Abstract
Anticoccidial effects of Galla rhois (GR) extract were evaluated in chickens after oral infection with Eimeria tenella. This study was performed using 3-day-old chickens (n=30). The animals were divided into 3 groups as follows: GR 0.5%/infected (n=10), untreated/infected (n=10), and non-infected control (n=10). The chickens were fed a standard diet supplemented with or without GR for 1 week before infection with E. tenella (10,000 sporulated oocysts per chicken). The effects of GR on E. tenella infection were assessed by 2 parameters, number of fecal oocysts and body weight gain, and the results of the polymerase chain reaction (PCR). The GR-fed chickens produced significantly lower number of fecal oocysts (P<0.05) than the E. tenella-infected chickens who were fed the standard diet. In addition, GR-based diet improved the loss of body weight caused by E. tenella infection. Positive findings of PCR were identified by distinct bands in the samples of E. tenella-inoculated chickens. However, PCR analysis revealed no E. tenella oocysts in the feces of GR-fed chickens. Our data showed that GR extracts had remarkable anticoccidial activities against E. tenella. This finding might have implications for the development of novel anticoccidial drugs.
Keywords: Anticoccidial activity, coccidiosis, eimeria, eimeria tenella, galla rhois
Coccidiosis is induced by infection with organisms belonging to Eimeria species, and it is an important parasitic disease of poultry [1]. Losses include mortality, morbidity, and cost of preventative or therapeutic drugs and/or vaccination. In addition, many of the in-feed medications commonly used for prevention of infections with Eimeria species have become less effective because some strains of parasites have developed reduced susceptibility to anticoccidials [2]. This finding suggests that coccidiosis is likely to have a greater impact on the profitability of broiler meat production in the future [2]. Eimeria infection causes extensive destruction of the intestinal epithelium, which results in reduced feed efficiency, body weight gain, and a temporary reduction in egg production [3,4]. Although coccidiosis is mainly controlled using chemotherapeutic agents, novel approaches are urgently required because of increasing number of drug-resistant parasite strains in the commercial poultry production settings [5-7].
Our recent in vitro and in vivo studies showed that the extract of Galla rhois (GR) had strong antiviral and antibiotic effects [8-10]. The constituents of GR extract include methyl gallate; 3-galloyl-gallic acid; 4-galloyl-gallic acid isomers; 1,2,3,4,6-penta-O-galloyl-β-D-glucose; and 2 inactive phenolic compounds, gallic acid methyl ester and gallic acid [11]. Methyl gallate, a major component of GR, also exhibits strong antimicrobial activity. GR extract inhibits the growth of Escherichia coli, without adversely affecting the growth of lactic acid-producing bacteria, and its activity is more pronounced with the presence of methyl gallate [12].
Although a different types of natural products have been investigated as alternative to control coccidiosis in chickens [3,5], the effects of GR extract on Eimeria infection has not been studied. In this work, we aim to evaluate the anticoccidial effects of GR extract on Eimeria tenella infection in Chicken
Materials and Methods
Experimental materials
To develop a natural herbal antimicrobial compound, over 1,000 kinds of herbal materials were tested by Center for Animal Resources Development, Wonkwang University. The herbal specimens were purchased at the University Oriental Drugstore, Iksan, Korea. Voucher specimens were deposited at the Herbarium of the College of Pharmacy, Wonkwang University. Plant material was extracted with ethanol under ultrasonic conditions for 3 h followed by paper filtration. The filtrates were evaporated in vacuum to yield water-soluble extracts. We employed in vitro antibacterial and in vitro antiviral screening tests to evaluate the effect of the medicinal herbal extracts on the pathogenic bacteria and viruses. We found that GR extract had very strong antibacterial and antiviral effects in vitro and in vivo [8-10]. In this study, we administered GR extract to the experimental animals.
Experimental animals
The study was performed using 3-day-old chickens (n=30) in the animal facility of Center for Animal Resources Development, Wonkwang University, Korea. Animals were acclimatized and maintained in an animal facility room with regulated temperature (28±2℃), humidity (50±5%) and light/dark cycle (12/12 h). The animals were fed a commercial post-broiler diet without antibiotics and coccidiostat (Hanil Feed Co., Yongin, Korea) and tap water ad libitum. The chickens were maintained in wire-floored grower cages during the study period. All studies were performed in accordance with the Guide for Animal Experimentation by Wonkwang University, and the protocol was approved by the Institutional Animal Care and Use Committee of Wonkwang University (WKU11-007). All efforts were made to minimize pain or discomfort to the animals used.
