Abstract
Rationale
Dominance hierarchies affect ethanol self-administration, with greater intake among subordinate animals compared to dominant animals. Excessive ethanol intake disrupts circadian rhythms. Diurnal rhythms of the hypothalamic–pituitary–adrenal axis have not been characterized in the context of ethanol self-administration with regard to social rank.
Objective
This study aimed to determine whether diurnal pituitary–adrenal hormonal rhythms account for differences between social ranks in ethanol self-administration or are differentially affected by ethanol self-administration between social ranks.
Methods
During alternating individual (n=11–12) and social (n=3 groups) housing of male cynomolgus monkeys (Macaca fascicularis), diurnal measures of cortisol and adrenocorticotropic hormone (ACTH) were obtained from plasma samples three times per week. Social rank was determined, ethanol (4 %, w/v) self-administration was induced, and then the monkeys were allowed a choice of water or ethanol for 22 h/day for 49 weeks.
Results
For all social ranks, plasma ACTH was elevated during social housing, but cortisol was stable, although greater among dominant monkeys. Ethanol self-administration blunted the effect of social housing, cortisol, and the diurnal rhythm for both hormones, regardless of daily ethanol intake (1.2–4.2 g/kg/day). Peak ACTH and cortisol were more likely to be observed in the morning during ethanol access. Ethanol, not vehicle, intake was lower during social housing across social ranks. Only dominant monkeys showed significantly lower blood–ethanol concentration during social housing.
Conclusions
There was a low threshold for disruption of diurnal pituitary rhythms by ethanol drinking, but sustained adrenal corticosteroid rhythms. Protection against heavy drinking among dominant monkeys may have constrained ethanol intoxication, possibly to preserve dominance rank.
Keywords: Ethanol, HPA axis, Stress, Monkey, Cortisol, ACTH
Introduction
Social interactions within all primate social groups are governed by hierarchical relationships in which dominant individuals benefit from favorable treatment such as access to food or preferential mating. Whereas social rank is inherited among females, social rank in males depends on factors including size (Morgan et al. 2000) and dominance-related behaviors (Flack and de Waal 2004). Hierarchical social structure among males varies between primate species, ranging from highly aggressive, strict hierarchies (e.g., baboons, rhesus macaques) to more permissive hierarchies (e.g., bonnet macaques), with cynomolgus monkeys being slightly less aggressive than the former (Thierry 2007). These social styles and the availability of social support for subordinate monkeys appear to be related to relative adrenocortical activity among dominant and subordinate monkeys as measured by cortisol (Abbott et al. 2003). The basic social structure of humans resembles that of nonhuman primates (Foley and Gamble 2009). While aggressively mediated dominance obviously occurs in humans, a majority of dominance interactions are verbal or psychogenic and may be escapable due to a variety of social roles (e.g., work, school, home; Bohem 2004). Nonetheless, social stress in humans (e.g., harassment at work; Richman et al. 2002), Old World monkeys (social separation and social affiliation; Higley et al. 1996), and New World (social separation and subordinate status; McKenzie-Quirk and Miczek 2008) monkeys has been linked with greater alcohol consumption.
Activity of the hypothalamic–pituitary–adrenal (HPA) axis prepares animals to adapt to stressors (e.g., novel stimuli, exercise, injury) and to remember the conditions under which they occurred to anticipate future stressors (Wolf 2009). Adrenal production of cortisol due to stimulation by adrenocorticotropic hormone (ACTH) from the pituitary is a primary outcome of the HPA axis. In addition, circulating cortisol concentration fluctuates throughout the day, being greatest in the morning and lowest in the evening in primates (Urbanski 2011). The diurnal rhythm of cortisol is reflective of a healthy HPA axis and impaired rhythms are a medically important index of stress associated with heart disease and cancer (Matthews et al. 2006; Rosmond and Bjorntorp 2000; Sephton et al. 2000). Time of day and wakefulness correlate strongly with the timing of diurnal hormone rhythms (e.g., Edwards et al. 2001) and are regulated independently of the absolute concentration or magnitude of the rhythms. For example, in capuchin monkeys, the synthetic glucocorticoid dexamethasone, an ACTH suppressor, decreased the absolute concentration of cortisol without disrupting its magnitude or diurnal rhythm (Torres-Farfan et al. 2008). Thus, the timing and magnitude of diurnal rhythms reflect different aspects of endocrine physiology, the former relating to the light–dark cycle or feeding schedule (Krieger 1974) and the latter to the activity of components of the HPA axis.
Ethanol is well known to influence HPA axis activity, acutely stimulating pituitary release of ACTH (Lee et al. 2004), thus increasing adrenal steroid synthesis (Porcu et al. 2004), an effect that diminishes with ethanol dependence (Boyd et al. 2010). Excessive ethanol consumption has been related to interactions between HPA axis genes (e.g., CRHR1) and psychosocial stress in humans (Blomeyer et al. 2008) and using genetic models (Sillaber et al. 2002). In addition, chronic ethanol consumption can disrupt hormonal circadian rhythms (reviewed by Spanagel et al. 2005). Recently, mice in which the clock gene Per1 was mutated had deficient diurnal activity rhythms and showed increased ethanol intake after social defeat compared to wild-type mice (Dong et al. 2011), suggesting that diurnal rhythms could interact with social stress to influence ethanol consumption. To date, the interaction between diurnal endocrine activity, social stress, and ethanol consumption has not been studied in primates.
The HPA axis has been proposed as a mechanism by which social stress affects ethanol consumption (Barr et al. 2008, 2009). Within subjects, social separation is often used to manipulate social stress, whereas classifying by social rank compares social stress between subjects. Greater cortisol and ethanol consumption were observed among peer-reared rhesus macaques during social separation compared to social housing (Higley et al. 1991). Likewise, when socially isolated for 1 week, squirrel monkeys consumed a greater quantity of ethanol and had greater afternoon salivary cortisol compared to during social housing (McKenzie-Quirk and Miczek 2008). McKenzie-Quirk and Miczek interpreted their data to be consistent with the tension reduction hypothesis, which proposes that ethanol drinking is reinforced by anxiolytic effects that decrease social stress.
