Abstract
Background and Purpose
The functional outcome of traumatic brain injury (TBI) varies widely. The aim of this study was to identify the factors predicting outcome following TBI.
Methods
We prospectively enrolled acute TBI patients, and assessed them clinically and radiologically using brain magnetic resonance imaging (MRI). Functional outcome was measured using the Glasgow Outcome Scale (GOS) at 3 months after TBI. A GOS score of ≤4 was regarded as an unfavorable outcome. We performed multivariate analysis to investigate the association between clinicoradiological variables and outcome.
Results
Forty-two patients completed the clinical evaluation in the acute phase and outcome measurement at 3 months. Motorcycle accident was associated with unfavorable outcome [odds ratio (OR)=38.3, p=0.022]. If the patients were the victims of the accident, they were more likely to have an unfavorable outcome (OR=21.3, p=0.037). All seven patients with a low Glasgow Coma Scale (GCS) score (i.e., ≤8) at 24 or 48 h after TBI were also found to have an unfavorable outcome. The presence of diffuse axonal injury (DAI) was a significant predicting factor of an unfavorable outcome (OR=8.48, p=0.042).
Conclusions
Motorcycle accident, being an accident victim, and a lower GCS score at 24 hours or more after the accident were found to be unfavorable prognostic variables. DAI was the only radiologic variable predicting an unfavorable outcome. Thus, it is important to identify DAI by applying MRI in the acute phase.
Keywords: traumatic brain injury, outcome, magnetic resonance imaging
Introduction
The incidence of traumatic brain injury (TBI) is reported to be between 56 and 836 per 100000,1 and the burden of the disease attributable to TBI is high due to it being more common in young adults and causing significant disabilities. One of the distinguishing features of TBI is a wide variation in functional outcome. In addition, disabilities after TBI are mostly cognitive, behavioral, and subjective symptoms. The assessment of the disability associated with TBI and prediction of the outcome are frequently required for claiming damages and social support. Several prognostic factors of a poor outcome have been proposed, such as old age, low initial Glasgow Coma Scale (GCS) score, anisocoria, hypotension, or shock.2-4 Radiological findings, such as obliteration of the third ventricle or basal cistern, midline shift, subarachnoid hemorrhage (SAH),3,4 lesion burden,5 and grade of diffuse axonal injury6 are reported as being predictive of a poor outcome.
Computed tomography (CT) has remained the primary imaging tool for TBI in clinical practice because of its sensitivity in detecting acute hemorrhage and its rapid scanning ability. However, CT has limited sensitivity for diffuse axonal injury (DAI), nonhemorrhagic contusions, and brainstem lesions.7 Although many studies have used delayed magnetic resonance imaging (MRI) to assess clinical outcome, few studies have used early MRI for the assessment of the severity or probable outcome of trauma.2,6,8,9 Early diffusion-weighted image (DWI) is particularly valuable in evaluating DAI.10
We performed a prospective study of TBI, including clinical measurements and MRI in the acute phase and at 3 months postinjury. The objectives of the current study were to describe the clinical and radiological characteristics of TBI and to elucidate its early prognostic factors.
Methods
Population and procedures
TBI patients were prospectively evaluated and followed up for more than 3 months. We consecutively enrolled patients who had clinical or radiologic evidence of TBI and where this evidence was obtained within 1 week after the time of injury. Exclusion criteria were being older than 75 years and having previous central nervous system (CNS) illness or concussion. All subjects received a brain CT scan immediately after visiting the hospital. We performed brain MRI within 2 weeks of the initial visit (mean, 4.1 days) if the patient's condition allowed it. We investigated the cause of trauma, clinical symptoms, neurological signs, presence of seizure during hospitalization, and GCS score at the time of presentation, and at 24 h, 48 h, and 1 week later using a structured case-recording form. The GCS scores of patients who were sedated were excluded from the analysis of prognostic factors.
Functional outcome was evaluated using the Glasgow Outcome Scale (GOS) at 3 months after TBI in an outpatient clinic or by telephone interview. We dichotomized the outcome into favorable (GOS score=5, able to return to work or school) and unfavorable (GOS score ≤4).
