Abstract
Objective
To compare survival outcomes and treatment-related morbidities between radical hysterectomy (RH) and primary chemoradiation therapy (CRT) in patients with bulky early-stage cervical cancer.
Methods
We selected 215 patients with stage IB2 and IIA2 cervical cancer (tumor diameter > 4 cm on magnetic resonance imaging) who underwent RH followed by tailored adjuvant therapy (n=147) or primary CRT (n=68) at two tertiary referral centers between 2001 and 2010.
Results
About twenty nine percent of patients were cured by RH alone and these patients experienced the best survival outcomes with the lowest morbidity rates. After the median follow-up times of 40 months, 27 RH (18.4%) and 20 CRT (29.4%) patients had recurrence (p=0.068) and 23 (15.6%) and 17 (25%) patients died of disease (p=0.101). The 5-year progression-free survival were 77% and 66% (p=0.047), and the 5-year overall survival were 78% and 67% (p=0.048) after RH and primary CRT, respectively. In multivariate analysis, patients who received primary CRT was at higher risk for tumor recurrence (odds ratio [OR], 2.26; 95% confidence interval [CI], 1.24 to 4.14; p=0.008) and death (OR, 3.02; 95% CI, 1.53 to 5.98; p=0.001) than those who received RH. Grade 3-4, early (17% vs. 30.9%, p=0.021) and late (1.4% vs. 8.8%, p=0.007) complications were significantly less frequent after RH than primary CRT.
Conclusion
Thirty percent of patients were cured by RH alone. A treatment outcome was better in this retrospective study in terms of morbidity and survival. Randomized trials are needed to confirm this result.
Keywords: Bulky early-stage cervical cancer, Chemoradiation therapy, Radical hysterectomy, Stage IB2, Stage IIA2
INTRODUCTION
Cervical cancer is the third most common female cancer and the fourth leading cause of cancer death in women worldwide [1]. It is the sixth most common female cancer and the seventh leading cause of cancer death in Korea [2,3]. At present, radical hysterectomy (RH) followed by tailored adjuvant therapy and primary chemoradiation therapy (CRT) are the most frequent treatments employed for patients with bulky early-stage (stage IB2 and IIA2) cervical cancer [4]. However, the optimal treatment modality in these patients remains unclear.
Only a single randomized controlled trial reported to date has compared RH followed by tailored adjuvant therapy with primary radiation therapy (RT) in patients with early-stage cervical cancer [5]. However, as the cited trial included only 40 patients with bulky early-stage cervical cancer in each treatment group, and was conducted before the era of CRT, the results thereof cannot be generalized to all patients with bulky early-stage cervical cancer. To the best of our knowledge, only two small retrospective case-control studies have compared RH followed by tailored adjuvant treatment with primary CRT in patients with bulky early-stage cervical cancer [6,7]. The survival outcomes were similar in these studies [5-7]. However, recent larger series suggested that patients who underwent RH followed by tailored adjuvant therapy experienced better survival outcomes compared to primary CRT [4,8,9]. Moreover, it has been suggested by investigators in the USA that RH followed by tailored adjuvant therapy is potentially the most cost-effective treatment strategy for patients with bulky early-stage cervical cancer compared to other treatment strategies including CRT [10,11], indicating that the role of RH in such patients should be re-evaluated. It is necessary to clarify whether RH followed by tailored adjuvant therapy, or primary CRT, is the better treatment modality in such patients. We therefore compared survival outcomes and treatment-related morbidities in patients with bulky early-stage cervical cancer who underwent RH followed by tailored adjuvant therapy, and those who received primary CRT.
