Abstract
Purpose
To determine the influence of ethnicity and sociodemographic factors on disease characteristics of the Canadian Pediatric Lupus population.
Methods
Childhood-onset SLE (cSLE) patients at four pediatric centers in Halifax, Montreal, Toronto and Vancouver were consecutively recruited. Sociodemographics and disease data were collected. Patients were categorized by their primary self-selected ethnicity, and exploratory cluster analyses were examined for disease expression by ethnicity.
Results
We enrolled 213 cSLE patients, and ethnicity data were available for 206 patients: White (31%), Asian (30%), South Asian (15%), Black (10%), Latino/Hispanic (4%), Aboriginal (4%) and Arab/Middle Eastern (3%). The frequency of clinical classification criteria (malar rash, arthritis, serositis and renal disease) and autoantibodies significantly differed among ethnicities. Medications were prescribed equally across ethnicities: 76% were taking prednisone, 86% anti-malarials, and 56% required additional immunosuppressants. Cluster analysis partitioned three main groups – mild (N = 50), moderate (N = 82) and severe (N = 68) disease clusters. Only 20% of White patients were in the severe cluster compared to 51% of Asian and 41% of Black patients (p=0.03). However, disease activity indices and damage scores were similar across ethnicities.
Conclusion
Canadian cSLE patients reflect our multi-ethnic population, with differences in disease manifestations, autoantibody profiles and severity of disease expression by ethnicity.
Keywords: child, adolescent, race, socioeconomic status, sociodemographics, autoimmune disease, chronic illness, systemic lupus erythematosus, paediatric
Introduction
Systemic Lupus Erythematosus (SLE) is a multisystem autoimmune disease associated with significant morbidity, with up to 20% of all patients diagnosed in childhood. Recent studies suggest that childhood-onset SLE (cSLE) is more frequent and severe in non-White populations, especially Black, Asian, Hispanic and Aboriginal populations.1-5 Although many studies of North American cSLE cohorts have focused on multi-ethnic populations, they have primarily originated from single centers, or have been small cohort studies.1,3,4,6,7 Larger cohorts reported from Taiwan, India, and Thailand 2,8,9 represent more ethnically homogeneous populations than those seen in North America. In particular, descriptions of cSLE in North American Asian, South Asian and Aboriginal (Native Americans/First Nations Canadians) populations are sparse,1,10 despite the rapid growth of these ethnic groups in Western countries.
Canada is a country with significant growth due to recent immigration patterns, with almost 70% of the population increase between 2001 and 2006 accounted for by immigration. Compared to the rest of the Canadian population, visible minorities are growing at an almost five times faster rate, and will represent almost 20% of the population by 2017.11,12 South Asians (primarily from India, Pakistan, Sri Lanka and Bangladesh) recently surpassed Chinese as the largest visible minority group in Canada, with Blacks as the third largest group. These minority groups remain ethnoculturally diverse, for example 52% of the Black group reports Caribbean origins, 42% report African origins, 12% from the British Isles, 11% Canadian, and 4% of French origin.11 Although Canada's public healthcare system provides universal access to medical care, only 53% of Canadians have dental insurance,13 and 62% have prescription drug coverage.14 Prescription drug and dental coverage are provided through federal programs for Aboriginals, and through provincial programs for lower income earners and seniors. For the remainder, individual or group private insurance plans are required. Thus, sociodemographic factors such as access to prescription drugs and distance from a healthcare provider may influence healthcare utilization and disease outcomes.
The 1000 Faces of Canadian Lupus is a cross-Canada national prospective observational cohort of SLE patients (both adults and children) that began recruiting both incident and prevalent cases of SLE in 2005. The objectives were to determine the influence of ethnicity and socioeconomic factors on disease activity, organ involvement, and disease outcomes. This report focuses on the children and adolescents with SLE that were enrolled and presents the baseline description of this ethnically diverse cSLE cohort; the adult cohort has been previously described.15 We analyzed this pediatric cohort by self-selected ethnicity for sociodemographic and socioeconomic factors and disease characteristics.
Methods
Study Design and Setting
This cross-sectional study enrolled both incident and prevalent cases of cSLE at four participating Canadian pediatric rheumatology centres in Halifax, Montreal, Toronto and Vancouver. The study consisted of a baseline visit, and follow-up visits every six months; this report presents only the data at enrollment (“baseline”). Eligible patients were consecutively recruited starting in November 2005, at the time of a routine clinic visit. An interview with the patient and his or her parent was conducted, along with a physical examination and laboratory tests. Approval from local Research Ethics Boards were obtained at each participating site.
Participants
Patients with a clinical diagnosis of SLE prior to their 18th birthday and followed at one of the participating centers were eligible to enroll in the pediatric arm of this study. As was true for the adult arm of the study, patients were allowed to enroll with a clinical diagnosis of cSLE, rather than the requirement to meet the American College of Rheumatology (ACR) classification criteria for SLE.16 However, the majority of subjects (89%) fulfilled ≥4 criteria at enrollment.
Study variables
Patients provided detailed data including age, gender, country of birth, highest education level achieved, total household income, dental insurance (yes/no), prescription medication coverage (yes/no), medication history, access to health care and medications (each categorized by the parent as “no problem/a bit of a problem/a big problem”) and family history. Self-selected ethnic background utilized the format and categories used by Statistics Canada.17 In the current analysis, patients were categorized according to the primary self-selected ethnic category; however, patients were allowed multiple choices, and detailed information on extended family ethnic background and country of origin were also collected.
