Abstract
Fecal specimens collected from 121 laboratory mice, 30 striped field mice (Apodemus agrarius), 70 yellow-necked mice (Apodemus flavicollis), and 3 bank voles (Myodes glareolus) were tested in sample pools for the presence of murine noroviruses (MNV). Ten of 41 laboratory mice and 2 of 3 striped field mice pooled samples were positive for MNV. All laboratory mouse MNVs were closely related to previously described MNVs. The complete ORF2 (VP1) of both striped field mouse MNVs identified in this study was 1623 nt (541 aa) long and differed at 12% nt (8% aa) positions from each other, at 22–24% nt (15–18% aa) positions from the laboratory mouse MNVs and at 20–22% nt (13–14% aa) positions from the recently described wood mouse (Apodemus sylvaticus) MNVs. This study provides further evidence for the circulation of novel, genetically diverse MNVs in wild mice.
Keywords: mouse, calicivirus, murine norovirus
Introduction
The Norovirus genus of the Caliciviridae family contains both human and animal pathogens and is further subdivided into five genogroups (GI-V), from which GV represents the murine NoVs (MNV). The ~7.5 kb positive sense, single stranded norovirus RNA genome is organized into three major open reading frames (ORF): ORF1 encodes a large polyprotein that is cleaved to individual non-structural proteins; ORF2 encodes the viral capsid protein (VP1); and ORF3 encodes a minor structural protein (VP2). In the MNV genome, an additional ORF4 has also been identified (Thackray et al., 2007). Since the discovery of the first MNV in 2003 (Karst et al., 2003) it became evident that MNV infection is the most prevalent viral infection in laboratory mice (Hsu et al., 2005; Kim et al., 2011; Perdue et al., 2007). MNV infection in immunocompetent mice is subclinical but in impaired mice such as STAT1−/−, MNV establishes systemic infection that leads to high mortality (Karst et al., 2003). Due to its ability to grow in tissue culture, MNV became the most widely used surrogate model for human norovirus research (Wobus et al., 2006). All MNV strains isolated from laboratory mice worldwide belong to a single genotype with ~13% nt and ~7% aa diversity in the ORF2 (VP1) region indicating a very limited genetic diversity compared to the genetic diversity observed among human noroviruses (Thackray et al., 2007). Recent studies, either by serology or molecular detection suggest that MNVs are also circulating in wild rodents (Parker et al., 2009; Smith et al., 2011). However, the extent of their genetic diversity and biological similarities to MNVs is not well established. In this study, wild and laboratory mice were screened for MNVs, and two novel MNVs from striped field mice (Apodemus agrarius) were identified and characterized.
Materials and Methods
Sample collection
Thirty striped field mice (Apodemus agrarius), 70 yellow-necked mice (Apodemus flavicollis), and 3 bank voles (Myodes glareolus) were live trapped between July and October of 2010 in South-West Hungary. Two pellets collected from each of 10 individual animals of the same Apodemus species were mixed together and made into 20% suspensions in RPMI-1640, yielding 3 striped field mice and 7 yellow-necked mice sample pools. The bank vole samples were processed individually. In addition 121 stool samples were collected from sentinel and research mice between May and July of 2011 at the CCHMC animal facility, housing over 40,000 mice. Two to five pellets were collected from each animal, made into 20% (w/v) suspensions and stored at −80°C. For MNV testing 3 individual samples were pooled together yielding 40 pooled and one individual laboratory mouse samples.
RNA extraction and cDNA synthesis
Viral RNA was extracted with the QIAamp viral RNA mini kit (Qiagen Hilden, Germany) according to the manufacturer’s instructions. Wild mice RNA samples were converted to cDNA using an oligo-dT primer (Reverse transcription system; Promega, Madison, WI), ethanol precipitated, vacuum dried and shipped to Cincinnati where they were reconstituted in 20 µl molecular biology grade water (Thermo Fisher Scientific Inc., Waltham, MA). RNA and cDNA samples were stored at −80 °C.
