NSAIDs and mucinous tumors (RR 0.69, 95% CI 0.50–0.94) and a suggestive, but statistically non-significant, inverse association between non-aspirin NSAIDs and endometrioid tumors (RR 0.76, 95% CI 0.53–1.09 in ever users).
The ovary is often the site of metastases and 10–30% of ovarian tumors are actually secondary cancers [31]. In instances where the primary cancer is not readily detected, an ovarian metastasis may be misclassified as a primary cancer; for example, endometrioid and mucinous subtypes are occasionally metastases of endometrial and colorectal cancers, respectively, rather than ovarian primaries [32,33]. Modungo proposed inflammation as a potential mechanism for endometrial cancer development in 2005 [34]; however, few studies have examined the association between NSAIDs and endometrial cancer since. The studies which have been published on the topic are conflicting; several report a null effect between NSAIDs and endometrial cancer [35–37], while others report an inverse association in obese women [38,39] or an inverse association in all women [40]. On the other hand, aspirin and non-aspirin NSAIDs are both established to be inversely associated with colorectal cancer [41,42], with a stronger association observed between NSAID use and proximal compared to distal colorectal cancer [43,44]. This may partially explain the significant inverse association Merritt observed for mucinous subtypes, and the trend observed in our analysis.
In addition to commonly representing metastases from other anatomical locations, both endometrioid and mucinous tumors have been linked to inflammation inducing risk factors. Endometriosis has been consistently linked to endometrioid tumors [45,46]. Endometriosis is associated with chronic inflammation and may lead to inflammation-induced tumor promotion at the site of the ovaries [47]. Likewise, smoking status and duration have been linked to mucinous ovarian tumors [48–51]. Mucinous tumors differ histologically from other ovarian cancer subtypes in that they resemble epithelium of the colon [52]. Colon epithelium is highly susceptible to carcinogenesis induced by cigarette smoking [53,54], an activity known to induce inflammation. Given that these two tumor subtypes are strongly linked to inflammation inducing risk factors, it is conceivable that use of inflammation-reducing analgesics could protect against these tumor types.
The NIH-AARP Diet and Health Study dataset has allowed us to prospectively assess the associations between aspirin and non-aspirin NSAIDs and ovarian cancer risk in a large cohort of women containing a moderate number of cases. The study design reduces concerns about recall bias, while the sample size (the second largest prospective case set published to date) provides greater power to detect an association than many previously published studies. Though we noted concerns about incomplete histology data, our analysis was based on a cohort in which accrual of cases occurred over a short time period (average follow-up time of cases was less than 5 years), allowing for reduced concern over changes in histology classification and terminology with time. This indicates that while we may have incomplete histology data, the available histology data should be classified consistently.
Our analysis provides evidence for lack of association between aspirin use and ovarian cancer risk, and only a suggestive association between non-aspirin NSAIDs use and risk; we acknowledge several limitations that may contribute to these findings. Our dataset does not provide information about dose or duration of analgesic use; both of these attributes may be important factors contributing to potential associations. Regular low-dose use of NSAIDs indicated for cardiovascular disease, for example, could also be considered as a chemoprevention strategy if an association between use and decreased ovarian cancer risk was established. Unfortunately, dose and duration information have not been consistent across previously published studies, so it was not possible for us to explore these factors in our meta-analysis either. Furthermore, we do not have information on indication for use, a factor which could independently affect risk. If the pro-inflammatory effects of ovulation contribute to ovarian carcinogenesis, then knowing use patterns for aspirin and non-aspirin NSAIDs at younger ages may be more relevant than recent use for assessing cancer relationships.
It is not uncommon for a large proportion of tumor histologies to be unspecified and grouped as “other epithelial tumors” in any case series [55]; the majority of these are assumed to be serous tumors, but not all can be classified as such. In our dataset, approximately 31% of cases were classified as “other epithelial tumors”; the large proportion of histologically unspecified epithelial ovarian cancer cases limited our ability to examine histology-specific associations with sufficient power. Furthermore, the data relies on pathological diagnoses as recorded, rather than based on centralized pathology review; this could lead to misclassification.
Our cohort consisted of individuals who were eligible for AARP membership; thus we did not include women under the age of 50 in our analysis, limiting the assessment of exposures and outcomes at younger ages. Furthermore, this inclusion criterion limits the generalizability of our results.