Study investigators reviewed medical records at the baseline visit and clinical data were abstracted and entered onto a comprehensive clinical record form. Collected data included the classification criteria for SLE,16 other clinical manifestations of lupus, and current and past medications. Autoantibody status was recorded for anti-dsDNA, anti-Sm, anti-RNP, anti-La, anti-Ro, and antiphospholipid antibodies (aPLs) -including anticardiolipin antibodies and lupus anticoagulant. Investigators were asked to rate if non-compliance with medication had been a problem in caring for that patient in the past year, with choices of “no problem”, “a bit of a problem” or “a big problem”. Disease activity was measured using the SLE Disease Activity Index 2000 (SLEDAI-2K) and revised Systemic Lupus Activity Measure (SLAM-R); both scales are sensitive to change in cSLE.18 The SLEDAI-2K contains 24 descriptors in 9 organ systems and is weighted to reflect the degree of disease activity. Scores range from 0 – 105, with higher scores representing greater disease activity.19 The SLAM-R includes 24 clinical manifestations and 8 laboratory parameters weighted according to severity, with different maximal scores for each organ system. Scores range from 0 to 86, with higher scores representing greater disease activity.20 The SLAM-R also includes a 10-cm visual analog scale (VAS) of physician-assessed global disease activity. Disease damage was recorded using the Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI), an index of 41 items in 12 organ systems/domains. Damage is irreversible change that has been present for at least 6 months, and may be due to the disease itself, treatment, or a co-morbidity, and scores range from 0 to 49.21
Statistical analyses
We summarized the characteristics of the study sample using descriptive statistics, and differences between ethnic groups were assessed with the chi-squared test for categorical variables or with analysis of variance and pair wise testing for continuous variables. For identification of groups of patients with similar clinical characteristics we used exploratory cluster analysis.22 Hierarchical agglomerative linkage using Ward's method allowed identification of a meaningful number of clusters using the ACR SLE classification criteria (we excluded autoantibodies from the clustering). This was followed by a non-hierarchical K-means clustering procedure to refine the clusters. By specifying 3 and 4 clusters in the K-means procedure, the results of the analysis of 3 clusters had greater clinical relevance than that of 4 clusters. Chi-square and Fisher's exact test were used to determine differences of features between the resultant clusters. Bonferroni adjustments for multiple comparisons were not made, as the majority of our analyses were exploratory. All statistical analyses used STATA 12 statistical software (STATACorp, College Station, TX).
Results
Participants
Between November 2005 and February 2009, 218 pediatric and 1715 adult patients were enrolled into the 1000 Faces of Canadian Lupus Study. Three of the pediatric patients fulfilled only 2 ACR classification criteria for SLE, and one patient had no demographic data available. After reviewing the clinical data of 22 patients who fulfilled only 3 of 11 ACR classification criteria, we excluded one further patient as unlikely SLE (more consistent with primary antiphospholipid syndrome), for a total of 213 pediatric patients available for analysis. The number of patients enrolled at each site mirrored the size of the clinical center. At the Hospital for Sick Children in Toronto, 134 (63%) patients were enrolled, 54 (25%) from British Columbia (B.C.) Children's Hospital in Vancouver, 17 (8%) from Montreal Children's Hospital, and 8 (4%) from Halifax IWK Hospital. Overall, there were 176 (83%) females, the mean age at cSLE diagnosis was 12.5 ± 0.3 years, mean disease duration at enrollment was 2.5 ± 2.7 years, and 175 patients (82%) were born in Canada. These demographic data were similar across the four geographic sites (data not shown), except for disease duration. Patients in Toronto had a disease duration of 1.9 ± 2.2 years, in Vancouver 3.9 ± 3.6 years, Montreal 2.4 ± 2.2, and Halifax 1.7 ± 1.8 (p<.001).
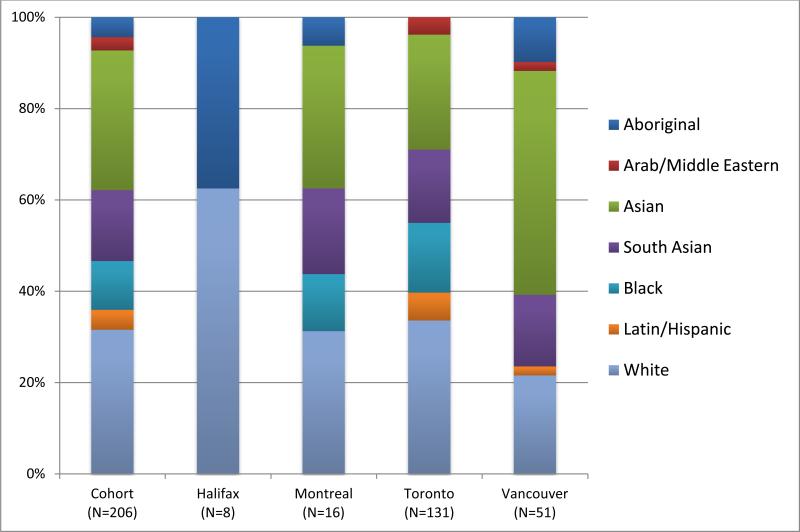
Using Statistics Canada guidelines for race and ethnicity,17 self-reported primary race/ethnicity data were available for 206 patients: 63 (31%) East and Southeast Asian patients (primarily from China, Korea, Taiwan, Philippines and Vietnam), and 32 (16%) South Asian patients (primarily from India, Pakistan, Sri Lanka and Bangladesh) , 22 (11%) Black, 9 (4%) Aboriginal Canadians, 65 (32%) White, 9 (4%) Latino/Hispanic and 6 (3%) Arab/Middle Eastern. The East/Southeast Asian group is labeled “Asian” for the remainder of this study, differentiating it from the “South Asian” group. The ethnic distribution of patients at the four centers (Figure 1) was significantly different (p<.001), reflecting existing differences in urban populations across Canada. The small Arab/Middle Eastern group was not included in subsequent analyses.