Molecular detection of murine noroviruses
Detection of caliciviruses in the wild mice samples were attempted with generic calicivirus primers (P289/P290) targeting nucleotide sequences encoding conserved amino acid motifs in the RNA dependent RNA polymerase (RdRp) region of ORF1 (Jiang et al., 1999) and with a MNV specific primer pair targeting a 396 bp region at the 5’ end of MNV ORF2 (VP1) (Kim et al., 2011). Two µl of cDNA was used in 25 µl PCR reactions using the GoTaq Green Master Mix (Promega, Madison, WI) according to the manufacturer’s protocol. Laboratory mouse RNA samples were tested only with the MNV specific primers in one step RT-PCR reactions (AccessQuick RT-PCR system; Promega, Madison, WI) using 2 µl RNA as template. Thermocycling conditions included reverse transcription at 50 °C for 50 min (for the RT-PCR) followed by an initial incubation at 94 °C for 2 min and 35 cycles of denaturation at 94 °C for 40 s, annealing at 50 °C for 40 s, and extension at 72 °C for 1 min. PCR products were analyzed on 2% agarose gels in the presence of ethidium bromide. The complete ORF2 sequences of the two Apodemus strains identified in this study were amplified with the MNV specific forward primer and a reverse primer (TGAGATCCTTCTGGGCCTGAAYTT) designed based on alignments of MNV sequences available in the GenBank.
Sequencing and phylogenetic analysis
PCR products were cloned into pGEM-T vector (Promega, Madison, WI) and sequenced using M13 forward and reverse primers on an ABI PRISM® 3730 DNA Analyzer (Applied Biosystems Inc, Foster city, CA). Each sample was sequenced in both directions from two independent clones. The complete ORF2 products were sequenced by primer walking. BLAST analyses were run against NCBI databases. Multiple sequence alignments of nucleotide and amino acid sequences were created using the Omiga v2.0 software (Oxford Molecular Ltd, Oxford, UK). Dendrograms were constructed by the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering method of the Molecular Evolutionary Genetics Analysis (MEGA version 3.1) software with Jukes-Cantor and Poisson correction distance calculations for nucleotide and amino acid sequence alignments, respectively (Kumar et al., 2004). The confidence values of the internal nodes were obtained by performing 1000 bootstrap analyses. Accession numbers of reference strains included in the analyses are listed in the figures (Fig 1 and 2). Nucleotide sequences obtained in this study were deposited to the GenBank database under the following accession numbers: JQ408727- JQ408738.
Figure 1.
Phylogenetic analysis of 356 nt partial ORF2 sequences of laboratory and wild mouse MNV isolates. The dendrogram was constructed by the UPGMA clustering method of MEGA version 3.1 with Jukes-Cantor distance calculations and 1000 bootstrap analyses. Strains described in this study are in bold.
Figure 2.
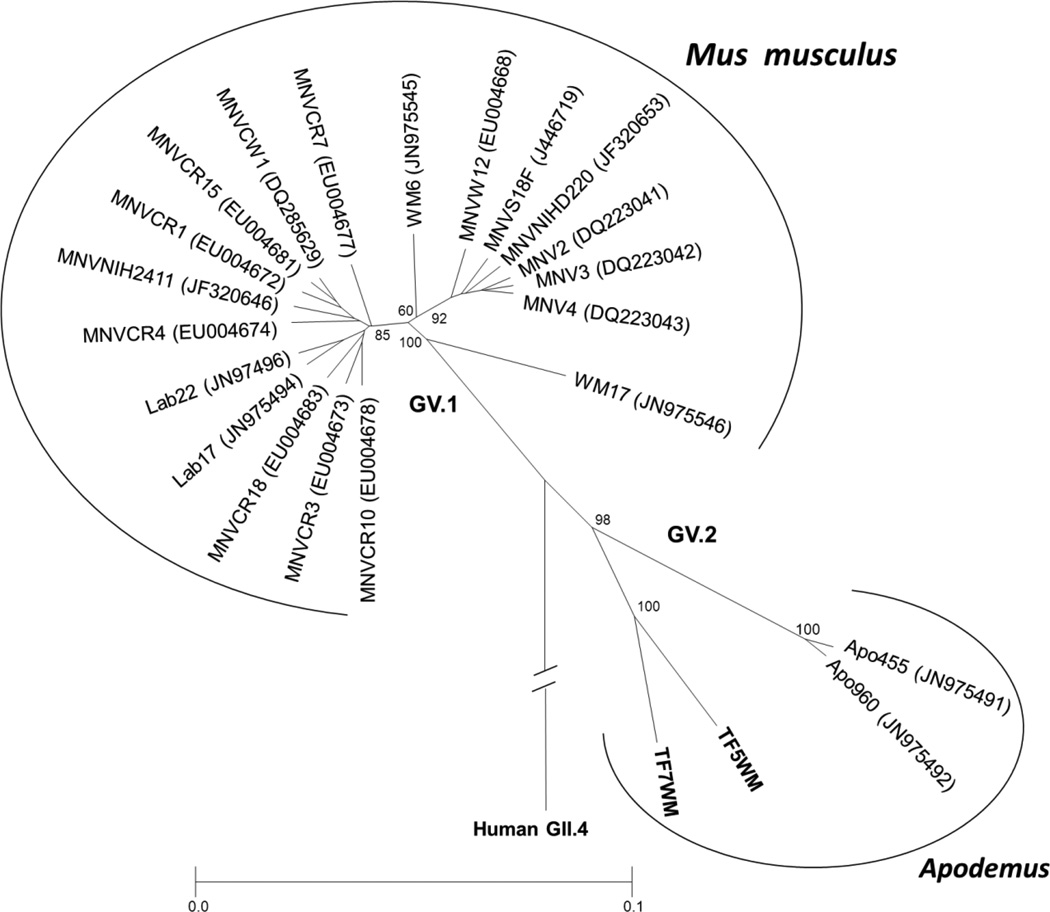
Phylogenetic analysis of complete VP1 sequences (541 aa) of laboratory and wild mouse MNVs and including Hu/NoV/Farmington Hills/2002/USA (AY502023) as out-group. The dendrogram was constructed by the UPGMA clustering method of MEGA version 3.1 with Poisson correction distance calculations and 1000 bootstrap analyses. Strains described in this study are in bold. WM6 and WM17 represent recently described isolates from house mouse (Smith et al., 2011).