Experimental design
Anticoccidial effects of GR extract were evaluated in chickens after infection with E. tenella via the oral route. This study was performed using 3-day-old chickens (n=30). The animals were divided into 3 groups: GR 0.5%/infected (n=10), untreated/infected (n=10), and non-infected control (n=10). Chickens were fed a standard diet supplemented with or without GR for 1 week before infection with E. tenella (10,000 sporulated oocysts per chicken). We examined the effects of GR on E. tenella infection using 2 parameters, number of fecal oocysts and body weight gain, and the findings of polymerase chain reaction (PCR).
Inoculation of Eimeria oocysts
Oocysts of E. tenella were cleaned by flotation on 5.25% sodium hypochlorite and washed 3 times using phosphate buffered saline. E. tenella was provided kindly by Professor Wongi Min at Gyeongsang National University in Korea. Chickens were treated by oral gavage using a 24-gauge stainless steel animal feeding tube (Popper & Sons, Inc., New York, USA) attached to a 3-mL syringe. The oral infectious dose of E. tenella has been approximated to be 104 oocysts of E. tenella in 1 mL of saline. The control chickens (n=10) received saline through the same route.
Clinical observation and weight measurements
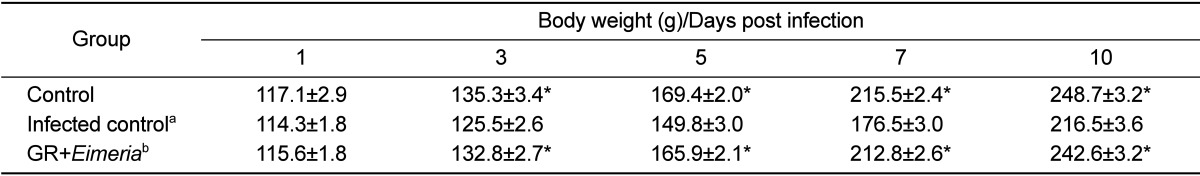
During the study period, the animals were examined twice daily for morbidity and mortality. Further, we compared clinical signs and changes in body weight of experimental animals. Body weights were individually measured for 2 weeks before infection and for 10 days post-infection.
Fecal sampling and oocyst counting
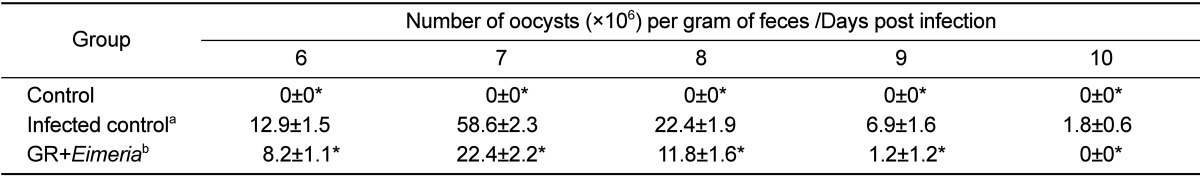
Fecal materials were collected from 6 to 10 days post-infection. The fecal samples were analyzed for the presence of coccidial oocysts using a standard fecal flotation technique [13]. Briefly, 1 g from each sample was suspended with 5 mL saline and pelleted by centrifugation at 1500×g for 5 min. The resulting pellet was resuspended in saturated sodium chloride (aqueous) and passed through a sieve with a mesh size of 1 mm to remove coarse fecal debris. The resulting filtrate was used in a standard gravity vial fecal flotation using 22 mm×22 mm cover slips. After flotation, the cover slip was mounted on a slide and examined in its entirety for the presence of coccidial oocysts. Total number of oocysts was calculated using the following formula: total number of oocysts=oocyst count×dilution factor×(fecal sample volume/counting chamber volume)/number of birds per cage.
Polymerase chain reaction
For DNA extraction, fecal samples were collected at 10 days after inoculation and cleaned with sodium hypoclorite solution (5%-6% active chlorine) for 10 min at 4℃, washed 3 times with deionized water, and resuspended in extraction buffer (10 mM Tris-Cl, pH 8.0; 50 mM EDTA, pH 8.0). The oocysts and sporocysts were completely broken down by vortexing with half the volume of 425-600 µm acid-washed glass beads (Sigma-Aldrich, St. Louis, Mo., USA). The lysate was centrifuged at 14,000×g for 10 min to eliminate debris and then digested using DNAse-free RNAse A (20 µg/mL) at 37℃ for 1 h. Further digestion was performed using proteinase K (100 µg/mL) and sodium dodecyl sulphate (SDS, 0.5%) at 50℃ for 2 h. The DNA was then extracted once with one volume of phenol, phenol/chloroform, and chloroform and precipitated using ethanol and ammonium acetate. The pellet was washed using 70% ethanol. The DNA was eluted in Tris-EDTA buffer (pH 8.0), and an aliquot was used for PCR amplification. The template DNA (50 ng) and 20 pmol of each primer were added to a PCR mixture tube (AccuPower PCR PreMix; Bioneer Corp., Korea) containing 2.5 U of Taq DNA polymerase, 250 µM each deoxynucleoside triphosphate, 10 mM Tris-HCl (pH 8.3), 40 mM KCl, 1.5 mM MgCl2, and the gel loading dye. The volume was adjusted using distilled water to 20 µL. The genomic DNA was amplified using species-specific forward primer (5'-AATTTAGTCCATCGCAACCCTTG-3') and reverse primer (5'-CGAGCGCTCTGCATACGACA-3') for the amplification of the 278-bp segment of E. tenella [13]. The reaction mixture was subjected to denaturation at 96℃ for 5 min followed by 35 cycles of denaturation at 95℃ for 30 s, annealing at 58℃ or 65℃ for 30 s, and extension at 72℃ for 1 min, and a final extension step of 72℃ for 3 min; the samples were maintained at 4℃ until analysis [13]. Reactions were performed using My Genie 32 Thermal Block PCR (Bioneer, Korea). Each sample (8 µL) was mixed with 2 µL loading buffer and was analyzed by electrophoresis in 2% agarose gels stained with 0.5 µg/mL ethidium bromide.
