Abstract
Background and Purpose
Anorectal dysmotility is common in advanced Parkinson's disease (PD), but there have been few evaluations in newly diagnosed PD patients.
Methods
We conducted anorectal manometric evaluations in 19 newly diagnosed, drug-naïve, early-stage PD patients. All of the PD patients were questioned regarding the presence of anorectal symptoms.
Results
Anorectal manometry was abnormal in 12 of the 19 patients. These abnormalities were more common in patients with more severe anorectal symptoms, as measured using a self-reported scale. However, more than 40% of patients with no or minimal symptoms also exhibited manometric abnormalities.
Conclusions
These results suggest that anorectal dysmotility manifests in many early-stage PD patients, which this represent evidence for the involvement of neuronal structures in such nonmotor manifestations in PD.
Keywords: Parkinson's disease, anorectal dysfunction, manometry
Introduction
Gastrointestinal (GI) dysmotility is common in Parkinson's disease (PD), manifesting mainly as dysphagia, impaired gastric emptying, constipation, and defecatory dysfunction. Constipation may precede the motor symptoms of PD by years.1 Recent neuropathological studies have clearly demonstrated the deposition of alpha-synuclein and dopaminergic neurons defects in the enteric nervous plexuses during the preclinical or early stages of this disease.2 It has also been suggested that these GI symptoms precede the typical motor symptoms of PD, and may therefore also be present in the preclinical stage of PD.3
Over the past few decades, numerous studies have revealed several anorectal motility dysfunctions in PD patients, including low basal and impaired squeezing pressures, prominent phasic fluctuations during squeeze, and a hypercontractile response to unchanged resting anal pressure, also known as the rectosphincteric reflex.4 In addition, altered voluntary anal contraction (squeezing) and paradoxical anal contraction during straining (anismus) have been established in PD patients.5-7 However, most previous analyses were performed in advanced PD patients and the patients enrolled in those studies were not free from drug effects. There have been only few evaluations in newly diagnosed PD patients.8
In the study described herein we evaluated anorectal manometric dysfunctions in newly diagnosed, early-stage PD patients and investigated the association with clinical symptoms.
Methods
Patients
Consecutive newly diagnosed PD patients were enrolled from April 2008 to May 2009 at the Movement Disorders Clinic, Seoul St. Mary's Hospital. A clinical diagnosis of PD was made according to the UK Brain Bank Criteria.9 At the time of the study, none of the patients had ever taken medication for Parkinsonism. The evaluation included a detailed medical and drug history, physical and neurological examinations, a brief neuropsychiatric assessment, and structural brain magnetic resonance imaging (MRI). Only patients with early-stage PD [i.e., ≤stage 2 according to the modified Hoehn and Yahr (H&Y) scale] and disease duration (i.e., manifestations of motor symptoms) not exceeding 2 years were included in this study. Excluded from the study were 1) patients with neurological abnormalities related to atypical PD or secondary Parkinsonism, 2) patients with diabetic neuropathy, and 3) patients taking medications known to influence anorectal motility, such as levodopa, dopamine agonists, anticholinergics, or GI dysmotility remedies.
This study was approved by the local ethics committee, and all patients gave their informed consent to participate.
Clinical assessments
All patients were subsequently evaluated using the Unified Parkinson's Disease Rating Scale (UPDRS), the H&Y scale, and the Korean version of the Mini-Mental Status Examination. In addition, the patients were asked to complete a self-administered scale to evaluate the presence and frequency or severity of large-bowel symptoms during the last 3 months. The self-administered questionnaire consisted of the following seven items: 1) low abdominal pain or discomfort, 2) constipation, 3) either excessive strain during defecation or difficulty with expulsion, 4) involuntary loss of stools (i.e., fecal incontinence), 5) incomplete bowel emptying (tenesmus) after having been to the toilet, 6) loose stool or diarrhea, and 7) difficulty relaxing the anal sphincter. Each symptom was scored with respect to either frequency (1=never or rare, 2=often, 3=frequent, and 4=always) or severity (1=no symptoms; 2=minimum or mild symptoms present but causing little distress or disturbance; 3=moderate symptoms causing some distress or disturbance; and 4=severe symptoms representing a major source of distress or disturbance).