Between October 2007 and September 2009, 93 individuals with TBI were registered. Of these, 51 patients were excluded for the following reasons: visiting the hospital more than 1 week after the injury (3 patients), being older than 75 years (10 patients), CNS illness (16 patients), concussion (10 patients), and refusal to provide consent or a missed follow-up (12 patients). Ultimately, 42 patients completed the clinical evaluation during the admission and at the 3-month follow-up.
Our institutional review board approved this study. Informed consent to participate was obtained from the patients and their caregivers.
MRI and CT protocols
All subjects were scanned using a 1.5-T magnetic resonance (MR) scanner (Gyroscan ACS-NT, Philips Medical Systems, Best, The Netherlands). Axial T1, T2, fluid-attenuated inversion recovery, gradient-echo, and diffusion, supplemented with coronal T2 and sagittal T1 images were obtained.
Single-shot spin-echo echo-planar DWI sequences were obtained by applying diffusion gradients in three orthogonal directions for each section, with two diffusion weightings (b=0 s/mm2 and b=1000 s/mm2). The parameters for DWI were as follows: repetition time/echo time, 3298/61 ms; matrix, 128×128; field of view, 24 cm; slice thickness, 5 mm; and intersection gap, 1.5 mm.
All CT scans were obtained using a helical CT scanner (LightSpeed Plus, GE Medical Systems, Milwaukee, WI, USA). The scanning parameters were 120 kV and 300 mA, with an image matrix of 512×512, a field of view of 22 cm, and a section thickness of 5 mm.
Imaging analysis
An experienced neuroradiologist (Kim SS) and neurologists (Lee SY and Yeo MJ) interpreted the imaging findings by consensus based on visual inspection, in accordance with predefined variables. We evaluated the presence of a contusion, subdural hemorrhage (SDH), epidural hemorrhage (EDH), SAH, DAI, midline shift, diffuse brain swelling, and transtentorial herniation, and the location and size of lesions using brain CT and MR images. Combined injury was defined when it had two or more component of TBI among contusion, SDH, and EDH.