Statistical analysis
Differences in the mean oocyst production and mean weight gain between the 4 groups were tested using oneway analysis of variance (ANOVA; GraphPad InStat; GraphPad Software Inc., San Diego, CA) and considered significant at P<0.05.
Results
The effects of GR on E. tenella infection were assessed using 2 parameters, number of fecal oocysts and body weight gain. The GR-fed chickens produced significantly lower number of fecal oocysts (P<0.05) than E. tenella-infected chickens fed a standard diet. In addition, GR-based diet improved body weight loss caused by E. tenella infection. Our results showed that compared to untreated controls, chickens treated with GR extracts had significantly lower levels of fecal oocyst shedding and higher anticoccidial activities (P<0.05).
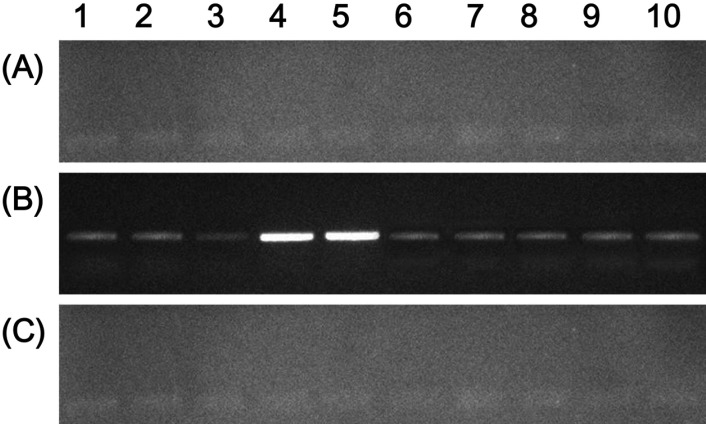
Fecal oocyst shedding was significantly higher in inoculated chickens than in control chickens (P<0.05). The number of fecal oocysts was the highest on day 7 post-inoculation (Table 1). Moreover, body weight gain was lesser in the animals in the inoculated group than in the control group (Table 2). Fecal oocyst shedding was observed in the inoculated animals. PCR assay, based on the amplification of internal transcribed spacer 1 (ITS1) regions of ribosomal DNA, was used to detect the chicken coccidian species E. tenella. The positive reactions were identified in the samples of E. tenella inoculated chickens. Although the strengths of PCR signals in the samples of E. tenella infected group were various, those of animal number 4 and 5 revealed distinct bands. Those results were suggested that the amounts of fecal shedding oocysts were various in the infected chickens. However, PCR analysis revealed no E. tenella oocysts in the feces of GR-fed chickens (Figure 1).
Table 1.
Results of number of oocysts in the feces of chickens with or without Galla rhois extracts
aThe chickens inoculated with Eimeria tenella oocysts.
bThe chickens inoculated with Eimeria live oocysts and treated with 0.5% Galla rhois extract (GR).
*Significantly different than that of control chickens not infected with Eimeria (P<0.05)
Table 2.
Results of body weight changes of chickens with or without Galla rhois extracts
aThe chickens inoculated with Eimeria tenella oocysts.
bThe chickens inoculated with Eimeria live oocysts and treated with 0.5% Galla rhois (GR).
*Significantly different than that in control chickens infected with Eimeria (P<0.05)
Figure 1.
Amplification of the sample DNAs by species-specific polymerase chain reaction (PCR) for Eimeria tenella identified on a 2% agarose gel electrophoresis. 1~10: Animal numbers in the Group A, B, C. (A) DNAs extracted from the fecal samples of control chickens. (B) DNAs extracted from the fecal samples of E. tenella-infected chickens. (C) The DNAs extracted from the fecal samples of E. tenella-infected chickens treated with 0.5% Galla rhois.