Finally, the patients were questioned about the onset of each described symptom that occurred prior to the onset of the motor symptoms of PD.
Anorectal manometry testing and analysis
The measurements were performed as described previously.10 All subjects were investigated in the left lateral position. The investigation was performed with a multilumen manometric catheter (outer diameter: 4 mm), with distal side openings spaced 5 mm apart. Three lumens were continuously perfused (0.5 mL/min) with bubble-free distilled water using a low-compliance pneumohydraulic capillary-infusion system (Arndorfer, Milwaukee, WI, USA) and were connected via an external transducer (P23 Db, Statham Instruments, Oxnard, CA, USA) to a multichannel polygraph (R511 A, Beckman, Irvine, CA, USA).
Both resting and squeeze anal sphincter pressures were obtained as follows: after a 3-min run-in period, the highest sphincter pressure in the anal canal at rest (normal=54-74 mm Hg) and during three squeezes. Patients were asked to squeeze the anal sphincter as strongly and for as long as possible; the longest time interval (in seconds) between the onset of increase in anal sphincter pressure and the return of the pressure curve to baseline was recorded and defined as the duration of maximum squeeze (normal=20-28 s). The rectal pressure and anal canal pressure during straining were also recorded by asking patients to bear down as if they were seated on the commode and attempting to open their bowels. Normal anal relaxation on strains was defined as >20% relaxation from baseline; otherwise, relaxation was recorded as absent or with paradoxical contraction.
The cough reflex was elicited by asking the patient to blow against a fixed pressure level into a manometer, to blow up a balloon, or simply to cough voluntarily. This test was repeated three times in each patient, with a 30-s rest interval between each attempt. For each maneuver the difference between the baseline pressure and the highest intrarectal pressure, and the difference between the baseline and the highest intra-anal pressure were measured. Among the three attempts, the profile showing the highest increase in these pressures was selected for analysis.
The rectal sensation threshold was tested using a compliant rectal balloon inflated with air at a continuous rate of 100 mL/min. Thresholds (in milliliters) for first sensation, urge to defecate, and maximum tolerated volume (pain) were recorded.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 (SPSS, Chicago, IL, USA). To determine whether bowel symptoms worsened with manometric abnormalities, the symptoms were first analyzed as a categorical variable according to the presence or absence of each symptom, and then treated with a continuous severity score. In addition, patients were categorized into the following two subgroups: 1) with no or mild problems (a score for each item of ≤1), and 2) with moderate-to-severe problems (a score for each item of ≥2). Finally, demographic or parkinsonian motor symptoms were compared. Statistical analyses were performed using independent-samples t-test or Pearson's chi-square test, and the statistical significance level was established at p<0.05. Data are presented as mean±SD values.