Results
None of the wild mice samples yielded calicivirus specific amplicon with the generic P289/P290 primers. When tested with the MNV specific primers, 2 of the 3 striped field mice (Apodemus agrarius) samples and 10 of the 41 laboratory mouse samples yielded RT-PCR amplicons, while the yellow-necked mouse (Apodemus flavicollis) and bank vole (Myodes glareolus) samples remained negative. Phylogenetic analysis of the 356 nt (without the primers) long amplicons revealed that all laboratory mouse MNVs detected in this study grouped closely with previously described MNVs (Fig 1). The diversity in this short region among the 10 MNV sequences was 0–4% nt differences which was similar to the diversity (2–5%) observed among the reference laboratory mouse MNVs used in the analysis. The two striped field mouse MNVs (TF5WM and TF7WM) grouped distantly from laboratory mouse MNVs and rooted together with the recently described wood mouse (Apodemus sylvaticus) MNVs (Smith et al., 2011) (Fig 1). Analysis of the 1623 nt (541 aa) long complete ORF2 (VP1) of TF5WM and TF7WM revealed 12% nt (8% aa) difference between each other, 22–24% nt (15–18% aa) difference with the laboratory mouse MNVs and 20–22% nt (13–14% aa) difference with wood mouse MNVs (Fig 2 and Table 1). Within VP1, the highest degree of divergence was observed in the hypervariable P2 domain (Table 1). Both the TF5WT and TF7WT ORF2 contained a 5’ double coding region with a 639 nt long ORF4.
Table 1.
Nucleotide/amino acid differences (%) between TF5WM and TF7WM and previously described wild and laboratory mouse MNVs.
| Host |
Apodemus agrarius |
Apodemus sylvaticus |
Mus musculus (wild) |
Mus musculus (Laboratory) |
Within MNVs |
|||
|---|---|---|---|---|---|---|---|---|
| Regiona | Strain | TF7WM | Apo455 | Apo90 | WM17 | WM16 | MNVs | |
| ORF2/VP1 | TF5WM | 12/8 | 21/13 | 22/14 | 24/18 | 25/20 | 22-24/16-18 | 4-14/2-7 |
| 5056-6678 | TF7WM | - | 20/13 | 21/14 | 24/18 | 24/18 | 22-23/15-17 | |
| N-terminus | TF5WM | 1/0 | 9/11 | 14/11 | 14/18 | 14/18 | 12-16/16-22 | 0-7/0-9 |
| 5056-5199 | TF7WM | - | 10/11 | 14/11 | 14/18 | 13/18 | 13-15/16-22 | |
| S-domain | TF5WM | 5/3 | 12/3 | 12/6 | 14/4 | 16/5 | 13-15/4-5 | 2-6/0-2 |
| 5200-5709 | TF7WM | - | 10/2 | 12/4 | 14/2 | 15/3 | 13-15/2-3 | |
| P1 domain | TF5WM | 3/6 | 21/11 | 22/12 | 22/17 | 25/17 | 20-23/12-16 | 6-18/2-10 |
| 5710-5886;6301-6678 | TF7WM | - | 21/9 | 22/9 | 24/15 | 23/15 | 19-23/10-14 | |
| P2 domain | TF5WM | 22/17 | 36/29 | 35/29 | 42/38 | 40/41 | 37-41/35-39 | 6-23/3-13 |
| 5887-6300 | TF7WM | - | 35/32 | 34/32 | 40/41 | 40/42 | 37-40/36-40 | |
| N-term +S-domain | TF5WM | 4/2 | 11/5 | 13/7 | 14/7 | 15/8 | 13-15/7-8 | 2-6/0-3 |
| 5056-5709 | TF7WM | - | 10/4 | 12/6 | 14/6 | 15/7 | 13-15/5-7 | |
| ORF4/VF1 | TF5WM | 5/10 | 14/29 | 15/32 | 14/34 | 16/35 | 13-15/31-33 | 2-6/3-14 |
| 5069-5707 | TF7WM | - | 13/28 | 15/32 | 14/34 | 15/34 | 13-15/31-35 | |
Nucleotide positions numbered as in MNV-CW1 (DQ285629).