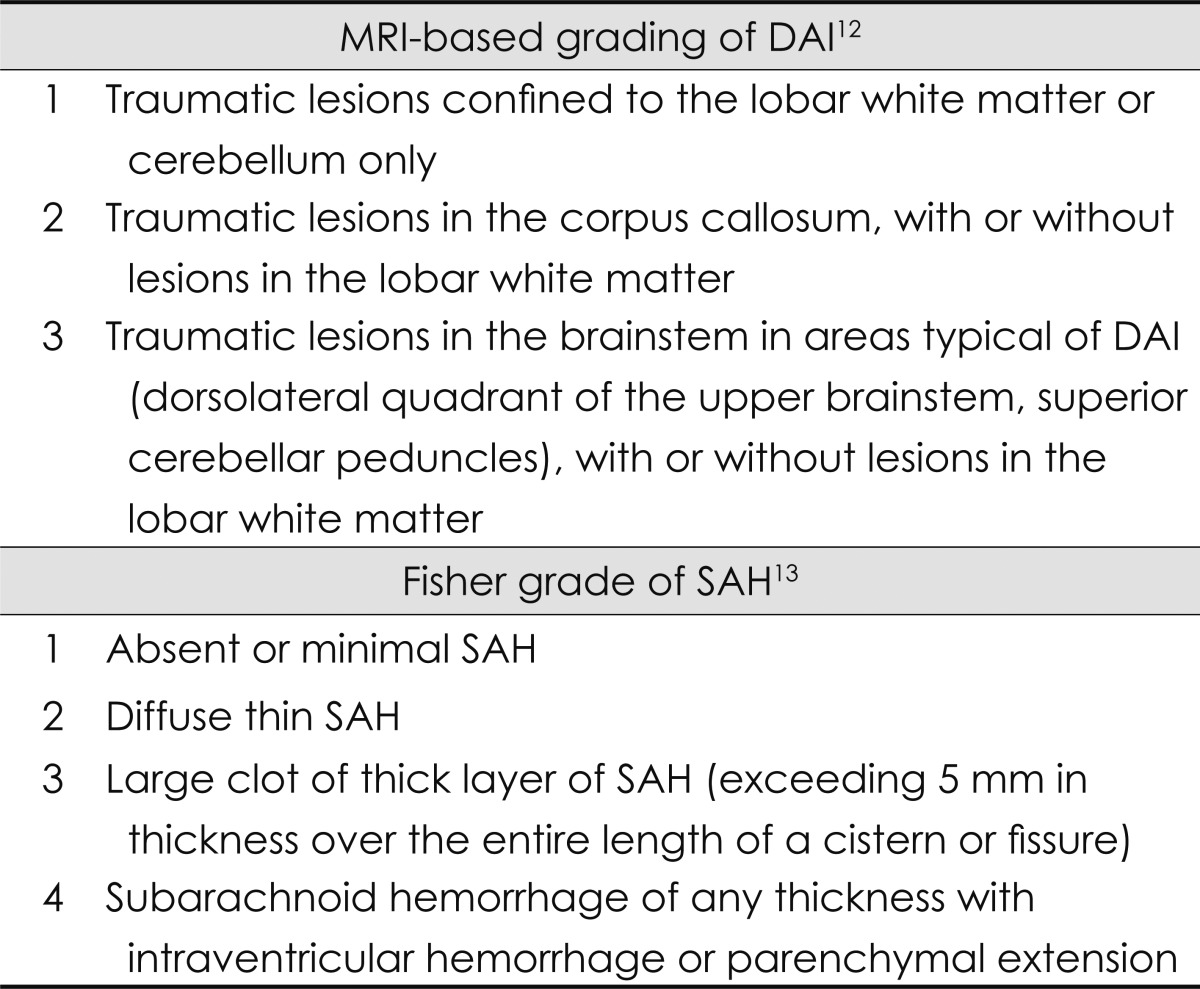
DAI appears on MR images as multiple, small, deeply situated, and elliptical lesions that spare the overlying cortex.11 DAI was defined as punctuate lesions of profound hypointensity on T2-weighted gradient-echo imaging, and as lesions of profound hyperintensity on fluid-attenuated inversion recovery and DWI.12,13 DAI was classified as stage 1, 2, or 3 using the modified Adams classification,14 according to its location. SAH was graded according to the 4-point Fisher scale (Table 1).15 The presence of diffuse brain swelling was determined based on the observation of diffuse cisternal obliteration on the brain CT image obtained during the acute period. Contusion was defined as parenchymal patchy lesion, which manifests as a low density on CT and a hyperintensity on T2-weighted MRI with or without hemorrhagic foci. We calculated the size of the contusion by measuring the diameter of the high signal intensity on T2-weighted MRI, since many of the contusions were not sufficiently visible on CT.
Table 1.
Grading system of diffuse axonal injury (DAI) and subarachnoid hemorrhage (SAH)
MRI: magnetic resonance imaging.
Statistics
We identified the independent predictors of unfavorable outcome by applying multiple logistic regression with stepwise selection. The regression model included the clinicoradiological variables that appeared to be associated with an unfavorable outcome in univariate analysis (p<0.1) or predictors suggested in previous studies. Multicollinearity was tested using the contingency coefficient, which represents the strength of association between categorical variables. A separate model was fit for each associated variable to avoid multicollinearity.
The magnitude of associations between the potential predictive variables and outcome was quantified using the odds ratio (OR) and the corresponding 95% confidence interval. A probability value of p<0.05 was considered significant in multivariate analysis. Statistical analysis was performed using the SPSS program (version 19.0, IBM SPSS Statistics, Chicago, IL, USA).