Results
Demographic data
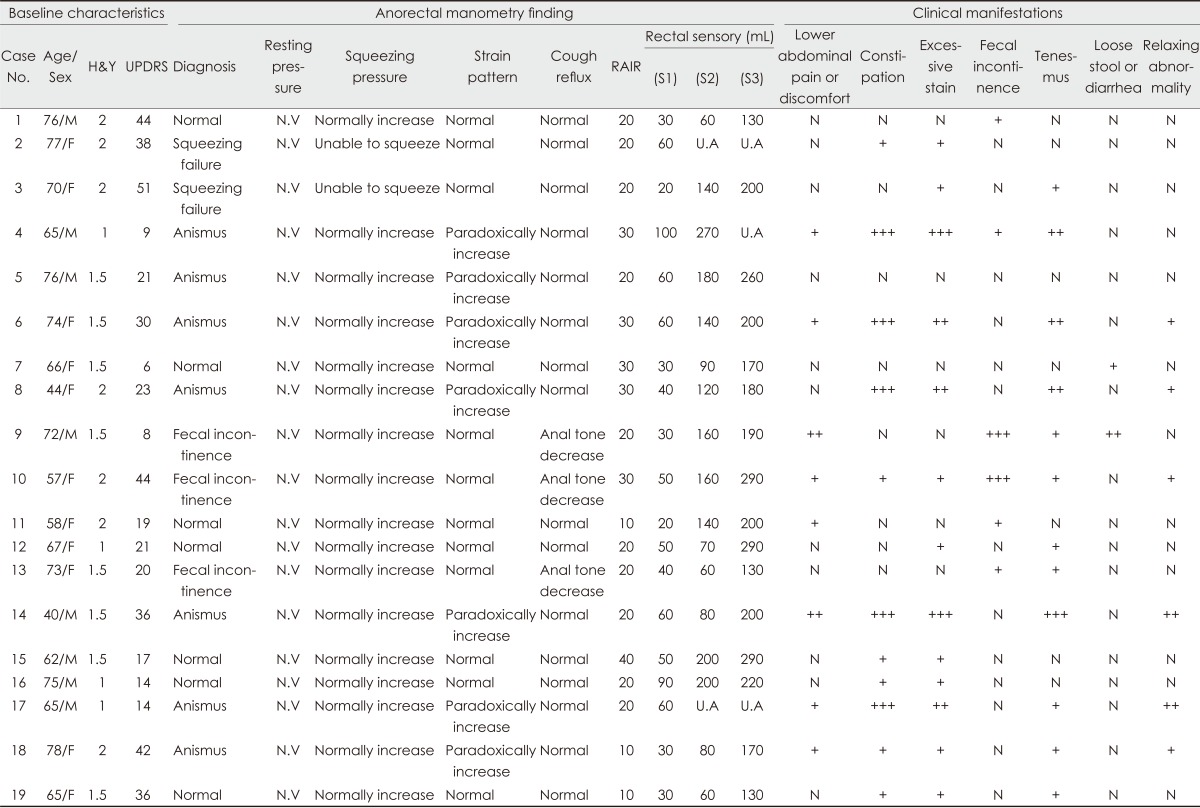
Eight of the 19 patients who were enrolled were male. The age at examination was 66.3±10.6 years, and the duration of parkinsonian motor symptoms was 1.0±0.2 years. The severity of parkinsonian motor symptoms was as follows: the H&Y stage was 1.6±0.4 and the total UPDRS score was 25.9±13.8. Four, eight, and seven patients were scored as H&Y stages 1, 1.5, and 2, respectively. The Korean version of the Mini-Mental Status Examination score was 27.2±2.0 (range, 24-30).
The frequencies of anorectal symptoms were as follows (Table 1): lower abdominal discomfort or pain, 42.1%; constipation, 57.9%; excessive straining for defecation, 68.4%; fecal incontinence, 31.6%; tenesmus, 63.2%; loose stool or diarrhea, 10.5%; and failure to relax the anal sphincter, 31.6%. Only one patient scored 0 on the self-administered scale. Eleven patients (57.9%) responded that the anorectal symptoms developed before the onset of parkinsonian motor symptoms and worsened after the onset, and two patients reported that the onset of the two types of symptoms (i.e., anorectal and neurological) occurred approximately simultaneously, with only months between them.
Table 1.
Clinical and anorectal manometric findings of each patient
+: often or mild, ++: frequent or moderate, +++: always or severe.
F: female, H&Y: Hoehn & Yahr stage, M: male, N: never or none, N.V: Normal value, RAIR: rectoanal inhibitory reflex, S1: first sensation, S2: continuous rectal sensation, S3: sensation of desire to defecate, U.A: unassessable (this means the patient does not feel the sense of filling with maximal testable volume [300 mL]), UPDRS: Unified Parkinson's Disease Rating Scale.
Results of anorectal manometry
The findings of the anorectal manometry test were abnormal in 12 of the 19 patients (Table 1). Manometric recordings during squeezing attempts showed inability to contract the anus in two patients (i.e., lack of anal contraction and short duration of lasting). Straining attempts revealed lack of anal inhibition and paradoxical contraction (anismus) in 7 patients; the remaining 12 patients exhibited normal anal inhibition and good relaxation of the external anal sphincter. In the cough-reflex test, 3 patients exhibited abnormally decreased anal tone with voluntary coughing, suggesting fecal incontinence; the other 16 patients exhibited a normal cough reflex (i.e., the anal tone increased reactively with voluntary coughing). Rectal sensory dysfunction was also observed in two PD patients with severe constipation. An increase in the threshold for first sensation was observed in these patients, and the threshold of continuous rectal sensation and sensation of desire to defecate were not detectable with the maximal tested volume (i.e., 300 mL).