The present study evaluated the interaction between social rank and housing conditions on ethanol self-administration and diurnal pituitary–adrenal rhythms in male cynomolgus monkeys. To manipulate housing conditions, we used the common manipulation of forming social groups by combining four individual housing units into a single social cage containing four monkeys (Grant et al. 1998; Morgan et al. 2000, 2002; Czoty et al. 2008; Riddick et al. 2009). Based on past studies, we hypothesized that all monkeys would drink more ethanol when individually compared to socially housed and that subordinate monkeys would drink more ethanol than dominant monkeys (McKenzie-Quirk and Miczek 2008). Previous studies suggested that activity rhythms during chronic, forced exposure to ethanol showed wide individual differences in rodents (Rosenwasser et al. 2005) and that cortisol secretion was elevated during withdrawal in alcoholics (Fonzi et al. 1994) and cynomolgus monkeys (Cuzon Carlson et al. 2011). The current study expanded upon these limited data by measuring the diurnal rhythm of pituitary–adrenal activity, which was expected to be disrupted particularly among heavy drinkers.
Materials and methods
Animals
The subjects were male cynomolgus monkeys (n=11–12, Macaca fascicularis, age 50–62 months, weight 3.8–5.7 kg) that were experimentally naïve at the onset of the study. Following 2 months of quarantine, the monkeys moved to the laboratory and were individually housed in quadrant cages (0.8×0.8×0.9 m) equipped with removable vertical and horizontal metal partitions, allowing for social housing (1.6×0.8×1.8 m). Each quadrant cage was individually equipped with an operant panel on the wall which dispensed and food and liquids. The room temperature was maintained at 20–22 °C, the humidity at 65 %, and the light cycle was 12 h (lights on at 7:00 AM). The monkeys were weighed weekly. All animal procedures were approved by the Wake Forest University IACUC and were performed in accordance with the NIH and the Guide for the Care and Use of Laboratory Animals. Portions of the ethanol self-administration data including blood–ethanol concentration (BEC) from these monkeys have been published (Grant et al. 2008).
Due to the influence of anesthesia on sampling of ACTH (e.g., Welker et al. 1992), over 5 months, the monkeys were first acclimated to the laboratory and then trained to participate in venipuncture to obtain blood samples without anesthesia (Porcu et al. 2006). All samples were obtained in the absence of anesthesia or fasting. During the first 27 weeks of the study, ethanol was not available. During this time, housing conditions were manipulated as shown in Table 1. Because adrenal activity in primates may be altered for more than 2 months following changes in social organization (Mendoza et al. 1979; Goo and Sassenrath 1980; Gonzalez et al. 1981), the housing conditions were changed after 3 months. For social housing, the cage partitions were removed for 2 h/day (9:00–11:00 AM). This limited social interaction was necessary to combine social housing with ethanol self-administration using our established protocol and was considered a daily reminder of social rank.
Table 1.
Timeline of experimental conditions
| Housing condition | Weeks |
|---|---|
| Before ethanol access | |
| Individual | 1–2 |
| Social | 3–15 |
| Individual | 16–27 |
| 22 h/day ethanol access | |
| Individual | 28–51 |
| Social | 52–76 |
Social rank classification
Social rank was determined by recording social behaviors during the last week of social housing and prior to ethanol access (week 15). Three trained observers completed 15-min observations of each monkey, with each observer monitoring a single rack of three to four monkeys. Behaviors were characterized as aggressive, submissive, or sexual. Minutes spent initiating and receiving aggressions, submissions, and grooming behaviors were also recorded. Outcomes of dyadic agonistic interactions between cage mates were used to identify social rank. Within each rack, the monkey with the most aggressions and to which most others submitted was designated the highest-ranking monkey. The monkey to which all but the highest-ranking monkey submitted was designated the second highest-ranking monkey, and so on. Thus, the monkeys were ranked as dominant, subordinate, or intermediate. A dominant monkey (i.e., 90) died 43 weeks into the experiment by causes unrelated to the experiment or chronic illness and was replaced in rank by the next highest-ranking monkey, 96 (Table 2).
Table 2.
Drinking status and behavioral scoring used in social rank assignment
| Social group | Monkey | Aggressive | Submissive | Sexual | Rank | Drinking status |
|---|---|---|---|---|---|---|
| 1 | 86 | 10 | 1 | 5 | Dominant | Nonheavy |
| 87 | 7 | 4 | 11 | Intermediate | Heavy | |
| 88 | 0 | 62 | 2 | Subordinate | Heavy | |
| 2 | 91 | 7 | 1 | 5 | Intermediate | Nonheavy |
| 92 | 20 | 0 | 11 | Dominant | Nonheavy | |
| 93 | 6 | 9 | 5 | Intermediate | Heavy | |
| 94 | 0 | 87 | 6 | Subordinate | Nonheavy | |
| 3 | 89 | 11 | 36 | 6 | Intermediate | Heavy |
| 90 | 33 | 3 | 11 | Dominant | Nonheavy | |
| 95 | 2 | 50 | 3 | Subordinate | Nonheavy | |
| 96 | 6 | 1 | 10 | Intermediatea | Nonheavy |
Monkeys drinking on average >3.0 g/kg/day throughout the 22 h/day access to ethanol were classified as heavy drinkers
96 became dominant 43 weeks into the experiment upon the death of 90 (see Table 1)
Ethanol access
The second part of the study (weeks 28–76) occurred after schedule-induced polydipsia was used to induce ethanol self-administration (Grant et al. 2008). Briefly, the monkeys consumed a required volume of water or 4 % ethanol (w/v, in water), resulting in the termination of a fixed-time 300-s schedule of pellet delivery. Every 30 days, the dose of ethanol consumed was increased from 0 g/kg/day (water volume equivalent to 1.5 g/kg ethanol) to 0.5, 1.0, and finally 1.5 g/kg/day. Following induction, ethanol (4 % w/v) and water were concurrently available for 22 h/day, with the monkeys first housed individually and then socially. Individual housing conditions were as previously described (Vivian et al. 2001), and during these periods, the monkeys were allowed visual, auditory, and olfactory contact with conspecifics. Ethanol was not available during social housing because the fluid reservoirs were replenished during this time. Details regarding training and meals are described by Grant et al. (2008). Drinking status was based on daily average intake over the 49 weeks of ethanol self-administration. Monkeys with a daily average >3.0 g/kg were defined as heavy drinkers (Vivian et al. 2001). Monkeys with intakes below this threshold were defined as nonheavy drinkers.