While our meta-analysis supports the evidence for the lack of association between regular aspirin use and epithelial ovarian cancer, it suggests an inverse association between regular non-aspirin NSAID use and epithelial ovarian cancer. Due to paucity of less common epithelial ovarian cancer subtypes in our data, we were unable to provide strong conclusive evidence about the associations between aspirin and non-aspirin NSAIDs and less common tumor subtypes. Among the studies identified in our review, only one other provided subtype analyses; data from this study provided evidence for an association between non-aspirin NSAIDs and mucinous tumors and for a suggestive effect on endometrioid tumors. The association between all classes of NSAIDs and histologic subtypes of epithelial ovarian cancer need to be examined in greater detail to parse out whether these effects are real; additionally, dose and duration of use should be examined properly in future studies, to determine whether the heterogeneity of effects we have observed among studies can be partially explained by differing patterns of use.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System under contract to the Department of Health (DOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the contractor or DOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis. Finally, we thank and remember the founder of the NIH-AARP Diet and Health Study, Dr. Arthur Schatzkin, who was a visionary and a dedicated investigator.
The research project was supported by the Intramural Research Program of the National Cancer Institute. Megan A. Murphy is supported in part by training grant NIH 5 T32 CA09001-35.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Molecular and cellular endocrinology. 2006;247(1–2):4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. Journal of the National Cancer Institute. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 4.Tzonou A, Polychronopoulou A, Hsieh CC, Rebelakos A, Karakatsani A, Trichopoulos D. Hair dyes, analgesics, tranquilizers and perineal talc application as risk factors for ovarian cancer. International journal of cancer Journal international du cancer. 1993;55(3):408–410. doi: 10.1002/ijc.2910550313. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351(9096):104–107. doi: 10.1016/S0140-6736(97)08064-1. [DOI] [PubMed] [Google Scholar]
- 6.Tavani A, Gallus S, La Vecchia C, Conti E, Montella M, Franceschi S. Aspirin and ovarian cancer: an Italian case-control study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2000;11(9):1171–1173. doi: 10.1023/a:1008373616424. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg L, Palmer JR, Rao RS, Coogan PF, Strom BL, Zauber AG, Stolley PD, Shapiro S. A case-control study of analgesic use and ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9(9):933–937. [PubMed] [Google Scholar]
- 8.Moysich KB, Mettlin C, Piver MS, Natarajan N, Menezes RJ, Swede H. Regular use of analgesic drugs and ovarian cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10(8):903–906. [PubMed] [Google Scholar]
- 9.Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Kato I, Koenig KL, Shore RE. Aspirin and epithelial ovarian cancer. Preventive medicine. 2001;33(6):682–687. doi: 10.1006/pmed.2001.0945. [DOI] [PubMed] [Google Scholar]
- 10.Schildkraut JM, Moorman PG, Halabi S, Calingaert B, Marks JR, Berchuck A. Analgesic drug use and risk of ovarian cancer. Epidemiology. 2006;17(1):104–107. doi: 10.1097/01.ede.0000190538.55645.f8. [DOI] [PubMed] [Google Scholar]
- 11.Meier CR, Schmitz S, Jick H. Association between acetaminophen or nonsteroidal antiinflammatory drugs and risk of developing ovarian, breast, or colon cancer. Pharmacotherapy. 2002;22(3):303–309. doi: 10.1592/phco.22.5.303.33189. [DOI] [PubMed] [Google Scholar]
- 12.Hannibal CG, Rossing MA, Wicklund KG, Cushing-Haugen KL. Analgesic drug use and risk of epithelial ovarian cancer. American journal of epidemiology. 2008;167(12):1430–1437. doi: 10.1093/aje/kwn082. [DOI] [PubMed] [Google Scholar]
- 13.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. International journal of cancer Journal international du cancer. 2008;122(1):170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 14.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. International journal of cancer Journal international du cancer. 2009;124(6):1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM. Inverse association of NSAID use and ovarian cancer in relation to oral contraceptive use and parity. British journal of cancer. 2008;98(11):1781–1783. doi: 10.1038/sj.bjc.6604392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairfield KM, Hunter DJ, Fuchs CS, Colditz GA, Hankinson SE. Aspirin, other NSAIDs, and ovarian cancer risk (United States) Cancer causes & control : CCC. 2002;13(6):535–542. doi: 10.1023/a:1016380917625. [DOI] [PubMed] [Google Scholar]
- 17.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. British journal of cancer. 2003;88(5):684–688. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. British journal of cancer. 2003;88(11):1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacey JV, Jr, Sherman ME, Hartge P, Schatzkin A, Schairer C. Medication use and risk of ovarian carcinoma: a prospective study. International journal of cancer Journal international du cancer. 2004;108(2):281–286. doi: 10.1002/ijc.11538. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro SP, Tworoger SS, Cramer DW, Rosner BA, Hankinson SE. Use of nonsteroidal antiinflammatory agents and incidence of ovarian cancer in 2 large prospective cohorts. American journal of epidemiology. 2009;169(11):1378–1387. doi: 10.1093/aje/kwp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(2):435–442. doi: 10.1158/1055-9965.EPI-09-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonovas S, Filioussi K, Sitaras NM. Do nonsteroidal anti-inflammatory drugs affect the risk of developing ovarian cancer? A meta-analysis. British journal of clinical pharmacology. 