Figure 1. Ethnicity by Geographic Location.
Each histogram bar depicts the relative proportion of subjects of each race/ethnicity at each center. Total number of subjects recruited at each site is indicated along the x-axis.
The distribution of socioeconomic and demographic data by ethnicity is shown in Table 1. Disease duration at enrollment was greater in the Aboriginal group (p=0.007), however, the age at diagnosis was not significantly different between the groups. A lower proportion of patients of Asian and South Asian descent were born in Canada compared to the other groups (p<0.001). Household income and prescription drug coverage were similar across groups; however, a lower proportion of Asian and South Asian patients had supplemental dental coverage (p=0.006). The average education level (highest grade completed) was 8.6 ± 2.7, which reflected the average age at enrollment, and did not differ by ethnicity (data not shown). Medication noncompliance categorized by the physician was “no problem” for 94% (188/200) of patients, “a bit of a problem” for 5% (10/200), and “a big problem” for 1% (2 patients). There were no significant differences in compliance ratings by ethnicity (data not shown).
Table 1.
Demographics by Ethnicity
| Ethnicity | Total (N=200) | Aboriginal (n=9) | Asian* (n=63) | South Asian* (n=32) | Black (n=22) | Latin/Hispanic (n=9) | White (n=65) | P-value |
|---|---|---|---|---|---|---|---|---|
| Female (%) | 166 (83) | 7 (78) | 51 (81) | 25 (78) | 19 (86) | 9 (100) | 55 (85) | 0.73 |
| Age at Baseline (y) | ||||||||
| Mean ± SD (min, max) | 14.9 ± 3.0 (5.4, 21.7) | 16.8 ± 1.6 (14.8, 19.0) | 15.2 ± 2.9 (7.7, 21.7) | 14.7 ± 3.7 (5.4, 19.8) | 15.1 ± 2.8 (5.7, 19.0) | 13.2 ± 4.0 (6.3, 17.6) | 14.7 ± 2.7 (7.2, 20.4) | 0.17 |
| Age at Diagnosis (y) | ||||||||
| Mean ± SD (min, max) | 12.6 ± 3.3 (3.0 – 17.7) | 11.7 ± 3.8 (4.3 – 15.2) | 12.6 ± 2.7 (5.9, 17.2) | 12.5 ± 3.5 (4.7, 17.3) | 12.4 ± 3.5 (4.2, 16.4) | 10.7 ± 4.7 (3.0, 15.8) | 13.0 ± 3.4 (3.0, 17.7) | 0.43 |
| Disease Duration (y) | ||||||||
| Mean ± SD (min, max) | 2.4 ± 2.6 (0, 14.6) | 5.1 ± 5.0 (0.1, 14.6) | 2.7 ± 2.4 (0.1, 8.6) | 2.3 ± 2.4 (0.2, 10.4) | 2.7 ± 2.6 (0, 8.8) | 2.6 ± 3.6 (0.2, 10.6) | 1.7 ± 2.1 (0, 11.1) | 0.007 |
| Born in Canada (%) | (N = 196) | |||||||
| 167 (85) | 9 (100) | 46 (73) | 24 (75) | 18 (90) | 6 (75) | 64 (100) | <0.001 | |
| Household Income (%) | (N=175) | 0.08 | ||||||
| <$15 000 | 11 (6) | 0 | 5 (10) | 2 (7) | 2 (11) | 1 (14) | 1 (2) | |
| $15 000 - $29 999 | 13 (7) | 0 | 6 (12) | 3 (10) | 1 (6) | 2 (29) | 1 (2) | |
| $30 000 - $49 999 | 36 (21) | 0 | 13 (25) | 5 (17) | 6 (33) | 1 (14) | 11 (18) | |
| >$50 000 | 115 (66) | 8 (100) | 28 (54) | 19 (66) | 9 (50) | 3 (43) | 48 (79) | |
| Dental Insurance (%) | (N=190) | |||||||
| 143 (75) | 9 (100) | 37 (64) | 18 (62) | 16 (76) | 6 (75) | 57 (88) | 0.006 | |
| Prescription Drug Coverage (%) | (N=189) | |||||||
| 148 (78) | 8 (89) | 40 (70) | 19 (66) | 18 (86) | 6 (75) | 57 (85) | 0.07 |
Asian includes East and Southeast Asian patients (primarily from China, Korea, Taiwan, Philippines and Vietnam), and South Asian includes patients primarily from India, Pakistan, Sri Lanka and Bangladesh
Clinical and Laboratory Characteristics
We observed differences between ethnic groups in the frequencies of some of the SLE classification criteria16 as seen in Table 2. Aboriginals and Blacks had a lower frequency of malar rash (p=0.002), and Asians demonstrated a lower frequency of arthritis (p=0.02). Serositis was more common in Aboriginals and Blacks (p=0.008), and renal disease was more common in Asian and Black patients (p=.009). Analysis of the autoantibody profiles as seen in Table 3 demonstrated that fewer White patients had dsDNA antibodies (p=0.01), anti-Sm antibodies (0.02), and anti-Ro antibodies (p=0.008) compared to patients of other ethnicities.
Table 2.