Apparatus
Within each monkey's home cage, an operant panel on one wall permitted access to all fluid and food and has been extensively described (Vivian et al. 2001). Panels were controlled via a computerized system (Macintosh G4, Apple Computer Inc., Cupertino, CA, USA, with interface and programming environment from National Instruments Corporation, Austin, TX, USA). Each panel contained two drinking spouts, a set of three lights (red, white, and green) located below one of the spouts, and a centrally located opening containing a dowel with an associated stimulus light. Each spout was connected by tubing to a fluid reservoir placed on a digital scale (N1B110, Ohaus Corporation, Pine Brook, NJ, USA) connected to the computer interface.
Assays
Blood draws (3 ml) provided the plasma for the assays of ACTH and cortisol. Blood samples were set on ice until centrifuged (3,000 rpm, 15 min, 4 °C) and stored at –20 °C (cortisol) or –80 °C (ACTH). ACTH and cortisol were assayed by Yerkes Endocrine Core Laboratory. BEC was measured approximately every fifth day 7 h into the 22 h/day drinking session, resulting in 68 observations per monkey evenly split between individual and social housing (21 observations for monkey 90 during individual housing, see above).
Statistical analysis
The independent variables were housing rack, housing condition (three levels pre-ethanol: first individual, social, and second individual; two levels during ethanol access: individual and social), social rank (three levels: dominant, intermediate, and subordinate), and where appropriate, time of day (three levels: AM, noon, and PM) for the plasma samples. The dependent variables were the mean, weekly maximums (peak), and weekly minimums (nadir) of ACTH (in picograms per milliliter) and cortisol (in micrograms per deciliter), mean daily ethanol intake (in grams per kilogram), and BEC (in milligrams per deciliter). ACTH and cortisol concentrations were log-transformed to meet distributional assumptions prior to analysis. All dependent variables were analyzed using linear mixed models in which monkey was the subject variable. Univariate analyses were conducted with each independent variable prior to multivariate modeling in which nonsignificant factors were placed in the error term. For drinking measures, a univariate model for cage rack was conducted, and then housing condition, social rank, and the interaction term were included in the full model because time of day was not relevant. In all analyses, cage rack was a nonsignificant factor. On a majority (69 %) of experimental days, monkey 96 was intermediate-ranked, and only this rank was included in analyses of endocrine measures. However, 96 was dominant during a majority (67 %) of sessions during 22 h/day access to ethanol, and this rank was included in the analysis of the effect of social rank on drinking. The results reported are from the final models using the optimal covariance structure as determined by –2 log likelihood criteria. Significant main effects and interactions were assessed using Bonferroni-corrected comparisons. For all analyses, α=0.05. All analyses were conducted using SAS 9.2.
Results
Effect of housing on ACTH and cortisol prior to ethanol access
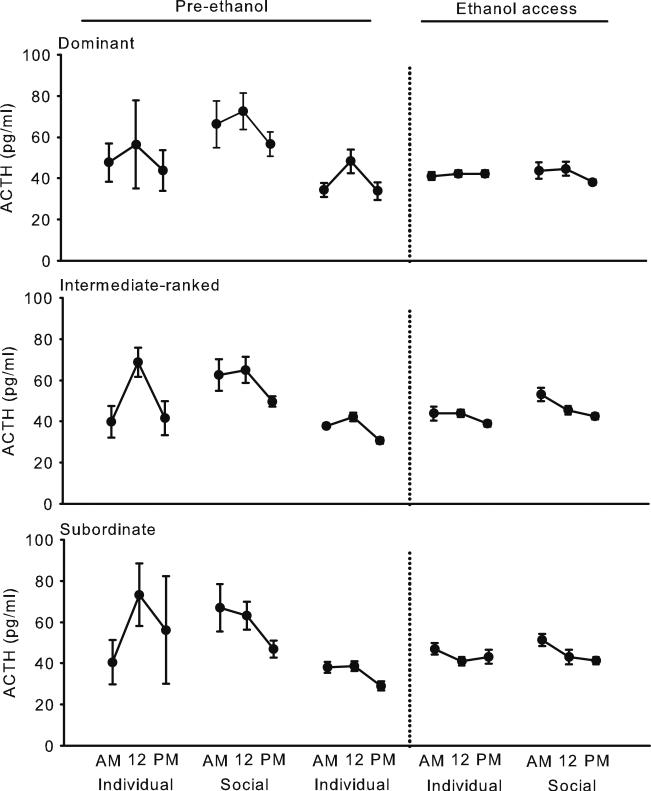
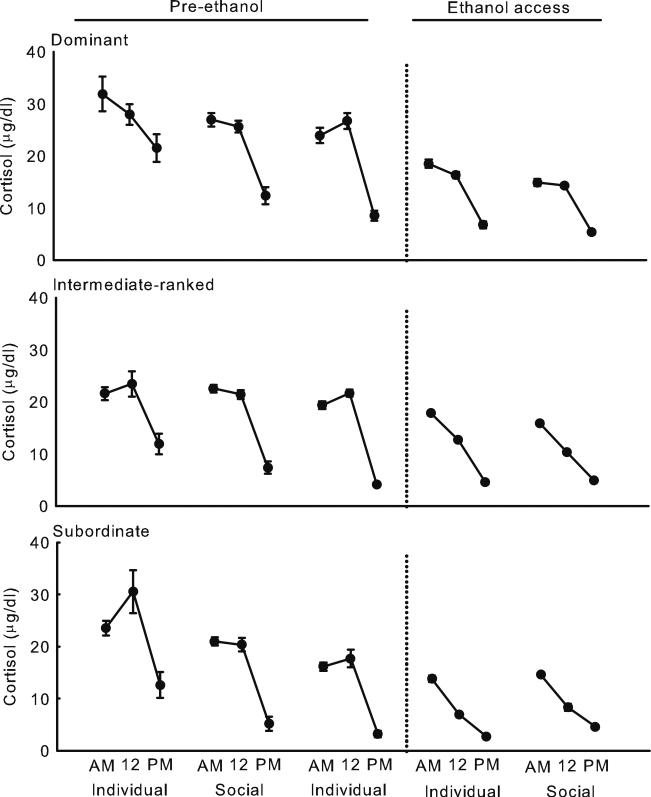