Correlation between clinical and manometric findings
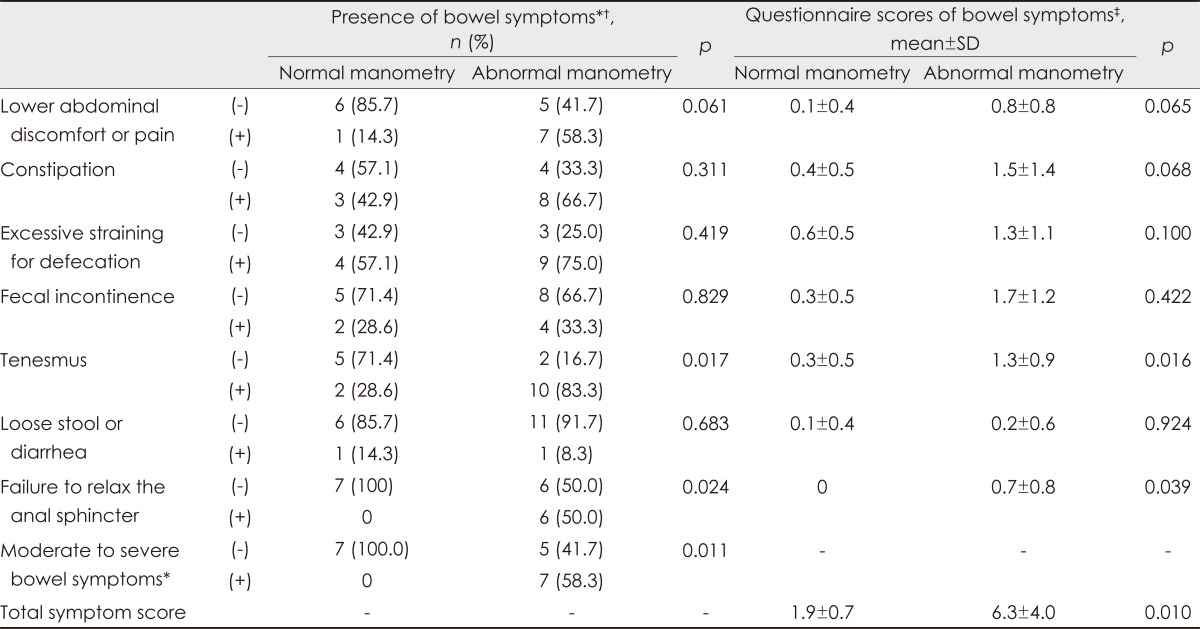
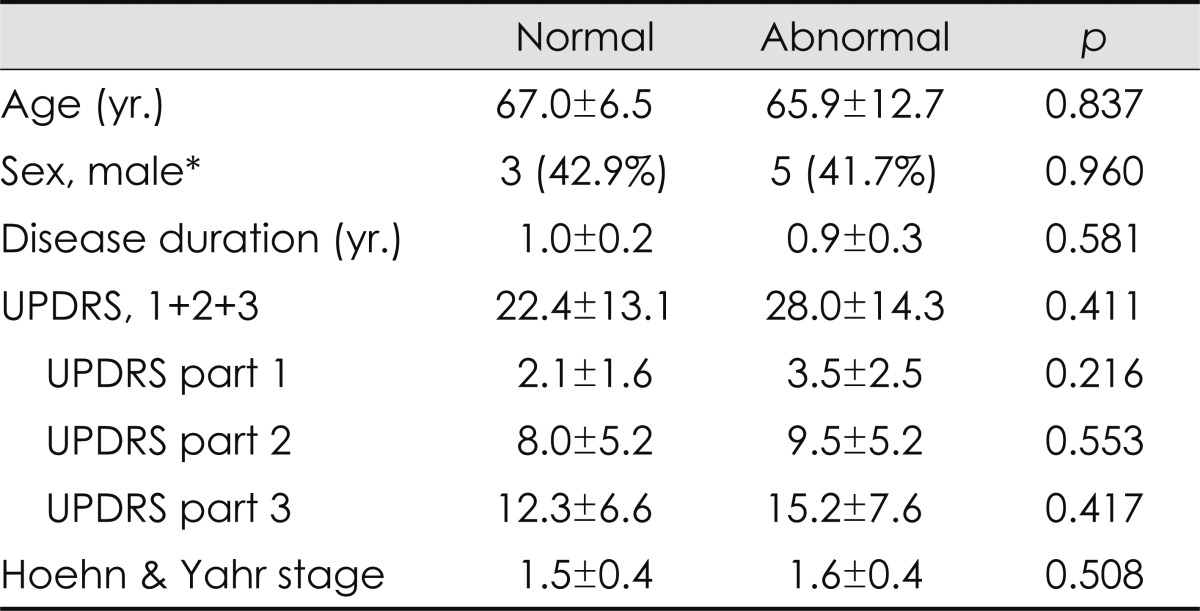
These abnormalities were more common in patients with more severe anorectal symptoms, as measured using the self-reported autonomic scale; however, more than 40% of the patients with no or minimal symptoms also exhibited manometric abnormalities (Table 2). With regard to each item in the questionnaire, the severities of constipation, straining for defecation, and failure to relax the anal sphincter were strongly correlated with the pattern of anismus in anorectal manometry (anismus group vs. normal group: 5.1±2.7 vs. 1.0±1.0; p=0.003), whereas fecal incontinence and fecal urgency symptoms were associated with cough-reflex abnormalities (fecal incontinence group vs. normal group: 2.3±1.2 vs. 0.3±0.5; p=0.003). These abnormalities were marginally associated with UPDRS; however, no correlation was found between anorectal dysmotility and the patients' age or illness duration (Table 3).
Table 2.
Anorectal manometry findings in patients according to the bowel symptoms
*Indicates group with (the score for each item ≥ 2), †Analyses were performed with the chi-square test, ‡Analysis were performed with the independent sample t-test.
Table 3.
Association among clinical parameters and manometric diagnosis, mean+S.D., n (%)
Analyses were performed with independent sample t-test.
*Analyses was performed with chi-square test.
UPDRS: Unified Parkinson's Disease Rating Scale.
Discussion
Defecatory dysfunction in PD is a consequence of uncoordinated action of the muscles involved in defecation. Relaxation of the puborectalis muscle allows opening of the anorectal angle and perineal descent, facilitating fecal expulsion. During defecation, failure of relaxation in both the external anal sphincter and the puborectalis muscles can produce a functional outlet obstruction. This has been considered as a focal dystonic phenomenon.11,12 The functional outlet obstruction may cause both excessive straining and a sense of incomplete emptying; it may also make defecation painful.