Discussion
Until very recently MNVs were only described in laboratory mice and with limited genetic diversity compared to other noroviruses, either indicating a recent introduction of MNVs to mouse (Mus musculus) and/or limited evolutionary pressure on MNVs compared to noroviruses of other hosts.
In 2010, we initiated a study for the molecular detection of caliciviruses in stool samples of wild and laboratory rodents. Based on literature search and sequence alignments, two primer pairs, a broadly reactive and an MNV specific, were selected for calicivirus detection. Primer pair P289/P290 targets nucleotide sequences encoding conserved amino acid motifs in the calicivirus RdRps and it was successfully used in our previous studies for the molecular detection of noro-, sapo-, vesi- and recoviruses (Farkas et al., 2010; Farkas et al., 2000; Farkas et al., 2008; Farkas et al., 2004). Tests with a few MNV positive samples indicated that P289/P290 is also able to detect MNVs. Despite its broader detection range, none of the wild mouse cDNA samples tested positive with P289/P290, while two of the striped field mice (Apodemus agrarius) samples yielded MNV specific amplicons with the MNV specific primers. Since the two primer sets target different regions of the MNV genome, for comparative reasons all of the laboratory mouse samples were tested only with the MNV specific primers. Ten of the 41 pooled samples collected from laboratory mice yielded MNV sequences. Since BLAST search and phylogenetic analysis indicated that all of the MNV sequences obtained from the CCHMC mouse colony were genetically closely related to each other and to previously described MNVs (Fig 1), individual samples of the positive pools were not retested. Wild mouse individual samples were not available. Phylogenetic analyses of the 356 nt partial ORF2 sequences indicated that the two striped field mice (Apodemus agrarius) MNVs, TF5WM and TF7WM are more closely related to MNVs recently described in wood mouse (Apodemus sylvaticus) in the UK (Smith et al., 2011), than to laboratory mouse MNVs (Fig 1). However, while the difference among the laboratory mouse MNVs was less than 5 % nt positions, TF5WM/TF7WM and Apo455/Apo960 differed from each other in 8–11% nt positions indicating the existence of higher genetic diversity among MNVs in wild mice than described in laboratory mice. Since norovirus classification is based on the analyses of complete capsid (VP1) protein sequences full length ORF2 (VP1) sequences were obtained for both TF5WM and TF7WM. Analysis of the 1623 nt (541 aa) long ORF2 (VP1) sequences of TF5WM/TF7WM revealed 22–24% nt (15–18% aa) differences with the laboratory mouse MNV strains. More interestingly, the differences between TF5WM/TF7WM and Apo455/Apo960 (20–22% nt and 13–14% aa) ranged at a very similar level. For comparison, the diversity among the laboratory mouse MNVs in the full length ORF2 (VP1) was 4–13% nt (2–7% aa) (Table 1). According to a large scale analysis of norovirus VP1 sequences, norovirus strains within the same genetic cluster (genoptype) differ at 0–14.1 % aa positions while the difference among the different genotypes of the same genetic group (genogroup) is 14.3–43.8 % and among the various genogroups is 44.9–61.4 % (Zheng et al., 2006). Based on these, the Apodemus MNVs are clearly separated from the laboratory mouse MNVs at the genetic cluster/type level (15–18%), while the difference among the UK and Hungarian Apodemus strains (13–14%) is slightly below the cut-off value proposed for genotype distinction. Pairwise distance calculations including over 70 GI and GII human NoVs corroborated this observation (data not shown). Based on these observations, we propose the classification of Mus and Apodemus MNVs into two genetic clusters, GV.1 and GV.2, respectively (Fig. 2). To clearly establish whether genotype level variation exist among the Apodemus MNVs more sequence data is needed.