Prior to ethanol access, the diurnal rhythm of ACTH was variable, F(2, 22)=14.4, p=0.0001 (Fig. 1), in contrast to the strong diurnal rhythm of cortisol, F(2, 18)=265.8, p<0.0001 (Fig. 2). Both hormones were greater in the morning (mean±SD: ACTH, 54.7±37.7 pg/ml; cortisol, 19.7±6.2 μg/dl) and at noon (ACTH, 54.1±30.3 pg/ml; cortisol, 17.5±8.2 μg/dl) compared to the evening (ACTH, 44.8±21.5 pg/ml; cortisol, 7.6±6.4 μg/dl). At all times of day before the monkeys had access to ethanol, mean ACTH was greater during social housing (68.9±54.4 pg/ml) compared to when monkeys were housed individually [41.1±19.4 pg/ml; housing condition, F (4, 41)=20.1, p<0.0001; housing condition×time of day, F(8, 81)=2.9, p=0.008]. Both the peak, F(4, 31)=44.0, p<0.0001, and nadir, F(4, 31)=30.5, p<0.0001, of ACTH were also elevated during social housing (Fig. 3). Greater ACTH as measured by the mean, peak, and nadir during social housing was unrelated to social rank. Concentrations of ACTH prior to ethanol access were similar between dominant (56.0±38.3 pg/ml), intermediate-ranked (54.3±34.4 pg/ml), and subordinate (53.7±34.4 pg/ml) monkeys when averaged across all times of day and housing conditions. In contrast, prior to ethanol access, averaged across all times of day and housing conditions, mean cortisol was greater among dominant (21.1±9.6 μg/dl) compared to intermediate-ranked (17.1±9.0 μg/dl) or subordinate monkeys (15.5±9.4 μg/dl), F(2, 9)=6.6, p=0.02. Both the peak and nadir of cortisol were greater among dominant compared to intermediate-ranked or subordinate monkeys [F(2, 8)=4.7, p=0.046 and F(2, 8)=11.1, p=0.005, respectively].
Fig. 1.
Mean ± SEM ACTH at 7:00 (AM), noon (12), and 5:30 (PM) during manipulations of housing before and after access to ethanol among dominant (top), intermediate-ranked (middle), and subordinate (bottom) monkeys. Error bars are near the symbols during ethanol access due to low variance in ACTH. Means are computed from 183 to 275 samples per monkey
Fig. 2.
Mean ± SEM cortisol at 7:00 (AM), noon (12), and 5:30 (PM) during manipulations of housing before and after access to ethanol among dominant (top), intermediate-ranked (middle), and subordinate (bottom) monkeys. Error bars are near the symbols during ethanol access due to low variance in cortisol. Means are computed from 188 to 278 samples per monkey
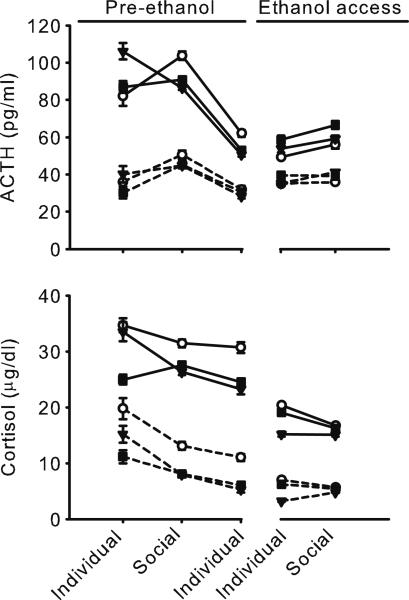
Fig. 3.
Mean ± SEM peak (solid lines) and nadir (dashed lines) ACTH (183–275 samples per monkey) and cortisol (188–278 samples per monkey) during manipulations of housing before and during access to ethanol among dominant (circle), intermediate-ranked (square), and subordinate (triangle) monkeys
Cortisol was markedly elevated only during the first day of social housing compared to the prior 2 weeks of individual housing (Supplemental Figs. 1, 2, and 3). The first day of social housing coincided with an evening blood draw, and 11 out of 12 monkeys showed increased cortisol ranging from 7.7 to 52.1 μg/dl (mean ± SD, 20.5±16.8 μg/dl) compared to evening cortisol during the preceding 2 weeks of individual housing. Elevated cortisol on the first day of social housing was unrelated to social rank. In contrast, cortisol decreased (–4.5±3.2 μg/dl) between the last 2 weeks of social housing and the first week of individual housing that followed. When all the data were included, the mean (Fig. 2), peak, and nadir (Fig. 3) of cortisol decreased across the housing manipulations, and concentrations did not differ selectively between social and individual housing [mean, F(4, 32)=44.9, p<0.0001; peak, F(4, 33)=110.3, p<0.0001; nadir, F(4, 33)=16.6, p<0.0001].
Consistent with the variable rhythm of ACTH, prior to ethanol access, peak ACTH was measured most frequently at noon, but peak was also measured in approximately 20–30 % of morning and evening samples, irrespective of housing condition. During individual housing, the distribution of peak cortisol between morning and noon samples was almost equal. During individual housing that followed social housing, however, a shift in the timing of peak cortisol occurred in which peak cortisol occurred most frequently at noon (Table 3).