The results of this study suggest that anorectal manometric dysfunctions are present in newly diagnosed, nonmedicated PD patients. The most frequent manometric abnormalities found in our patients were either paradoxical anal contraction or insufficient anal relaxation during straining. In patients with PD, the "paradoxical" hypercontractile response (anismus) or insufficient relaxation of the external anal sphincter during straining can be one of the main causes of functional outlet-type constipation.13 Furthermore, it may result in the inability to relax the pelvic floor and therefore prevent straightening of the rectoanal angle, as demonstrated previously.14 A minority of our patients exhibited an abnormal cough reflex (i.e., decreased sphincter tone with voluntary cough). Since the anal sphincter tone reactively increases with voluntary coughing under normal conditions, the presence of decreased sphincter tone with voluntary coughing is suggestive of fecal incontinence. This can result either from supraspinal dysregulation or involvement of the sacral parasympathetic and Onuf's nucleus neurons; it may also occur as a result of a more direct loss of neurons in the enteric nervous system, eliciting defective coordination of the anorectal musculature.15-17
Most of the early-stage PD patients in our study exhibited altered bowel habits and defecatory frequency. The most bothersome complaints were reduced bowel frequency, difficulty in defecation, and occasional incontinence. The severities of constipation, straining for defecation, and failure to relax the anal sphincter were strongly correlated with the pattern of anismus in anorectal manometry, whereas the symptoms of fecal incontinence were associated with cough-reflex abnormalities. Therefore, we consider that the use of manometric analysis in PD can be useful for the clinical diagnosis of anorectal symptoms.
In addition, anorectal manometric abnormalities were more common in patients with more severe anorectal symptoms, as measured by a self-reported autonomic scale; however, more than 40% of patients with no or minimal symptoms also had manometric abnormalities. This suggests that anorectal dysmotility presents evidence of the involvement of neuronal structures in these nonmotor manifestations in PD.