Results
General characteristics
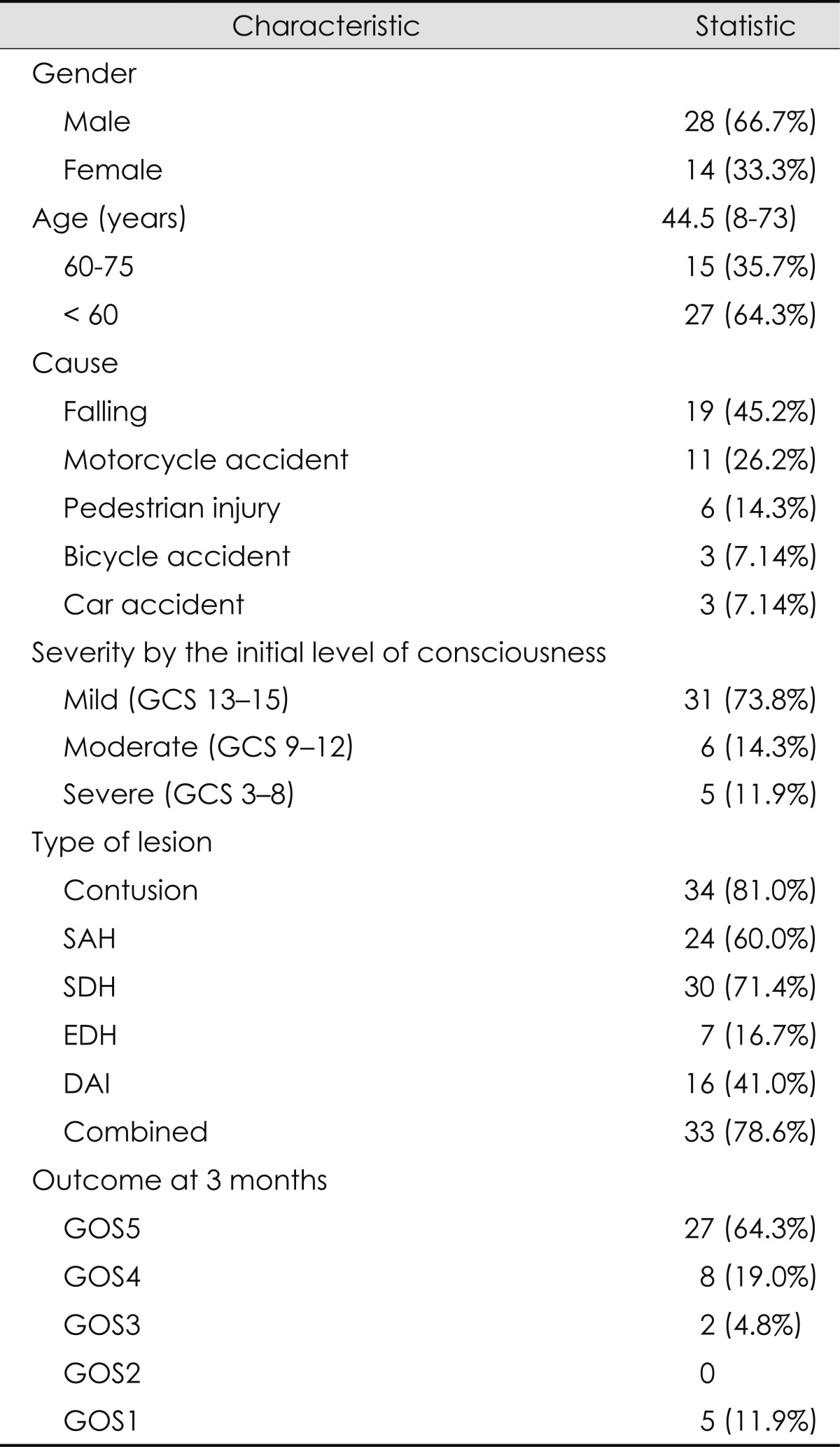
The 3-month outcome after TBI was available for 42 patients. Their mean age was 44.5 years (range, 8-73 years), and 28 (66.7%) were men and 14 (33.3%) were women. The causes of the TBIs were falling (19 patients, 45.2%), motorcycle accident (1 patients, 26.2%), pedestrian injury (6 patients, 14.3%), bicycle accident (3 patients, 7.14%), and car accident (3 patients, 7.14%). Ten (23.8%) of the patients were victims of the accidents, while others were perpetrators or those whose responsibility of the accident was ambiguous.
Severity according to the GCS at presentation was mild (GCS score, 13-15) in 31 patients (73.8%), moderate (GCS score, 9-12) in 6 patients (14.3%), and severe (GCS score, 3-8) in 5 patients (11.9%). Acute seizure occurred in 6 patients (14.3%), within two weeks of onset of TBI.