Table 3.
Mean ± SD percentage of times of day with peak ACTH (183–275 samples per monkey) or cortisol (188–278 samples per monkey) across housing conditions before and during access to ethanol for self-administration
| Housing condition | ACTH |
Cortisol |
||||
|---|---|---|---|---|---|---|
| AM | Noon | PM | AM | Noon | PM | |
| Pre-ethanol access | ||||||
| Individual | 17±25 | 50±31 | 33±33 | 50±30 | 43±20 | 7±17 |
| Social | 37±13 | 45±14 | 18±15 | 50±12 | 44±11 | 6±4 |
| Individual | 27±14 | 58±18 | 15±10 | 28±20 | 72±20 | 0 |
| Ethanol access | ||||||
| Individual | 41±19 | 37±10 | 22±15 | 73 ±17 | 25±15 | 2±4 |
| Social | 54±19 | 27±15 | 19±9 | 80±20 | 19±18 | 1±2 |
Effect of housing on ACTH and cortisol during ethanol access
Access to ethanol was associated with a flattened diurnal rhythm for ACTH and cortisol in all monkeys. Specifically, the peak and nadir were more similar compared to when the monkeys did not have access to ethanol (Fig. 3). In addition, cortisol was suppressed during ethanol self-administration. The timing of peak ACTH and cortisol also shifted during ethanol access, most frequently occurring in the morning (Table 3). Ethanol self-administration blunted the endocrine effects of the housing manipulation. That is, when the monkeys had access to ethanol, social housing did not increase ACTH compared to individual housing at any time of day, as indicated by mean ACTH (individual, 46.0±22.2 pg/ml; social, 48.7±23.6 pg/ml; Fig. 2) or the nadir of ACTH (individual, 34.5±17.1 pg/ml; social, 35.4±15.1 pg/ml; Fig. 3). Peak ACTH was slightly greater during social (65.3±30.6 pg/ml) compared to individual (59.8±29.9 pg/ml) housing, but the magnitude of increase was blunted during ethanol access. Ethanol intake was not correlated with the decrease in ACTH from social housing before and during ethanol access, suggesting that ethanol dampened ACTH below an average daily intake of 1.8 g/kg.
On average, cortisol was similar across housing conditions during access to ethanol (Fig. 2), and there was no evidence of a transient increase on the first day of social housing (Supplemental Figs. 1, 2, and 3). When housed individually and drinking ethanol, subordinate monkeys had lower peak cortisol (17.7±9.8 μg/dl) compared to intermediate-ranked (20.8±6.9 μg/dl) or dominant (23.7±8.5 μg/dl) monkeys, F(8, 33)=3.1, p=0.01, and the nadir of cortisol was greater in both intermediate-ranked (5.8±4.1 μg/dl) and dominant (8.1±4.3 μg/dl) compared to subordinate (3.4±2.1 μg/dl) monkeys, F(8, 33)=2.8, p=0.02. None of these measures differed between the social ranks when they were housed socially and drinking ethanol (Fig. 3). Averaged across all times of day and social ranks, cortisol was blunted during ethanol self-administration (11.4±6.4 μg/dl) compared to before ethanol access (18.6±10.5 μg/dl).
Effect of housing condition on ethanol self-administration
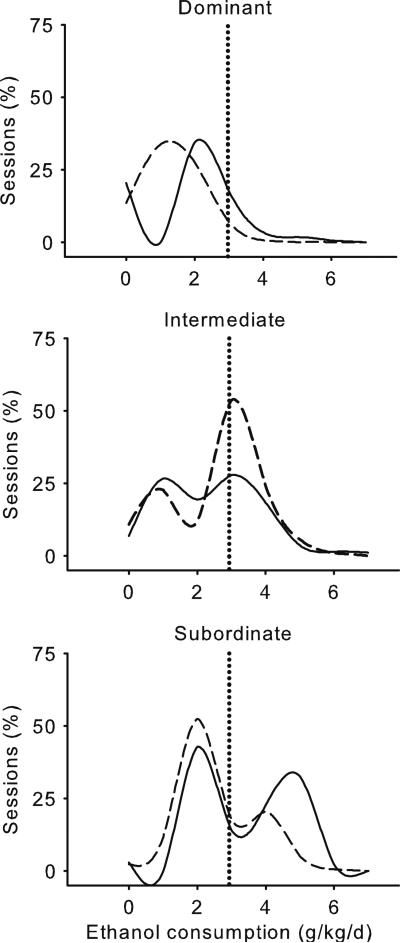
None of the dominant monkeys were heavy drinkers, and all had lower ethanol intakes when housed in a social group compared to those individually housed (Table 2, Fig. 4). Lower daily ethanol intake among dominant (mean±SD, 2.0±1.0 g/kg/day) compared to subordinate (3.0±1.3 g/kg/day) and intermediate-ranked (3.1±1.3 g/kg/day) monkeys was not significantly different when averaged across housing conditions, F(2, 8)=2.0, similar to BEC, F(2, 8)=2.1. Overall, average daily ethanol intake was slightly but significantly lower during social (mean±SD, 2.6±1.1 g/kg/day) compared to individual (2.8±1.1 g/kg/day) housing, F(1, 7)=100.9, p<0.0001, especially among dominant monkeys [housing condition×rank, F(2, 7)=75.9, p<0.0001]. BEC differed between housing conditions only among dominant monkeys, for whom it was lower during social (48±55 mg/dl) compared to individual (66±71 mg/dl) housing [housing condition×rank, F(2, 7)=14.5, p=0.003, t(5)=4.3, p=0.02; Fig. 4]. In contrast, BEC was similar between individual and social housing among intermediate-ranked (88±61 and 104±68 mg/dl, respectively) and subordinate (119±51 and 130±62 mg/dl, respectively) monkeys. For each social rank, ethanol intake decreased significantly between the week before and the first week of social housing, F(1, 7)=56.8, p= 0.0001. However, only dominant monkeys maintained lower ethanol intake throughout social housing. In contrast, vehicle intake did not change significantly, F(1, 7)=5.2, p=0.06, among any social rank, F(2, 7)=4.5, p=0.06 (Supplemental Figs. 4, 5, and 6).