While anorectal manometric evaluation can be useful for the quantitative analysis of anorectal dysfunction, the associated limitations should be noted. First, in addition to anorectal dysmotility due to PD itself, other structural abnormalities such as obstetrical injuries in female patients should be considered. Second, we attempted to reduce the effects of drugs by enrolling patients with no history of taking PD drugs; however, it is possible that some unidentified drugs affected the condition of the patients. Finally, our study did not have a case-controlled design. Many elderly patients over the age of 60 years can have bowel problems in the absence of PD. Therefore, larger case numbers and comparison with controls are required to confirm the present findings.
In conclusion, most of the included patients with early-stage PD exhibited anorectal dysfunctions before the clinical motor manifestations of PD. The use of manometry allows quantitative analysis of anorectal dysfunctions and provides objective evidence for involvement of the enteric nervous system in PD. The screening of anorectal function in patients with early-stage PD can facilitate prompt recognition and effective therapeutic interventions for significant defecatory dysfunctions.
Footnotes
The authors have no financial conflicts of interest.
Part of this work was published as a special issue in the Journal of the Neurological Sciences. However, data was fully presented only in this paper.
References
- 1.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashraf W, Pfeiffer RF, Quigley EM. Anorectal manometry in the assessment of anorectal function in Parkinson's disease: a comparison with chronic idiopathic constipation. Mov Disord. 1994;9:655–663. doi: 10.1002/mds.870090612. [DOI] [PubMed] [Google Scholar]
- 5.Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson's disease. Am J Gastroenterol. 1994;89:15–25. [PubMed] [Google Scholar]
- 6.Stocchi F, Badiali D, Vacca L, D'Alba L, Bracci F, Ruggieri S, et al. Anorectal function in multiple system atrophy and Parkinson's disease. Mov Disord. 2000;15:71–76. doi: 10.1002/1531-8257(200001)15:1<71::aid-mds1012>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Sung HY, Lee KS, Kim YI, Kim HT. Anorectal dysfunctions in Parkinson's disease. J Neurol Sci. 2011;310:144–151. doi: 10.1016/j.jns.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G, Maggio D, Battaglia E, Giulietti O, Spinozzi F, Reboldi G, et al. Manometric investigation of anorectal function in early and late stage Parkinson's disease. J Neurol Neurosurg Psychiatry. 2000;68:768–770. doi: 10.1136/jnnp.68.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 11.Mathers SE, Kempster PA, Swash M, Lees AJ. Constipation and paradoxical puborectalis contraction in anismus and Parkinson's disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry. 1988;51:1503–1507. doi: 10.1136/jnnp.51.12.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathers SE, Kempster PA, Law PJ, Frankel JP, Bartram CI, Lees AJ, et al. Anal sphincter dysfunction in Parkinson's disease. Arch Neurol. 1989;46:1061–1064. doi: 10.1001/archneur.1989.00520460037010. [DOI] [PubMed] [Google Scholar]
- 13.Jost WH. Gastrointestinal dysfunction in Parkinson's Disease. J Neurol Sci. 2010;289:69–73. doi: 10.1016/j.jns.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Bharucha AE. Constipation. Best Pract Res Clin Gastroenterol. 2007;21:709–731. doi: 10.1016/j.bpg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Meagher AP, Lubowski DZ, King DW. The cough response of the anal sphincter. Int J Colorectal Dis. 1993;8:217–219. doi: 10.1007/BF00290310. [DOI] [PubMed] [Google Scholar]
- 16.Amarenco G, Ismael SS, Lagauche D, Raibaut P, Rene-Corail P, Wolff N, et al. Cough anal reflex: strict relationship between intravesical pressure and pelvic floor muscle electromyographic activity during cough. Urodynamic and electrophysiological study. J Urol. 2005;173:149–152. doi: 10.1097/01.ju.0000147305.00443.df. [DOI] [PubMed] [Google Scholar]
- 17.Deffieux X, Raibaut P, Rene-Corail P, Katz R, Perrigot M, Ismael SS, et al. External anal sphincter contraction during cough: not a simple spinal reflex. Neurourol Urodyn. 2006;25:782–787. doi: 10.1002/nau.20228. [DOI] [PubMed] [Google Scholar]