Classification Criteria14 by Ethnicity
| Criterion N (%) | Total (N=200) | Aboriginal (N=9) | Asian (N=63) | South Asian (N=32) | Black (N=22) | Latino/Hispanic (N=9) | White (N=65) | P-value |
|---|---|---|---|---|---|---|---|---|
| Malar Rash | 132 (66) | 3 (33) | 41 (65) | 21 (66) | 8 (36) | 8 (89) | 51 (78) | 0.002 |
| Discoid Rash | 5 (3) | 0 | 3 (5) | 0 | 2 (9) | 0 | 0 | 0.15 |
| Photosensitivity | 50 (25) | 2 (22) | 20 (32) | 9 (28) | 2 (9) | 1 (11) | 16 (25) | 0.36 |
| Ulcers | 54 (27) | 2 (22) | 17 (27) | 9 (28) | 7 (32) | 3 (33) | 16 (25) | 0.97 |
| Arthritis | 121 (61) | 7 (78) | 29 (46) | 23 (72) | 13 (59) | 3 (33) | 46 (71) | 0.02 |
| Serositis | 35 (18) | 4 (44) | 10 (16) | 5 (16) | 9 (41) | 0 | 7 (11) | 0.008 |
| Renal | 72 (36) | 3 (33) | 32 (51) | 9 (28) | 11 (50) | 3 (33) | 14 (22) | 0.009 |
| Neurologic | 26 (13) | 1 (11) | 8 (13) | 2 (6) | 3 (14) | 0 | 12 (18) | 0.54 |
| Hematologic | 134 (67) | 7 (78) | 45 (71) | 21 (66) | 17 (77) | 6 (67) | 38 (58) | 0.37 |
| Immunologic | 162 (81) | 9 (100) | 55 (87) | 31 (97) | 18 (82) | 7 (78) | 42 (65) | 0.001 |
| ANA | 195 (98) | 9 (100) | 62 (98) | 32 (100) | 22 (100) | 9 (100) | 61 (94) | 0.59 |
ANA = antinuclear antibody
Table 3.
Autoantibodies and Disease Measures by Ethnicity
| AUTOANTIBODY N (%) | Total (N=191) | Aboriginal (N=9) | Asian (N=63) | South Asian (N=32) | Black (N=22) | Latino/Hispanic (N=9) | White (N=65) | P-value |
|---|---|---|---|---|---|---|---|---|
| anti- dsDNA | 132 (66) | 5 (56) | 47 (75) | 26 (81) | 16 (73) | 6 (67) | 32 (49) | 0.01 |
| anti-Smith (anti-Sm) | 65 (33) | 5 (56) | 27 (43) | 7 (22) | 9 (41) | 4 (44) | 13 (20) | 0.02 |
| anti-Ribonuclear Protein (anti-RNP) | 74 (37) | 4 (44) | 25 (40) | 11 (34) | 11a (52) | 5 (56) | 18 (28) | 0.26 |
| anti-Ro (anti-SSA) | 69(35) | 3 (33) | 28 (44) | 13 (41) | 7b (35) | 6 (67) | 12 (18) | 0.008 |
| anti-La (anti-SSB) | 38 (19) | 2 (22) | 19 (30) | 7 (22) | 3b (15) | 1 (11) | 6 (9) | 0.07 |
| anti-phospholipid antibody (any) | 95 (48) | 3 (33) | 31 (49) | 17 (53) | 12 (55) | 5 (56) | 27 (42) | 0.74 |
| ACL | 88 (44) | 3 (33) | 30 (48) | 15 (47) | 12 (55) | 4 (44) | 24 (37) | 0.67 |
| LAc | 37 (19) | 1 (11) | 11 (17) | 5 (16) | 5 (23) | 1 (11) | 14 (22) | 0.95 |
| SLEDAI (mean ± SD, range) | 3.1 ± 4.1 (0, 24) | 4 ± 4 (0, 12) | 3.7 ± 5.1 (0, 24) | 2.1 ± 1.9 (0, 6) | 3.6 ± 5.0 (0, 19) | 3 ± 2.5 (0, 8) | 2.9 ± 3.7 (0, 16) | 0.52 |
| SLAM-R | 3.4 ± 3.7 (0, 21) | 4.1 ± 3.9 (0, 11) | 3.0 ± 2.9 (0, 12) | 2.7 ± 2.2 (0, 7) | 4.7 ± 5.8 (0, 21) | 4.1 ± 2.7 (1, 9) | 3.6 ± 4 (0, 21) | 0.38 |
| SDI (median, range) | 0 (0, 5) | 0 (0, 1) | 0 (0, 5) | 0 (0, 2) | 0 (0, 3) | 0 (0, 1) | 0 (0, 5) | 0.78 |
Where indicated, antibody results not available for all patients:
N=21
N=20
ACL: IgG anticardiolipin antibodies; LAc: lupus anticoagulant; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SLAM-R: Revised Systemic Lupus Activity Measure; SDI: Systemic Lupus Collaborating Clinics/American College of Rheumatology Damage Index
We conducted an exploratory cluster analysis to determine if the clinical features associated together (Table 4). The clustering procedure demonstrated 3 clinically distinct clusters. Cluster 1 contained 68 patients (34%), and was characterized by multiorgan (“severe”) disease – all had renal disease, 76% had hematologic involvement, and 41% arthritis. The largest group was Cluster 2, with 82 patients (41%), representing “moderate” disease with predominant features of hematologic involvement (100%) and arthritis (69%). Cluster 3 represented 50 patients (25%), and was characterized by “mild” disease – patients predominantly had mucocutaneous involvement and arthritis. Once these clusters were established we then determined if the ethnic and autoantibody distributions differed by clusters (Table 5). We observed a significant difference in the ethnicity distribution between clusters (p=0.03). White patients had milder disease (only 20% partitioned to Cluster 1 – “severe” group), while conversely only 9% of Black patients and 16% of Asian patients were in the “mild” group (Cluster 3). Autoantibodies did not follow a particular cluster distribution, although significantly more patients in Cluster 1 were anti-dsDNA antibody positive than in the other clusters (92% versus 62% and 36%, p<0.001).