Fig. 4.
Frequency distributions of daily ethanol consumption for dominant (top), intermediate-ranked (middle), and subordinate (bottom) monkeys during individual and social housing calculated across 1-g/kg intervals from 170 to 175 sessions, respectively, per housing condition (except monkey 90). After the death of monkey 90 during individual housing (week 43), monkey 96 became dominant
Discussion
The hormonal data in the current study suggested that individual housing was less stressful than quadrant social housing in young adult male cynomolgus monkeys. However, it should be emphasized that the quadrant caging is relatively restricted space and does not allow animals to leave the presence of any other member of the group. Ethanol self-administration was then assessed during individual housing or daily social grouping. In contrast to the tension reduction hypothesis, continuous individual housing, which was less stressful, was associated with greater ethanol consumption. Greater ethanol self-administration was previously reported during social isolation compared to social housing in rats (Parker and Radow 1974; Wolffgramm and Heyne 1991; Ehlers et al. 2007) and monkeys (Kraemer and McKinney 1985; McKenzie-Quirk and Miczek 2008; Higley et al. 1991). From a behavioral economics viewpoint, ethanol self-administration may have increased during individual housing due to the absence of alternative sources of reinforcement, including physical interaction with conspecifics (Crowley 1983). This seems unlikely, however, because in the current study, ethanol access from the panel occurred when all monkeys were individually housed, after the daily social grouping (i.e., barriers prevented direct physical interaction as a source of reinforcement). The decreased ethanol drinking among dominant monkeys was more likely due to associative processes related to the daily reminder of social rank, rather than the proximal environment. Dominant monkeys may avoid ethanol intoxication during social housing because it is incompatible with behaviors that maintain dominance (Winslow and Miczek 1985).
The current study showed that protection against heavy ethanol drinking by dominant social status was unrelated to diurnal pituitary–adrenal activity, which was similar during daily social grouping among the ranks. The lower ethanol intake of dominant compared to nondominant monkeys is similar to previous studies showing lower self-administration of cocaine (Morgan et al. 2002; but see Czoty et al. 2004) and ethanol (McKenzie-Quirk and Miczek 2008). Apart from an involvement of pituitary–adrenal activity, differences in dopamine activity between social ranks (e.g., female cynomolgus monkeys; Grant et al. 1998) could explain lower self-administration of drugs among dominant monkeys. Lower synaptic dopamine and dopamine D2 receptors in the striatum of subordinates (Grant et al. 1998) could enhance drug reinforcement or behavioral processes involved in the acquisition of operant self-administration. Indeed, once self-administration was acquired, male cynomolgus monkeys of different social ranks did not differ in self-administration of cocaine (Czoty et al. 2004). Additional studies are needed to determine the extent to which striatal dopamine activity provides a biological basis for protection against consumption of excessive quantities of ethanol by dominant social status.
Two hours of social housing in the morning had a lasting effect on pituitary activity, as indicated by increased ACTH in evening samples. Indeed, ACTH remained elevated throughout social housing prior to ethanol exposure. The average concentrations of ACTH in the current study are lower than concentrations after an acute stressor (presentation of “catch gloves,” >100 pg/ml; female cynomolgus monkeys; Herod et al. 2011). Overall, the levels of ACTH found in the present study appear to be indicative of the vigilance associated with living in a social setting, rather than a “fight or flight” response. In addition, average cortisol measured across all social ranks was <40 μg/dl, consistent with normal, healthy macaques trained to participate in venipuncture (Wilson et al. 2005). On the first day of social housing, cortisol was transiently elevated for monkeys of all social ranks (maximum, 60 μg/dl). Similar to Czoty et al. (2008), cortisol concentrations returned to normal within days after a switch to social housing. In another study, when socially housed squirrel monkeys were subsequently housed alone, cortisol increased nearly twofold in the first hour, peaking after 1 day, and remained elevated above baseline for almost a week (Lyons et al. 1999). The current study showed that transiently elevated cortisol was related to housing condition, rather than just a change of housing, because rather than increasing, cortisol decreased slightly when the monkeys were switched from social to individual housing.
The maintenance of cortisol rhythms despite fluctuating ACTH is not surprising as adrenocortical rhythms are generated in part independently of pituitary ACTH (Meier 1976) and adrenocortical sensitivity to ACTH is adaptable (Oster et al. 2006). Indeed, adrenal sensitivity to ACTH has been reported to vary with social status in monkeys. In females (Shively 1998) and males (Czoty et al. 2008), socially housed subordinate cynomolgus monkeys had greater plasma cortisol following the administration of ACTH compared to dominant and intermediate-ranked monkeys. In the current study, similar to past studies (e.g., Czoty et al. 2008), diurnal adrenocortical rhythms only slightly differed between the social ranks. We found that subordinate and intermediate-ranked monkeys had more similar cortisol (peak and nadir) before ethanol access, but dominant and intermediate-ranked monkeys had more similar cortisol during ethanol access before social housing. This appeared to be due to the maintenance of cortisol peaks and nadirs among intermediate-ranked monkeys during ethanol access.
Intermediate-ranked monkeys may have a distinct temperament. For example, previous studies showed that intermediate-ranked monkeys initiated grooming more frequently than dominant or subordinate monkeys (Morgan et al. 2000) and had lower adrenal cortisol response to ACTH during individual housing (Czoty et al. 2008). The data in the current study could indicate that adrenocortical rhythms are more dynamic among individuals at the extremes of social rank. Insofar as social rank is an outcome of trait-like behaviors, endocrine activity and social behaviors should be consistent even if a monkey changes rank. Social upheaval due to the death of the dominant monkey (90) in rack 3 resulted in a prolonged, but reversible, increase in ACTH in the next highest-ranking monkey (96), suggesting that social rank is a trait. Physical contact was not possible during the upheaval, so the transient increase in ACTH could have been related to increased vocal aggression. While monkey 90 was alive and dominant, monkey 96 was an intermediate-ranked low drinker, and he remained a low drinker after becoming dominant. The data suggest that low ethanol consumption may be a behavioral trait of dominant nonhuman primates.