We detected a contusion in 34 (81.0%), SAH in 24 (60.0%), SDH in 30 (71.4%), EDH in 7 (16.7%), and DAI in 16 (41.0%) patients. Thirty-three patients (78.6%) had combined injuries with multiple pathologic features. The contusion was located in the frontal, temporal, occipital, and parietal lobes in 28, 25, 5, and 4 patients, respectively. Fourteen, six, two, and two patients had grade 1, 2, 3, and 4 SAH, respectively. Ten, two, and four patients had grade 1, 2, and 3 DAI, respectively. Midline shift (>5 mm) was detected in 16 (38.1%) patients, transtentorial herniation was observed in 7 (16.7%) patients, and diffuse swelling was observed in 7 (16.7%) patients.
Three months after their accident, 27 patients (64.3%) were able to return to work or school (GOS score=5), 8 patients had moderate disability (GOS score=4), and 2 patients could not live independently (GOS score=3). Five patients died within 3 months (GOS score=1); the time to death ranged from 2 to 37 days. Two patients died as a result of systemic trauma, two patients died because of either the TBI itself or sepsis after a prolonged bedridden state due to the TBI, and one patient died unexpectedly of an unknown cause (Table 2).
Table 2.
General characteristics of the patients
DAI: diffuse axonal injury, EDH: epidural hemorrhage, GCS: Glasgow Coma Scale, GOS: Glasgow Outcome Scale, SAH: subarachnoid hemorrhage, SDH: subdural hemorrhage.
Predictive factors
Clinical variables
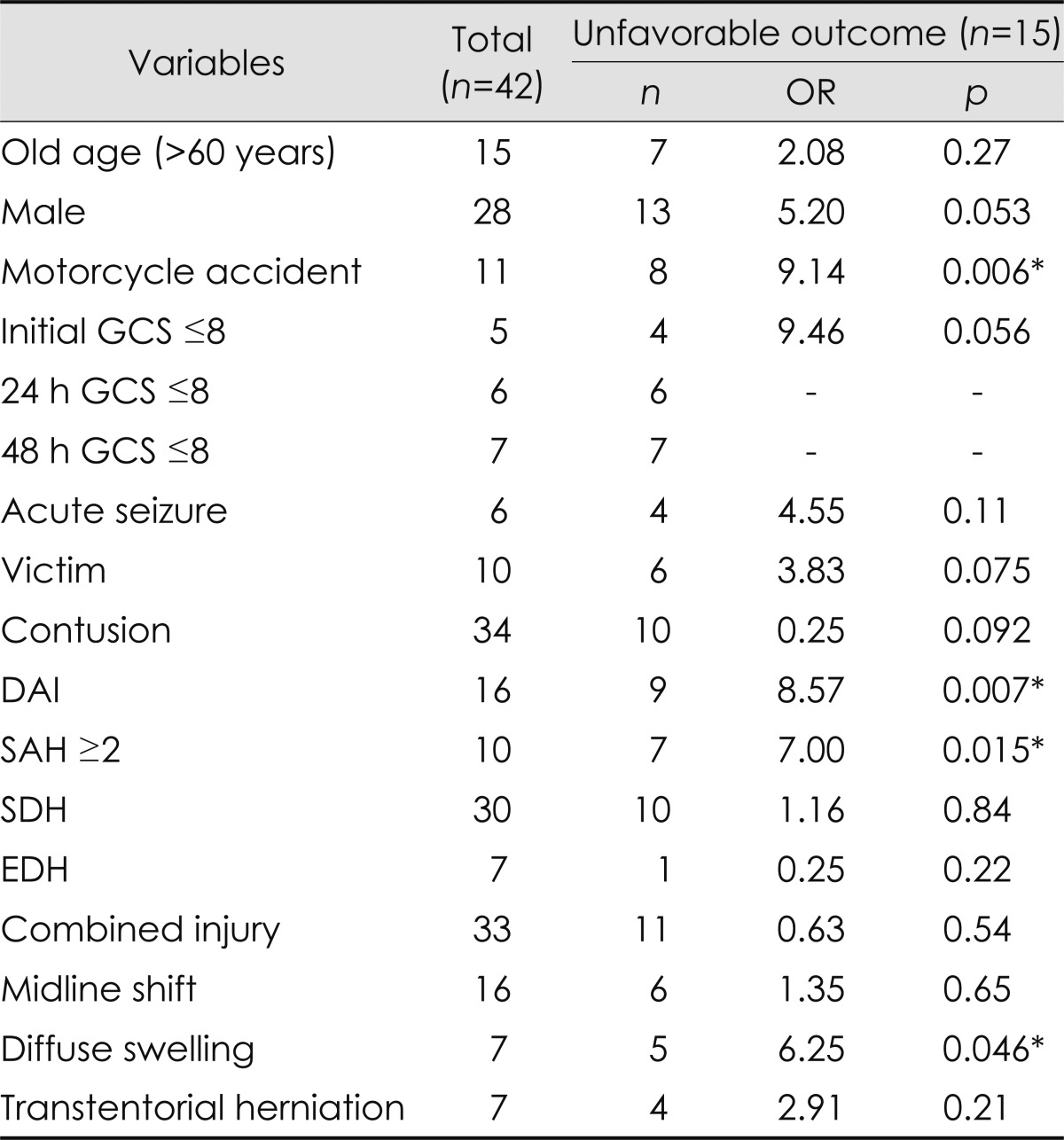
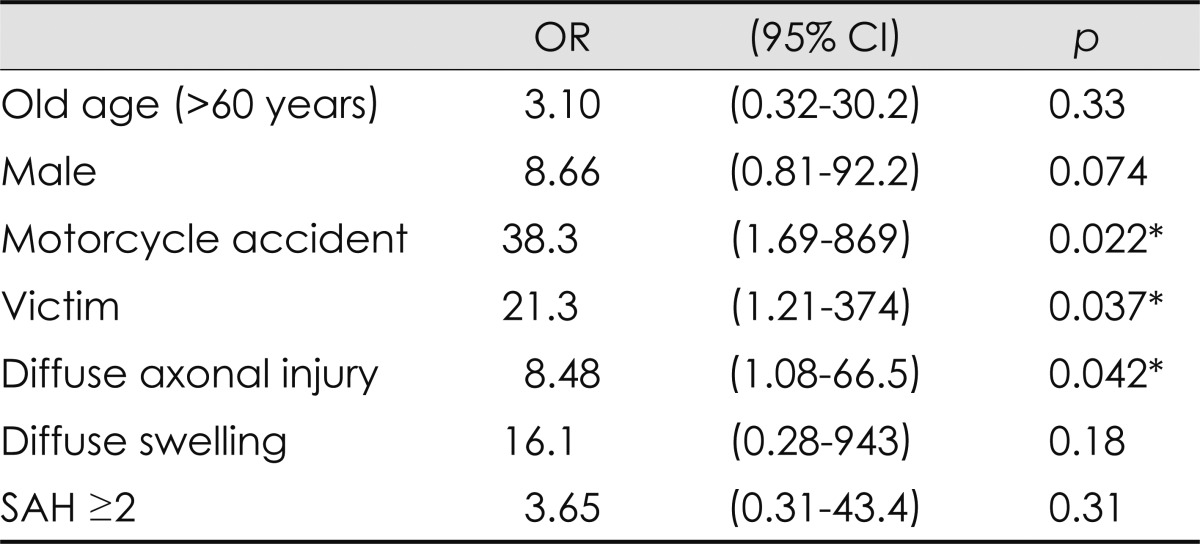
The cause of motorcycle accident was an independent prognostic factor of an unfavorable outcome (OR=44.6; p=0.012, multivariate analysis). If the patients were victims of the accidents they were more likely to have an unfavorable outcome (OR=68.7; p=0.013, multivariate analysis). Four out of five patients who had an initial GCS score of ≤8 had an unfavorable outcome, and six patients with a GCS score of ≤8 at 24 h and seven patients with a GCS score of ≤8 at 48 h had all unfavorable outcomes. Age and gender were not associated with outcome. Acute seizure did not predict an unfavorable outcome (Table 3 and 4).
Table 3.
Univariate analysis of the clinicoradiological variables that predicted outcomes
*Indicates the results considered statistically significant (p<0.05).
DAI: diffuse axonal injury, EDH: epidural hemorrhage, GCS: Glasgow Coma Scale, OR: odds ratio, SAH: subarachnoid hemorrhage, SDH: subdural hemorrhage.