Table 4.
Clustering by Clinical Criteria
| Cluster 1 (N=68) | Cluster 2 (N=82) | Cluster 3 (N=50) | P-value | |
|---|---|---|---|---|
| Malar Rash | 43 (63) | 48 (59) | 41 (82) | 0.02 |
| Discoid Rash | 1 (2) | 2 (2) | 2 (4) | 0.73 |
| Photosensitivity | 18 (26) | 12 (15) | 20 (40) | 0.005 |
| Oral/Nasal Ulcers | 21 (31) | 18 (22) | 15 (30) | 0.39 |
| Arthritis | 28 (41) | 56 (69) | 37 (74) | <0.001 |
| Serositis | 12 (18) | 16 (20) | 7 (14) | 0.75 |
| Pleuritis | 10 (15) | 13 (16) | 5 (10) | 0.71 |
| Pericarditis | 4 (6) | 9 (11) | 5 (10) | 0.54 |
| Renal Disease | 68 (100) | 3 (4) | 1 (2) | <0.001 |
| Proteinuria | 63 (93) | 0 | 0 | <0.001 |
| Casts | 42 (62) | 0 | 0 | <0.001 |
| Neuropsychiatric | 5 (7) | 10 (12) | 11 (22) | 0.07 |
| Disease | ||||
| Seizures | 1 (2) | 3 (4) | 1 (2) | 0.85 |
| Psychosis | 4 (6) | 4 (5) | 7 (14) | 0.15 |
| Hematologic | 52 (76) | 82 (100) | 0 | <0.001 |
| Anemia | 20 (29) | 32 (39) | 0 | <0.001 |
| Leucopenia | 24 (35) | 20 (24) | 0 | <0.001 |
| Lymphopenia | 35 (51) | 50 (61) | 0 | <0.001 |
| Thrombocytopenia | 24 (35) | 46 (56) | 0 | <0.001 |
Cluster 1 is characterized by multiorgan (“severe”) disease – all have renal disease, and the majority hematologic and mucocutaneous involvement, as well as a significant proportion with arthritis. Cluster 2 is “moderate” disease, with predominant features of hematologic involvement and arthritis, and Cluster 3 is “mild” disease – patients predominantly have mucocutaneous involvement and arthritis.
Table 5.
Ethnicity and Autoantibody Distribution Using Clustering by Clinical Criteria
| Cluster 1 (N=68) | Cluster 2 (N=82) | Cluster 3 (N=50) | P-value | |
|---|---|---|---|---|
| Ethnicity (N,%*) | 0.03 | |||
| Aboriginal | 3 (33) | 5 (56) | 1 (11) | |
| Asian | 32 (51) | 21 (33) | 10 (16) | |
| South Asian | 8 (25) | 15 (47) | 9 (28) | |
| Black | 9 (41) | 11 (50) | 2 (9) | |
| Latino/Hispanic | 3 (33) | 3 (33) | 3 (33) | |
| White | 13 (20) | 28 (43) | 24 (37) | |
| Autoantibodies | ||||
| ANA | 68 (100) | 79 (96) | 48 (96) | 0.22 |
| dsDNA | 63 (93) | 51 (62) | 18 (36) | <0.001 |
| Anti-Smith | 25 (37) | 26 (32) | 14 (28) | 0.61 |
| Anti-RNP | 26 (38) | 32 (39) | 16 (32) | 0.68 |
| Anti-Ro | 31 (46) | 27 (33) | 11 (22) | 0.03 |
| Anti-La | 17 (25) | 14 (17) | 7 (14) | 0.31 |
| Anticardiolipin Antibodies | 37 (54) | 36 (44) | 15 (30) | 0.03 |
| Lupus Anticoagulant | 9 (13) | 24 (29) | 4 (8) | 0.004 |
The percentage listed is that of that ethnic group. For example, South Asian patients in Cluster 1 – there were 8 patients, which represents 25% of all South Asian patients in the study.
Treatment
At the time of the baseline visit there were no differences between the different ethnic groups in the current use of antimalarials, prednisone and all immunosuppressive medications. Eighty-six percent (171/200) of patients were taking antimalarials (hydroxychloroquine or chloroquine), and 76% (151/200) prednisone. Thirty percent were prescribed azathioprine (59/200), 13% (25/200) mycophenolate mofetil (MMF) or mycophenolic acid (MPA), 7% (13/200) cyclophosphamide, and 7% (13/200) methotrexate. Additionally, there were no differences in the proportion of patients in each ethnic group who had “ever” taken any of these medications (data not shown). Ninety-one percent of patients had taken prednisone either now or in the past, while 90% had taken antimalarials. For the immunosuppressants azathioprine, MMF/MPA, cyclophosphamide and methotrexate, 48%, 17%, 22% and 10% respectively had “ever” taken these drugs.