Despite a constant light–dark cycle, the current study showed that peak cortisol was more likely in the morning during ethanol access. This result contrasts with flattened diurnal corticosterone in mice that consumed an ethanol liquid diet up to 100 mg/dl (Kakihana and Moore 1976). The shift in daily peak cortisol levels from a mix of morning/afternoon to predominantly morning samples during ethanol self-administration is an interesting effect. Diurnal hormone rhythms can be entrained to food access, and therefore, possibly ethanol access. For example, in food- and water-restricted rats, corticosteroids peaked immediately before food and water access (Krieger 1974). However, in the current study, ethanol access began 4 h after (11:00 AM) the start of the light cycle. The light cycle began at the same time as the blood draw for morning cortisol (7:00 AM). Thus, if diurnal cortisol was entrained to the first meal of the day (and to ethanol access), then peak cortisol would be expected to coincide with the noon sample, not the 7:00 AM sample. However, the data suggest that the increased consistency of cortisol rhythms during ethanol access reflects greater entrainment to the light cycle rather than the feeding schedule.
Although the endogenous rhythm of the adrenal gland became quite consistent during ethanol self-administration, the absolute concentration of cortisol was suppressed, especially at morning and noon. Lower cortisol levels associated with chronic ethanol self-administration is consistent with our previous results in male cynomolgus monkeys (Cuzon Carlson et al. 2011). In social drinkers, self-reported heavy alcohol drinking (men, >3 drinks/day and women, >2 drinks/day) was associated with greater cortisol at awakening and in the evening compared to moderate drinkers (men, ≤3 drinks/day and women, ≤2 drinks/day; Boschloo et al. 2011). From this study, it would appear that the results in humans are at odds with those reported here in the monkey. However, morning cortisol can reflect neural control over diurnal rhythm variation or accelerated production of cortisol that follows awakening and reflects morning activities (Wilhelm et al. 2007). This is an important distinction because different neural mechanisms mediate the awakening response (e.g., brain stem, thalamus; Balkin et al. 2002) versus morning concentrations due to diurnal variation (e.g., suprachiasmatic nucleus; Krieger et al. 1977), and the different neural circuitry could be differentially affected by ethanol.
Although this study did not directly investigate hypothalamic signals for adrenal activation, the function of the pituitary was assessed by measuring ACTH. In short, ethanol self-administration was accompanied by blunted diurnal pituitary rhythms that were not correlated with the quantity of ethanol consumed or housing conditions. These data suggest a very low threshold for ethanol disruption of diurnal pituitary activity. Few studies have examined the threshold for ethanol modulation of circadian processes, and to our knowledge, none in humans. Rats consuming 8 g/kg ethanol/day (estimated maximum BEC, 50–100 mg/dl) had decreased entrainment of activity rhythms to light compared to controls (Rosenwasser et al. 2005). Two weeks of an ethanol liquid diet (35 % of daily calories) in rats flattened the circadian mRNA expression of pro-opiomelanocortin (POMC) and period genes in the arcuate nucleus of the hypothalamus (Chen et al. 2004). Lastly, 6 weeks of exposure to 11.5 g/kg/day, but not 5.5 g/kg/day, ethanol in a liquid diet disrupted sleep patterns in rats (Mukherjee et al. 2008). Compared to the studies above, the current results appear to indicate an even lower threshold for disruption of diurnal rhythms of pituitary ACTH in cynomolgus monkeys. In men, Ekman et al. (1994) reported that 1.0 g/kg, but not 0.5 g/kg, ethanol stimulated POMC activity in the afternoon, although the cleavage products β-endorphin and ACTH were not elevated. In contrast, excessive quantities of alcohol, e.g., during alcoholism, are associated with decreased expression of clock genes in peripheral blood mononuclear cells (Huang et al. 2010). During severe ethanol withdrawal in humans, cortisol was elevated throughout the day, obliterating its diurnal rhythm in one study (Risher-Flowers et al. 1988) or delaying peak cortisol in another study in which the severity of withdrawal was not described (Iranmanesh et al. 1989). We have also reported increased morning cortisol in cynomolgus monkeys during abstinence from ethanol self-administration (Cuzon Carlson et al. 2011).
In summary, the main findings of this study are that social housing in quadrant cages resulted in a state-dependent increase in ACTH across all social ranks, whereas cortisol was transiently increased, then returned to baseline. After ethanol self-administration was established, dominant monkeys were at low risk for chronic heavy consumption. Ethanol self-administration resulted in a dampening of cortisol and ACTH concentrations and ACTH circadian rhythm. Finally, limited daily social housing resulted in a significant decrease in ethanol intake compared to individual housing, but interestingly, BEC was lower only among dominant monkeys. The results suggest that social dominance is a protective feature, particularly in a social setting, against chronic heavy ethanol drinking, even though pituitary–adrenal activity appears to be similarly affected by ethanol self-administration across housing conditions and social ranks.
Supplementary Material
Acknowledgments
This work and preparation of the manuscript was supported by RR000163, AA019431, AA019355, AA10760, AA13541, AA13510, and T32AA007468.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-012-2707-z) contains supplementary material, which is available to authorized users.