Table 4.
Multivariate analysis of the clinicoradiological variables that predicted unfavorable outcome
Motorcycle accident and diffuse axonal injury were separately analyzed because they are significantly related with each other. Iinitial Glasgow Coma Scale ≤8 was excluded from this analysis, because the number was too small for multivariate analysis and it was significantly colinear with diffuse axonal injury and diffuse swelling.
*Indicates the results considered statistically significant (p<0.05).
SAH: subarachnoid hemorrhage, OR: odds ratio, CI: confidence interval.
Neuroimaging variables
DAI was a prognostic factor of unfavorable outcome (OR=8.48; p=0.042, multivariate analysis). Grade 2 or more DAI was particularly strongly associated with an unfavorable outcome (OR=30.5; p=0.018, multivariate analysis). However, DAI did not always predict an unfavorable outcome. Seven (43.8%) DAI patients had a good recovery, including one patient with grade 3 DAI. DAI was significantly associated with motorcycle accidents (contingency coefficient, 0.327; p=0.031) and an initial GCS score of ≤8 (contingency coefficient, 0.418; p=0.004). Diffuse swelling and grade 2 or more SAH were related to an unfavorable outcome in univariate analysis (OR=6.25; p=0.046 and OR=7.00; p=0.015, respectively), but the association was not statistically significant in multivariate analysis. Diffuse swelling covaried with an initial GCS score of ≤8 (contingency coefficient, 0.53; p<0.001). All four patients with grade 3 or more severe SAH had an unfavorable outcome.
The presence and size of the SDH or EDH, the presence, size, and location of the contusion, combined injury, and midline shift were not significantly associated with an unfavorable outcome. Seven of the nine patients with a contusion larger than 60 cm3 regained good function (Table 3 and 4).
Discussion
Relatively few studies have focused on the relationship between the cause of injury and functional outcome. The present study found that a TBI cause of motorcycle accident predicted an unfavorable outcome. Motorcycle accidents accompany marked acceleration-deceleration without protection, and commonly result in severe TBI, including DAI.16 In the IMPACT study,17 falling was associated with a poorer outcome than traffic accidents and assaults. However, this relationship was lost after adjustment for age, and TBI associated with motorcycle accidents was not analyzed separately.
The GCS score was associated with outcome in this study, which is consistent with the results of previous studies. All patients who had low GCS score at 24 or 48 h had an unfavorable outcome, which suggests a low probability of favorable outcome following coma persisting for 24 h or more after TBI, although the sample was too small to allow generalization.
Being the victim of an accident was shown to be a significant independent prognostic factor of an unfavorable outcome. Most studies4,18 investigating predictive factors of TBI categorized good and poor outcomes as GOS scores of ≥4 and ≤3, respectively. However, the most frequent and problematic symptoms after TBI are cognitive and behavioral symptoms. Therefore, we classified a GOS score of ≤4 (able to live independently, but unable to return to work or school) as an unfavorable outcome. However, since the level of social functioning is influenced by emotion and can be manipulated by secondary gain, it is important to determine the objective variables that are correlated with outcome in order to establish whether or not social dysfunction is concordant with the TBI.
DAI was the only neuroimaging variable associated with unfavorable outcome. DAI was observed in 41.0% of the subjects in our study, and was reported to range between 48% and 72% according to the severity of the subjects' injury in other studies.14,19 DAI results from the tearing of axons due to shearing forces during acceleration, deceleration, and rotation of the brain,13 and appears to be the frequent cause of the poor outcome.6 Brainstem lesions were found to be associated with a poor outcome in previous studies.2,6,8,9 brainstem lesions were detected in only four of the patients in the present study, of which three had an unfavorable outcome, although we were unable to estimate the statistical significance of this finding.
Lesion burden was associated with outcome in several studies,6 but it was not applicable to individual patients. In cases of extra-axial hematoma, timely surgery can result in good outcome. Even for parenchymal lesions, seven out of nine patients with a large contusion (>60 cm3) had a good outcome in this study.
Early MRI improves the outcome prediction in TBI because it can detect DAI-which is a frequent form of TBI and a strong prognostic factor-much more sensitively than can CT and delayed MRI. DWI and gradient-echo sequences are the most useful MR sequences for evaluating TBI in the acute stage due to their superior resolution for DAI and the short scan time, which permits the evaluation of clinically unstable patients.