Disease Activity and Damage
At the baseline visit, average disease activity was low, as measured by SLEDAI-2K score (overall mean 3.1 ± 4.1) or SLAM-R score (3.4 ± 3.7). Physician global disease activity scores rated on a visual analog scale (VAS) demonstrated an overall mean of 15.1 mm (range 0 – 99). Mean disease damage, as measured by SDI score was 0.3 ± 0.8 (range 0 – 5), and median SDI was 0 (IQR 0). These measures did not differ by ethnicity (Table 3). Evidence of any damage, (SDI score > 0) was observed in 16% of patients (32/199), and 7% of patients (14/199) had a score of ≥ 2. Damage scores were most frequently accounted for by cognitive impairment (7 patients), cataracts (7 patients), avascular necrosis (8 events in 6 patients), and stroke (5 events in 3 patients).
Discussion
The aim of the 1000 Faces of Canadian Lupus Study was to document the variable phenotype of SLE in Canada, across a multi-ethnic population of both children and adults. Although the pediatric arm of this project enrolled subjects in only 4 of the 13 provinces and territories, the largest clinical centers representing the Canadian cities with the greatest ethnic diversity participated. In the most recent census, 16% of all Canadians identified themselves as belonging to a visible minority.11 In the current study, 69% of the patients enrolled self-identified as non-White ethnicity, which is consistent with previous reports of a greater prevalence of SLE in non-White ethnicities,1,23-25 but is greater than the reported percentage of visible minorities in these Canadian cities (which range from 8% in Halifax to 43% in Toronto).11 It is also greater than that reported in the adult 1000 Faces of Lupus cohort which was 62% White.15 The ethnic diversity of each center was representative of their urban surroundings. Although only 4% percent of our cohort identified themselves as primarily Aboriginal, this is greater than the 3% of Canadians who are Aboriginals. Traditionally, Aboriginal Canadians have lower health status, with poorer access to primary and specialist care,26-28 so it is possible that we have in fact underrepresented this population. In addition, we did not recruit cSLE patients from Manitoba or Saskatchewan, two provinces with high percentages of Aboriginal Canadians.
Sociodemographic characteristics were similar across ethnicities, although patients of Asian and South Asian descent were less likely to have prescription drug and dental coverage. These patient groups were also less likely to have been born in Canada and, therefore, as newer immigrants to Canada perhaps fewer were able to obtain drug coverage and dental insurance generally provided by full-time employment. Alternatively, a greater percentage of these groups may have been self-employed or part of a small business, where supplemental coverage would not be provided. The lower coverage rates in these groups may be a proxy for income; however, we did not observe a significant difference in total household income by ethnicity. In this study the upper income limit was set at $50,000 yearly, lower than the 2008 median Canadian household income of $68,86029 and, therefore, we may not have been able to detect differences which may have been present. Unlike studies from the United States and the GLADEL cohort, we did not find significant differences in disease activity, damage or treatment based on ethnicity, but socioeconomic status (SES) and access to care may be more important determinants of outcome.30-32
Our cluster analysis identified three distinct clusters based on clinical features, which we then explored for their ethnicity distributions. While severe disease was rare in the White patients (only 20% fell into the severe disease cluster), it was prevalent in the non-White ethnicities, especially the Asian (51%) and Black (43%) patients. Comparing our data to the adult SLE patients enrolled in the 1000 Faces of Lupus Study, the overall clinical features were similar, however, the adult patients had a greater proportion of White patients with renal disease (40%), and South Asian patients were included in the Asian ethnic group and not analyzed separately.15 Our findings of more severe disease in Blacks, Asians and Aboriginals (Clusters 1 and 2 - severe and moderate disease) are consistent with the adult literature.33-35 Neuropsychiatric disease was uncommon in our clinical clusters, and was likely underestimated as we did not ascertain the prevalence of the 19 neuropsychiatric syndromes of SLE (NPSLE),36 only that of seizures and psychosis. Instead, we might have expected between 35 and 65% of the cohort to report NPSLE, depending on the criteria employed.8,37,38
The current study confirms previous reports of a higher prevalence of anti-Sm and anti-RNP antibodies in non-White cSLE patients.6,39 A recent cluster analysis of autoantibodies in 156 cSLE patients (from Toronto, which includes 80 of the patients enrolled in the current study) also demonstrated that non-White patients were more likely to have detectable autoantibodies, including anti-Sm and anti-U1RNP.22 Further, when the autoantibody clusters were analyzed, the cluster with the highest percentage of White patients also had the mildest disease, characterized predominantly by malar rash, thrombocytopenia, and a low incidence of renal disease (<20%) in the presence of anti-dsDNA antibodies but no other autoantibodies.22 In our current study, we observed a higher proportion of patients with anti-dsDNA antibodies in Cluster 1 (100% of patients had renal disease in this cluster); however, there were no differences in the frequency of anti-Sm, RNP and other autoantibodies between clusters. Therefore, the emerging data would suggest that the presence of specific autoantibodies may be less important than ethnicity in determining the risk of specific organ involvement in cSLE. However, recent SLE literature would instead suggest that genotypic differences predict variations in phenotypic disease expression,40-42 although only one large study has examined non-Caucasian ethnic groups.
Although we observed differences in clinical manifestations between the ethnic groups, we did not observe greater irreversible damage in non-White ethnicity groups.1,3,30,43 In fact, this entire cohort had very little damage, with an average SDI score of 0.6 at 2.4 years of disease. Thus, we may attribute the low SDI scores to the relatively short disease duration, as damage generally accrues with greater duration of disease.44-46
We recognize potential limitations of our study methods and interpretation. As this was a cross-sectional examination of cSLE we were unable to collect and analyze for possible differences between ethnicities at disease presentation and track these differences through the disease course. Additionally, due to limitations in the cohort size and definition of the ethnic groups we were unable to take into account mixed parental heritage and heterogeneous background; we chose to use only the primary designation by the patient (parent). Notably, when the parental ethnic designations were one parent White and one non-White, we used the non-White designation as the primary ethnicity. Since we did not correct our exploratory statistical analyses for multiple testing, the significance levels of some of our findings should be interpreted cautiously.