2005;60(2):194–203. doi: 10.1111/j.1365-2125.2005.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonovas S, Filioussi K, Sitaras NM. Paracetamol use and risk of ovarian cancer: a meta-analysis. British journal of clinical pharmacology. 2006;62(1):113–121. doi: 10.1111/j.1365-2125.2005.02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. American journal of epidemiology. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 26.Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, Hartge P, Hollenbeck A, Park Y, Wentzensen N. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP diet and health study. International journal of cancer Journal international du cancer. 2011 doi: 10.1002/ijc.26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. Journal of Registry Management. 2005;32:70–75. [Google Scholar]
- 28.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. American journal of epidemiology. 2010;171(1):45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kir G, Gurbuz A, Karateke A, Kir M. Clinicopathologic and immunohistochemical profile of ovarian metastases from colorectal carcinoma. World journal of gastrointestinal surgery. 2010;2(4):109–116. doi: 10.4240/wjgs.v2.i4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(Suppl 2):S99–S111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 33.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Advances in anatomic pathology. 2007;14(3):149–177. doi: 10.1097/PAP.0b013e3180504abf. [DOI] [PubMed] [Google Scholar]
- 34.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(12):2840–2847. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 35.Danforth KN, Gierach GL, Brinton LA, Hollenbeck AR, Katki HA, Leitzmann MF, Schatzkin A, Lacey JV., Jr Nonsteroidal anti-inflammatory drug use and endometrial cancer risk in the NIH-AARP Diet and Health Study. Cancer Prev Res (Phila) 2009;2(5):466–472. doi: 10.1158/1940-6207.CAPR-08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodelon C, Doherty JA, Chen C, Rossing MA, Weiss NS. Use of nonsteroidal antiinflammatory drugs and risk of endometrial cancer. American journal of epidemiology. 2009;170(12):1512–1517. doi: 10.1093/aje/kwp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosetti C, Bravi F, Talamini R, Montella M, Negri E, La Vecchia C. Aspirin and risk of endometrial cancer: a case-control study from Italy. Eur J Cancer Prev. 2010;19(5):401–403. doi: 10.1097/cej.0b013e32833b4871. [DOI] [PubMed] [Google Scholar]
- 38.Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(12):2923–2928. doi: 10.1158/1055-9965.EPI-05-0457. [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer research. 2008;68(7):2507–2513. doi: 10.1158/0008-5472.CAN-07-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, Zauber AG, Olson SH. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(5):1448–1456. doi: 10.1158/1055-9965.EPI-08-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dube C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Annals of internal medicine. 2007;146(5):365–375. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 42.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Annals of internal medicine. 2007;146(5):376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 43.Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Archives of internal medicine. 1999;159(2):161–166. doi: 10.1001/archinte.159.2.161. [DOI] [PubMed] [Google Scholar]
- 44.Mahipal A, Anderson KE, Limburg PJ, Folsom AR. Nonsteroidal anti-inflammatory drugs and subsite-specific colorectal cancer incidence in the Iowa women's health study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(10):1785–1790. doi: 10.1158/1055-9965.EPI-05-0674. [DOI] [PubMed] [Google Scholar]
- 45.Sayasneh A, Tsivos D, Crawford R. Endometriosis and ovarian cancer: a systematic review. ISRN obstetrics and gynecology. 2011;2011 doi: 10.5402/2011/140310. 140310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. The lancet oncology. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei JJ, William J, Bulun S. Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2011;30(6):553–568. doi: 10.1097/PGP.0b013e31821f4b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112(5):1169–1177. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 49.Marchbanks PA, Wilson H, Bastos E, Cramer DW, Schildkraut JM, Peterson HB. Cigarette smoking and epithelial ovarian cancer by histologic type. Obstetrics and gynecology. 2000;95(2):255–260. doi: 10.1016/s0029-7844(99)00531-1. [DOI] [PubMed] [Google Scholar]
- 50.Green A, Purdie D, Bain C, Siskind V, Webb PM. Cigarette smoking and risk of epithelial ovarian cancer (Australia) Cancer causes & control : CCC. 2001;12(8):713–719. doi: 10.1023/a:1011297403819. [DOI] [PubMed] [Google Scholar]
- 51.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC. Association of cigarette smoking with the risk of ovarian cancer. International journal of cancer Journal international du cancer. 2004;111(1):124–130. doi: 10.1002/ijc.20242. [DOI] [PubMed] [Google Scholar]
- 52.Kumar VAA, Fausto N. Robbins & Cotran Pathologic Basis of Disease. 7th ed. Philadephia: Elsevier Saunders; 2005. [Google Scholar]
- 53.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicological sciences : an official journal of the Society of Toxicology. 2007;97(2):279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 54.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterology clinics of North America. 2002;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 55.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995–2004. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(1):132–139. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.