Both the TF5WT and TF7WT ORF2 sequences contained a second reading frame (ORF4) of the same length (639 nt) as in laboratory mouse MNVs which is 21 nt longer than the Apo455 and Apo960 ORF4s (618 nt). Recently, McFadden et al., demonstrated that ORF4 is efficiently translated during MNV infection and ORF4-derived protein, VF1 plays a role in promoting virus replication by interfering with interferon mediated host response pathways and apoptosis (McFadden et al., 2011). The ORF4/VF1 coding region almost completely overlaps with the N-terminal and S-domain (N/S) of the ORF2/VP1 with a +1 frame shift between the 2 ORFs. Interestingly, in the ORF2/N/S the differences among the various MNVs were higher at the nt than at the aa level (4–15% nt vs. 2–8% aa), while in the ORF4/VF1 the nt differences were significantly lower than the aa differences (5–16% nt vs. 10–35% aa) (Table 1). Multiple sequence alignments revealed that most of the nt substitutions (~75%) occurred at positions corresponding to the 3rd codon position in ORF2/N/S and the 2nd codon position in ORF4/VF1. This evolutionary conservation of the N/S domain of a structural protein (VP1) vs. a protein (VF1) with role in virulence remains to be explained. The larger number of non-synonymous substitutions in ORF4 may also suggest that the active, functional domain(s) of VF1 might be limited to a few conserved regions.
In summary, here we provided further evidence for the existence of genetically diverse MNVs in wild mice. Evaluation of more diverse host species is necessary.
ACKNOWLEDGEMENTS
We thank Susan Austin for helping with the sample collection. Grants from the National Institutes of Health (P01HD13021) and the Infectious Disease Scholar Fund of CCHMC to T.F., and the Hungarian National Research Grant OTKA (K81258) to L.E. were used to support this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The GenBank/EMBL/DDBJ accession numbers for the nucleotide sequences determined in this study are: JQ408727- JQ408738
REFERENCES
- Farkas T, Cross RW, Hargitt E, 3rd, Lerche NW, Morrow AL, Sestak K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol. 2010;84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T, Jiang X, Guerrero ML, Zhong W, Wilton N, Berke T, Matson DO, Pickering LK, Ruiz-Palacios G. Prevalence and genetic diversity of human caliciviruses (HuCVs) in Mexican children. J Med Virol. 2000;62:217–223. [PubMed] [Google Scholar]
- Farkas T, Sestak K, Wei C, Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T, Zhong WM, Jing Y, Huang PW, Espinosa SM, Martinez N, Morrow AL, Ruiz-Palacios GM, Pickering LK, X J. Genetic diversity among sapoviruses. Arch Virol. 2004;149(7):1309–1323. doi: 10.1007/s00705-004-0296-9. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol. 2005;12:1145–1151. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, Virgin HWt. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Kim JR, Seok SH, Kim DJ, Baek MW, Na YR, Han JH, Kim TH, Park JH, Turner PV, Chung DH, Kang BC. Prevalence of murine norovirus infection in Korean laboratory animal facilities. J Vet Med Sci. 2011;73:687–691. doi: 10.1292/jvms.10-0226. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- McFadden N, Bailey D, Carrara G, Benson A, Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P, Macdonald A, Goodfellow I. Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4. PLoS Pathog. 2011;7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Malone S, Bunte RM, Smith AL. Infectious diseases in wild mice (Mus musculus) collected on and around the University of Pennsylvania (Philadelphia) Campus. Comp Med. 2009;59:424–430. [PMC free article] [PubMed] [Google Scholar]
- Perdue KA, Green KY, Copeland M, Barron E, Mandel M, Faucette LJ, Williams EM, Sosnovtsev SV, Elkins WR, Ward JM. Naturally occurring murine norovirus infection in a large research institution. J Am Assoc Lab Anim Sci. 2007;46:39–45. [PubMed] [Google Scholar]
- Smith D, McFadden N, Blundell RJ, Meredith A, Simmonds P. Diversity of murine norovirus in wild rodent populations: species-specific associations suggest an ancient divergence. J Gen Virol. 2011 doi: 10.1099/vir.0.036392-0. [DOI] [PubMed] [Google Scholar]
- Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HWt. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol. 2007;81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]