In conclusion, we have shown that the cSLE population of Canada is ethnically diverse, with the majority of patients belonging to a visible minority. Although we observed ethnic differences in the severity of disease expression, similar to the Adult 1000 Faces of Lupus cohort15 we did not observe differences in the measured outcomes of treatment, disease activity and irreversible organ damage. These findings would suggest that universal access to healthcare in Canada is an important determinant of positive outcomes in cSLE. Disease activity was low, and damage was minimal in our cohort of relatively recently diagnosed patients, thus long-term study of a population with universal healthcare may further delineate the determinants of outcome in cSLE. Due to recent immigration patterns and the changing ethnocultural landscape, we expect that the incidence of cSLE in Canada will continue to increase, and that the 1000 Faces of Canadian Lupus will continue to evolve in the future. This type of analysis should be undertaken in other countries with different ethnic/cultural mixes and different healthcare systems to determine if our findings are unique to Canada.
Significance and Innovation.
This Canadian cSLE cohort represents one of the largest multicenter, nationally-acquired and ethnically diverse cohorts of cSLE ever described.
We confirm that disease manifestations, autoantibody profiles and severity of disease expression differ by ethnicity, although treatment, disease activity and irreversible organ damage are similar. This suggests that universal access to healthcare in Canada may be an important determinant of favorable disease outcomes in cSLE.
A greater proportion of patients of non-White ethnicity develop severe organ manifestations, confirming findings of studies from other North and South American cSLE cohorts.
Acknowledgement
The CaNIOS 1000 Faces Investigators, coordinators and research assistants, in addition to the authors are as follows:
BC Children's Hospital: Ross Petty, MD
Montreal Children's Hospital: Sarah Campillo, MD, Karen Duffy, MD, and Rosie Scuccimarri, MD
IWK Health Centre: Bianca Lang, MD, Suzanne Ramsey, MD, and Aleasha Warner
Hospital for Sick Children: Lawrence Ng, BSc
Toronto Western Hospital, University Health Network: Paul Fortin, MD, PhD, Murray Urowitz, MD, Dafna
Gladman, MD, Lori Albert, MD, Simon Carette, MPhil, MD , Rob Inman, MD, and Tamara McKenzie
Montreal General Hospital: Ann Clarke, MD, MSc, Christian Pineau, MD, Sasha Bernatsky, MD, PhD, Michele Tobaly and Tania Santopietro
Ottawa General Hospital: C. Douglas Smith, MD, Sherri Simpson and Katrin Smith
Hopital Maisonneuve-Rosemont: Michel Zummer, MD and Diane Ferland
Lethbridge Regional Health Centre: Hector Arbillaga, MD and Annella Wehlage
Jewish General Hospital: Marie Hudson, MD, Murray Baron, MD, and Laeora Berkson, MD and Jessica Bernstein
Halifax Queen Elizabeth II: John Hanly, MD
St. Joseph's Health Sciences Centre: Sara Hewitt and Janine Ouimet
Health Sciences Centre, University of Manitoba: Carol Hitchon, MD, MSc, Andrea Craig (1000 Faces National Coordinator) and Mellissa Moyen
Financial Support: Supported by an operating grant from The Arthritis Society National Office #TAS04/0049. CaNIOS is supported in part by Lupus Canada, Lupus Ontario and BC Lupus as well as the Arthritis and Autoimmune Research Centre Foundation. Dr. Levy's work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K23AR053202)
References
- 1.Hiraki LT, Benseler SM, Tyrrell PN, Harvey E, Hebert D, Silverman ED. Ethnic differences in pediatric systemic lupus erythematosus. J Rheumatol. 2009;36:2539–46. doi: 10.3899/jrheum.081141. [DOI] [PubMed] [Google Scholar]
- 2.Huang JL, Yao TC, See LC. Prevalence of pediatric systemic lupus erythematosus and juvenile chronic arthritis in a Chinese population: a nation-wide prospective population-based study in Taiwan. Clin Exp Rheumatol. 2004;22:776–80. [PubMed] [Google Scholar]
- 3.Tucker LB, Uribe AG, Fernandez M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus. 2008;17:314–22. doi: 10.1177/0961203307087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miettunen PM, Ortiz-Alvarez O, Petty RE, et al. Gender and ethnic origin have no effect on longterm outcome of childhood-onset systemic lupus erythematosus. J Rheumatol. 2004;31:1650–4. [PubMed] [Google Scholar]
- 5.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092–4. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gedalia A, Molina JF, Molina J, Uribe O, Malagon C, Espinoza LR. Childhood-onset systemic lupus erythematosus: a comparative study of African Americans and Latin Americans. J Natl Med Assoc. 1999;91:497–501. [PMC free article] [PubMed] [Google Scholar]
- 7.Hersh AO, von Scheven E, Yazdany J, et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2009;61:13–20. doi: 10.1002/art.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Gupta MK, Ahluwalia J, Singh P, Malhi P. Neuropsychiatric manifestations and antiphospholipid antibodies in pediatric onset lupus: 14 years of experience from a tertiary center of North India. Rheumatol International. 2009;29:1455–61. doi: 10.1007/s00296-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 9.Vachvanichsanong P, Dissaneewate P, McNeil E. Twenty-two years’ experience with childhood-onset SLE in a developing country: are outcomes similar to developed countries? Arch Dis Child. 2011;96:44–9. doi: 10.1136/adc.2010.183699. [DOI] [PubMed] [Google Scholar]
- 10.Houghton KM, Page J, Cabral DA, Petty RE, Tucker LB. Systemic lupus erythematosus in the pediatric North American Native population of British Columbia. J Rheumatol. 2006;33:161–3. [PubMed] [Google Scholar]
- 11.Canada S. Canada's Ethnocultural Mosaic, 2006 Census. 2008.