Discussion
Coccidiosis of domestic fowl is a worldwide disease caused by obligate intracellular protozoan of the genus Eimeria. Coccidiosis results in economic losses in poultry production. E. tenella is an important pathogen causing avian coccidiosis in the poultry industry [1,14]. This disease is characterized by enteric lesions of variable extent and severity, which reduce the absorptive function of the intestinal mucosa and lead to weight loss, diarrhea, poorer feed conversion, and a higher mortality in the affected flocks [15].
Prophylactic medication has been used to control and prevent coccidiosis in commercially grown chickens. However, organisms belonging to Eimeria rapidly develop resistance to drugs, and an increasing concern of consumers about the drugs used to control has prompted development of alternative control strategies against avian coccidiosis. The new approaches include the use of natural products, probiotics, improved farm management practices, and modulation of the immune system of chicken [3,5].
To develop a natural herbal antimicrobial additive, we performed a screening study of over 1,000 kinds of herbal materials. Previously, we performed antibacterial and antiviral studies to evaluate the effect of the medicinal herbal extracts on pathogenic bacteria and viruses. We found that GR, a herbal antimicrobial compound, had very strong antibacterial and antiviral effects [8-10]. Assessment of host disease susceptibility to avian coccidiosis has been evaluated by counting the number of fecal oocysts and body weight changes after challenge infection with live coccidia parasites [16,17]. In this study, anticoccidial effects of the GR extract were evaluated in chickens after oral infection with E. tenella. Our results showed that GR-fed chickens produced significantly lower number of fecal oocysts (P<0.05) than the E. tenella-infected chicken fed the standard diet. In addition, GR-based diet improved body weight loss caused by E. tenella infection. Furthermore, PCR analysis revealed no E. tenella oocysts in the feces of GR-fed chickens.
Our data showed that GR extracts had remarkable anticoccidial activities against E. tenella. This finding might have implications for development of a new anticoccidial drug. To our knowledge, this is the first study that showed the anticoccidial effect of GR on Eimeria parasites.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021940).
References
- 1.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006;5(1):143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PA, Fairbairn D. Biochemistry of sporulation in oocysts of Eimeria acervulina. J Protozool. 1961;8(4):410–416. [Google Scholar]
- 3.Dalloul RA, Lillehoj HS. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49(1):1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- 4.Min W, Dalloul RA, Lillehoj HS. Application of biotechnological tools for coccidia vaccine development. J Vet Sci. 2004;5(4):279–288. [PubMed] [Google Scholar]
- 5.Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15(1):58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman HD. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26(2):221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 7.Williams RB. Fifty years of anticoccidial vaccines for poultry (1952-2002) Avian Dis. 2002;46(4):775–802. doi: 10.1637/0005-2086(2002)046[0775:FYOAVF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Hong SH, Song J, Son MW, Kim O. The effects of natural herbal antibacterial compound against enteropathogenic Escherichia coli. Lab Anim Res. 2008;24(3):341–346. [Google Scholar]
- 9.Lee HA, Park H, Kim YC, Kim O. The effects of natural herbal antiviral compound against porcine epidemic diarrhea virus. Lab Anim Res. 2008;24(2):179–185. [Google Scholar]
- 10.Lee HA, Hwang KK, Song JH, Kim O. The effects of an herbal antimicrobial feed additive on the health status and performance of growing and finishing pigs. Lab Anim Res. 2008;24(2):187–192. [Google Scholar]
- 11.An RB, Oh H, Kim YC. Phenolic constituents of galla Rhois with hepatoprotective effects on tacrine- and nitrofurantoin-induced cytotoxicity in Hep G2 cells. Biol Pharm Bull. 2005;28(11):2155–2157. doi: 10.1248/bpb.28.2155. [DOI] [PubMed] [Google Scholar]
- 12.Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, Shin DW, Kwon DY. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules. 2009;14(5):1773–1780. doi: 10.3390/molecules14051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HA, Hong S, Chung Y, Kim O. Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res. 2011;27(3):255–258. doi: 10.5625/lar.2011.27.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDougald LR. Protozoal infections. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of Poultry. Ames: Iowa State Press; 2003. pp. 973–991. [Google Scholar]
- 15.Stotish RL, Wang CC, Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol. 1978;64(6):1074–1081. [PubMed] [Google Scholar]
- 16.Chapman HD, Matsler PL, Muthavarapu VK, Chapman ME. Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts. Avian Dis. 2005;49(3):426–429. doi: 10.1637/7359-032805R1.1. [DOI] [PubMed] [Google Scholar]
- 17.Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. Adjuvant effects of IL-1beta, IL-2, IL-8, IL-15, IFN-alpha, IFN-gamma TGF-beta4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine. 2001;20(1-2):267–274. doi: 10.1016/s0264-410x(01)00270-5. [DOI] [PubMed] [Google Scholar]