The major limitation of this study was the small number of patients and the absence of a long-term outcome measurement. Our population was relatively old and many patients were excluded because of preexisting CNS illness. In addition, loss of follow-up is more frequent in TBI than in chronic illness. Large-scale multicenter studies are therefore needed to confirm our findings. The GOS score is usually determined at 3, 6, and 12 months after TBI;20 we measured it at 3 months as a midterm outcome. Many studies have found that the symptoms of mild TBI resolve within 3 months.22 The time course of recovery for moderate-to-severe TBI was not closely examined. Three months is sufficient for stabilization of the lesion and longer times after TBI may permit interventions of confounding factors such as the environment and restoration ability of the individuals, although recovery can occur after more than 3 months following TBI.
In summary, motorcycle accident, being an accident victim, decreased level of consciousness after 24 h, and DAI rather than the type and size of lesion are unfavorable prognostic variables.
Acknowledgements
This study was supported by a 2008 Kangwon National University Hospital Grant.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Lagares A, Ramos A, Pérez-Nuñez A, Ballenilla F, Alday R, Gómez PA, et al. The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir (Wien) 2009;151:341–356. doi: 10.1007/s00701-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 3.MRC CRASH, Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano G, Bachevalier J, Levin HS, Song JX, Scheibel RS, Fletcher JM. Volume of focal brain lesions and hippocampal formation in relation to memory function after closed head injury in children. J Neurol Neurosurg Psychiatry. 2000;69:210–216. doi: 10.1136/jnnp.69.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss N, Galanaud D, Carpentier A, Naccache L, Puybasset L. Clinical review: Prognostic value of magnetic resonance imaging in acute brain injury and coma. Crit Care. 2007;11:230. doi: 10.1186/cc6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–682. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- 8.Weiss N, Galanaud D, Carpentier A, Tezenas de, Naccache L, Coriat P, et al. A combined clinical and MRI approach for outcome assessment of traumatic head injured comatose patients. J Neurol. 2008;255:217–223. doi: 10.1007/s00415-008-0658-4. [DOI] [PubMed] [Google Scholar]
- 9.Skandsen T, Kvistad KA, Solheim O, Lydersen S, Strand IH, Vik A. Prognostic value of magnetic resonance imaging in moderate and severe head injury: a prospective study of early MRI findings and one-year outcome. J Neurotrauma. 2011;28:691–699. doi: 10.1089/neu.2010.1590. [DOI] [PubMed] [Google Scholar]
- 10.Huisman TA. Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur Radiol. 2003;13:2283–2297. doi: 10.1007/s00330-003-1843-6. [DOI] [PubMed] [Google Scholar]
- 11.Gentry LR. Imaging of closed head injury. Radiology. 1994;191:1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- 12.Ezaki Y, Tsutsumi K, Morikawa M, Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol. 2006;47:733–740. doi: 10.1080/02841850600771486. [DOI] [PubMed] [Google Scholar]
- 13.Parizel PM, Ozsarlak, Van Goethem JW, van den Hauwe L, Dillen C, Verlooy J, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol. 1998;8:960–965. doi: 10.1007/s003300050496. [DOI] [PubMed] [Google Scholar]
- 14.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113:556–563. doi: 10.3171/2009.9.JNS09626. [DOI] [PubMed] [Google Scholar]
- 15.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shuaeib FM, Hamouda AMS, Umar RSR, Hamdan MM, Hashmi MSJ. Motorcycle helmet. Part I. Biomechanics and computational issues. J Mater Process Technol. 2002;123:406–421. [Google Scholar]
- 17.Butcher I, McHugh GS, Lu J, Steyerberg EW, Hernández AV, Mushkudiani N, et al. Prognostic value of cause of injury in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:281–286. doi: 10.1089/neu.2006.0030. [DOI] [PubMed] [Google Scholar]
- 18.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 19.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150:663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 20.Zink BJ. Traumatic brain injury outcome: concepts for emergency care. Ann Emerg Med. 2001;37:318–332. doi: 10.1067/mem.2001.113505. [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]