- 12.Belanger A, Malenfant EC. Ethnocultural diversity in Canada: Prospects for 2017. Adapted from Population projections of visible minority groups, Canada, provinces and regions: 2001-2017 (Statistics Canada Catalogue no 91-541-XIE) 2005.
- 13.Millar WJ, Locker D. Dental insurance and use of dental services. Health Rep. 1999;11:55–67(Eng). 59–72(Fre). [PubMed] [Google Scholar]
- 14.Kapur V, Basu K. Drug coverage in Canada: who is at risk? Health Policy. 2005;71:181–93. doi: 10.1016/j.healthpol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Peschken CA, Katz SJ, Silverman E, et al. The 1000 Canadian faces of lupus: determinants of disease outcome in a large multiethnic cohort. J Rheumatol. 2009;36:1200–8. doi: 10.3899/jrheum.080912. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Canada S. Census dictionary - Internet version. 2001.
- 18.Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42:1354–60. doi: 10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 20.Bae SC, Koh HK, Chang DK, Kim MH, Park JK, Kim SY. Reliability and validity of systemic lupus activity measure-revised (SLAM-R) for measuring clinical disease activity in systemic lupus erythematosus. Lupus. 2001;10:405–9. doi: 10.1191/096120301678646146. [DOI] [PubMed] [Google Scholar]
- 21.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 22.Jurencak R, Fritzler M, Tyrrell P, Hiraki L, Benseler S, Silverman E. Autoantibodies in pediatric systemic lupus erythematosus: ethnic grouping, cluster analysis, and clinical correlations. J Rheumatol. 2009;36:416–21. doi: 10.3899/jrheum.080588. [DOI] [PubMed] [Google Scholar]
- 23.Kurahara D, Tokuda A, Grandinetti A, et al. Ethnic differences in risk for pediatric rheumatic illness in a culturally diverse population. J Rheumatol. 2002;29:379–83. [PubMed] [Google Scholar]
- 24.McCarty DJ, Manzi S, Medsger TA, Jr., Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38:1260–70. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 25.Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus. 2006;15:715–9. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 26.Adelson N. The embodiment of inequity: health disparities in aboriginal Canada. Can J Public Health. 2005;96(Suppl 2):S45–61. doi: 10.1007/BF03403702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao S, Manns BJ, Culleton BF, et al. Access to health care among status Aboriginal people with chronic kidney disease. CMAJ. 2008;179:1007–12. doi: 10.1503/cmaj.080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah BR, Gunraj N, Hux JE. Markers of access to and quality of primary care for aboriginal people in Ontario, Canada. Am J Public Health. 2003;93:798–802. doi: 10.2105/ajph.93.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Median total income, by family type, by census metropolitan area. [August 27, 2011].
- 30.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83:1–17. doi: 10.1097/01.md.0000104742.42401.e2. [DOI] [PubMed] [Google Scholar]
- 31.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37:1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trupin L, Tonner MC, Yazdany J, et al. The role of neighborhood and individual socioeconomic status in outcomes of systemic lupus erythematosus. J Rheumatol. 2008;35:1782–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Alarcon GS, Calvo-Alen J, McGwin G, Jr., et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis. 2006;65:1168–74. doi: 10.1136/ard.200X.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcon GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 36.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Sibbitt WL, Jr., Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29:1536–42. [PubMed] [Google Scholar]
- 38.Olfat MO, Al-Mayouf SM, Muzaffer MA. Pattern of neuropsychiatric manifestations and outcome in juvenile systemic lupus erythematosus. Clin Rheumatol. 2004;23:395–9. doi: 10.1007/s10067-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 39.Barron KS, Silverman ED, Gonzales J, Reveille JD. Clinical, serologic, and immunogenetic studies in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1993;36:348–54. doi: 10.1002/art.1780360310. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez E, Nadig A, Richardson BC, et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1752–7. doi: 10.1136/ard.2011.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richman IB, Chung SA, Taylor KE, et al. European population substructure correlates with systemic lupus erythematosus endophenotypes in North Americans of European descent. Genes and immunity. 2010;11:515–21. doi: 10.1038/gene.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor KE, Chung SA, Graham RR, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7:e1001311. doi: 10.1371/journal.pgen.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutcliffe N, Clarke AE, Gordon C, Farewell V, Isenberg DA. The association of socio-economic status, race, psychosocial factors and outcome in patients with systemic lupus erythematosus. Rheumatology (Oxford) 1999;38:1130–7. doi: 10.1093/rheumatology/38.11.1130. [DOI] [PubMed] [Google Scholar]
- 44.Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM. Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002;46:436–44. doi: 10.1002/art.10072. [DOI] [PubMed] [Google Scholar]
- 45.Bandeira M, Buratti S, Bartoli M, et al. Relationship between damage accrual, disease flares and cumulative drug therapies in juvenile-onset systemic lupus erythematosus. Lupus. 2006;15:515–20. doi: 10.1191/0961203306lu2316oa. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Suarez R, Ruperto N, Gastaldi R, et al. A proposal for a pediatric version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index based on the analysis of 1,015 patients with juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2989–96. doi: 10.1002/art.22048. [DOI] [PubMed] [Google Scholar]