Contributor Information
Christa M. Helms, Oregon National Primate Research Center, Oregon Health and Science University, 505 NW 185th Avenue, Beaverton, OR 97006, USA
Megan N. McClintick, Oregon National Primate Research Center, Oregon Health and Science University, 505 NW 185th Avenue, Beaverton, OR 97006, USA
Kathleen A. Grant, Oregon National Primate Research Center, Oregon Health and Science University, 505 NW 185th Avenue, Beaverton, OR 97006, USA Department of Behavioral Neuroscience, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, Belenky G, Herscovitch P. The process of awakening: a PET study of regional brain activity patterns mediating the reestablishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley JD, Heilig M, Suomi SJ, Goldman D. CRH haplotype predicts CSF CRH, HPA axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. PNAS. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bohem C. Hierarchy in the forest: the evolution of egalitarian behavior. Harvard University Press; Cambridge: 2004. [Google Scholar]
- Boschloo L, Vogelzangs N, Licht CMM, Vreeburg SA, Smit JH, van den Brink W, Veltman DJ, de Geus EJC, Beekman ATF, Penninx BWJH. Heavy alcohol use, rather than alcohol dependence, is associated with dysregulation of the hypothalamic–pituitary–adrenal axis and the autonomic nervous system. Drug Alcohol Depend. 2011;116:170–176. doi: 10.1016/j.drugalcdep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O’Buckley TK, Morrow AL. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. J Neurochem. 2010;115:142–152. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Crowley TJ. Substance abuse research in monkey social groups. Prog Clin Biol Res. 1983;131:255–275. [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J Neuroendocrinol. 2008;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke T-K, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G. Effects of the circadian rhythm gene period 1 (Per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry. 2011;168:1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CJ, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Ekman A-C, Vakkuri O, Vuolteenaho O, Leppäluoto J. Delayed pro-opiomelanocortin activation after ethanol intake in man. Alcohol Clin Exp Res. 1994;18:1226–1229. doi: 10.1111/j.1530-0277.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Flack JC, De Waal FBM. Dominance style, social power, and conflict management: a conceptual framework. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. Cambridge University Press; Cambridge: 2004. [Google Scholar]
- Foley R, Gamble C. The ecology of social transitions in human evolution. Phil Trans R Soc B. 2009;364:3267–3279. doi: 10.1098/rstb.2009.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, Albergati A, Montalbetti L, Savoldi F, Polleri A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21:109–112. [PubMed] [Google Scholar]
- Gonzalez CA, Gunnar MR, Levine S. Behavioral and hormonal responses to social disruption and infant stimuli in female rhesus monkeys. Psychoneuroendocrinology. 1981;6:53–64. doi: 10.1016/0306-4530(81)90048-2. [DOI] [PubMed] [Google Scholar]
- Goo GP, Sassenrath EN. Persistent adrenocortical activation in female rhesus monkeys after new breeding group formation. J Med Primatol. 1980;9:325–334. doi: 10.1159/000460162. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopa-mine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011;300:E28–E36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption in nonhuman primates. Alcohol. 1991;34:402–418. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebro-spinal fluid 5-hydroxyindoleaceetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Huang M-C, Ho C-W, Chen C-H, Liu S-C, Chen C-C, Leu S-J. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34:1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Veldhuis JD, Johnson ML, Lizarralde G. 24-hour pulsatile and circadian patterns of cortisol secretion in alcoholic men. J Androl. 1989;10:54–63. doi: 10.1002/j.1939-4640.1989.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976;46:301–305. doi: 10.1007/BF00421118. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. Social separation increases alcohol consumption in rhesus monkeys. Psychopharmacology. 1985;104:367–376. doi: 10.1007/BF00431706. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic–pituitary–adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic–pituitary–adrenal physiology resemble aspects of hyper-cortisolism in humans. Psychoneuroendocrinology. 1999;24:131–142. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology. 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier AH. Daily variation in concentration of plasma corticosteroid in hypophysectomized rats. Endocrinology. 1976;98:1475–1479. doi: 10.1210/endo-98-6-1475. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Coe CL, Lowe EL, Levine S. The physiological response to group formation in adult male squirrel monkeys. Psychoneuroendocrinology. 1979;3:221–229. doi: 10.1016/0306-4530(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fasicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Kazerooni M, Simasko SM. Dose–response study of chronic alcohol induced changes in sleep patterns in rats. Brain Res. 2008;1208:120–127. doi: 10.1016/j.brainres.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffman MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiol Behav. 1974;12:1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Ibba C, Piredda M, Tocco S, Marra C, Purdy RH, Biggio G, Concas A. Failure of γ-hydroxybutyric acid both to increase neuroactive steroid concentrations in adrenalectomized–orchiectomized rats and to induce tolerance to its steroidogenic effect in intact animals. Brain Res. 2004;1012:160–168. doi: 10.1016/j.brainres.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Porcu P, Rogers LS, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic–pituitary–adrenal axis. Pharmacol Biochem Behav. 2006;84:618–627. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Richman JA, Shinsako SA, Rospenda KM, Flaherty JA, Freels S. Workplace harassment/abuse and alcohol-related outcomes: the mediating role of psychological distress. J Stud Alcohol. 2002;63:412–419. doi: 10.15288/jsa.2002.63.412. [DOI] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158:1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher-Flowers D, Adinoff B, Ravitz B, Bone GH, Martin PR, Nutt D, Linnoila M. Circadian rhythms of cortisol during alcohol withdrawal. Adv Alcohol Subst Abus. 1988;7:37–41. doi: 10.1300/J251v07n03_06. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Int. 2005;22:227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic–pituitary–adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Nat Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psych. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking function CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res. 2005;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Thierry B. Unity in diversity: lessons from macaque societies. Evol Anthropol. 2007;16:224–238. [Google Scholar]
- Torres-Farfan C, Valenzuela FJ, Ebensperger R, Méndez N, Campino C, Richter HG, Valenzuela GJ, Serón-Ferré M. Circadian cortisol secretion and circadian adrenal responses to ACTH are maintained in dexamethasone suppressed capuchin monkeys (Cebus paella). Am J Primtol. 2008;70:93–100. doi: 10.1002/ajp.20461. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Welker C, Schäfer-Witt C, Voigt K. Social position and personality in Macaca fascicularis. Folia Primatol. 1992;58:112–117. [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Social status as a determinant of alcohol effects on aggressive behavior in squirrel monkeys (Saimiri sciureus). Psychopharmacology. 1985;85:167–172. doi: 10.1007/BF00428408. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.