MATERIALS AND METHODS
1. Study population
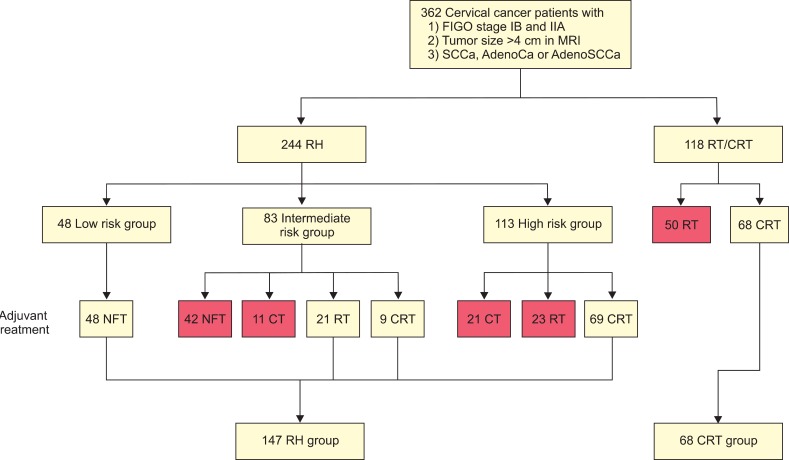
We retrospectively searched the records of two tertiary cancer centers located in Seoul, Korea (Asan Medical Center and Samsung Medical Center) and identified all consecutive patients with stage IB2 or IIA2 cervical cancer who underwent RH followed by tailored adjuvant therapy, or primary chemoradiation therapy. Patients were included if they had: 1) histologically confirmed cervical cancer of stage IB2 or IIA2 according to the International Federation of Obstetrics and Gynecology (FIGO) staging system revised in 2009 [12]; 2) a tumor diameter >4 cm on magnetic resonance imaging (MRI); and 3) squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Patients with small cell neuroendocrine carcinomas, those with occult cervical cancer detected after simple hysterectomy, and those who received neoadjuvant chemotherapy, were excluded. Because current standard tailored adjuvant therapy after RH is RT or CRT for intermediate risk groups [13] and CRT for high risk group [14], patients who did not receive RT or CRT after RH in intermediate risk group and patients who did not receive CRT after RH in high risk group were excluded. Fig. 1 shows the patient flow for this study. Of the 362 patients who were evaluated for the eligibility criteria of this study, 147 patients underwent RH and 68 patients received primary CRT. Demographic and clinicopathological data were gathered from medical records of the patients. The study protocol was approved by the Institutional Review Board of each center.
Fig. 1.
Patient flow. Red box, excluded data. AdenoCa, adenocarcinoma; AdenoSCCa, adenosquamous carcinoma; CRT, chemoradiation therapy; CT, chemotherapy; FIGO, International Federation of Obstetrics and Gynecology; MRI, magnetic resonance imaging; NFT, no further treatment; RH, radical hysterectomy; RT, radiation therapy; SCCa, squamous cell carcinoma.
2. Definitions
Intermediate risk group after RH was defined according to Gynecologic Oncology Group (GOG) protocol 92 [13]. High risk group after RH was defined as patients with parametrial involvement, lymph node metastasis, or positive resection margin [14]. Recurrence-free survival (RFS) was defined as the time, in months, from the date of RH or CRT, to the date of first documented recurrence, death or date of last contact. Overall survival (OS) was defined as the time, in months, from the date of RH or CRT to the date of death; or for living patients, the date of last contact regardless of whether or not this contact is on a subsequent protocol. All documented treatment-related toxicities were graded using the Radiation Therapy Oncology Group (RTOG) criteria and the NCI Common Toxicity Criteria for Adverse Events (CTCAE, version 3.0). Toxicities observed within 4 weeks of treatment were categorized as early complication, whereas those occurring later were considered to be late complication.
3. Radical hysterectomy
All patients underwent Piver-Rutledge type 3 hysterectomy with pelvic and/or para-aortic lymphadenectomy [15]. According to the pathologic risk factors, 48 (20.2%), 30 (33.8%), and 69 (46%) patients were low risk, intermediate risk, and high risk group, respectively. None of 48 patients in low risk group received adjuvant therapy. Of 30 patients in intermediate-risk group, 21 patients (13.1%) and 9 patients (26.2%) received adjuvant RT and CRT, respectively (Fig. 1). Of the 69 patients in high-risk group, received adjuvant CRT (Fig. 1). The radiation dose ranged from 4,010 to 5,040 cGy in patients who received adjuvant RT or CRT. No one received intracavitary brachytherapy or parametrial booster dose. Chemotherapy regimen in patients who received CRT consisted of weekly cisplatin in 30 patients, 5-fluorouracil/cisplatin in 36 patients, or paclitaxel/cisplatin in 12 patients.
4. Primary chemoradiation therapy
Patients received external pelvic RT (radiation dose range, 4,140 to 5,040 Gy), intracavitary brachytherapy (radiation dose range, 3,000 to 3,500 cGy), and parametrial booster dose (radiation dose range, 540 to 1,200 cGy). All patients received concurrent chemotherapy during external beam RT consisted of weekly cisplatin in 52 patients, 5-fluorouracil/cisplatin in 10 patients, or paclitaxel/cisplatin in 6 patients.
5. Statistical analysis
Oncologic outcomes and treatment-related complications were compared between patients who underwent RH and those who received CRT. Mean values in the two groups were compared using Student's t-test or the Mann-Whitney U-test. Frequency distributions were compared using the chi-squared test or Fisher's exact test. RFS and OS were estimated using the Kaplan-Meier method and group data were compared using Cox's proportional hazards models. The data were initially compared using univariate analysis, and all variables significant in this exercise were included in multivariate analysis, again using Cox's proportional hazards method. Two-sided p-values <0.05 were regarded as significant. All statistical analyses were performed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patients' characteristics
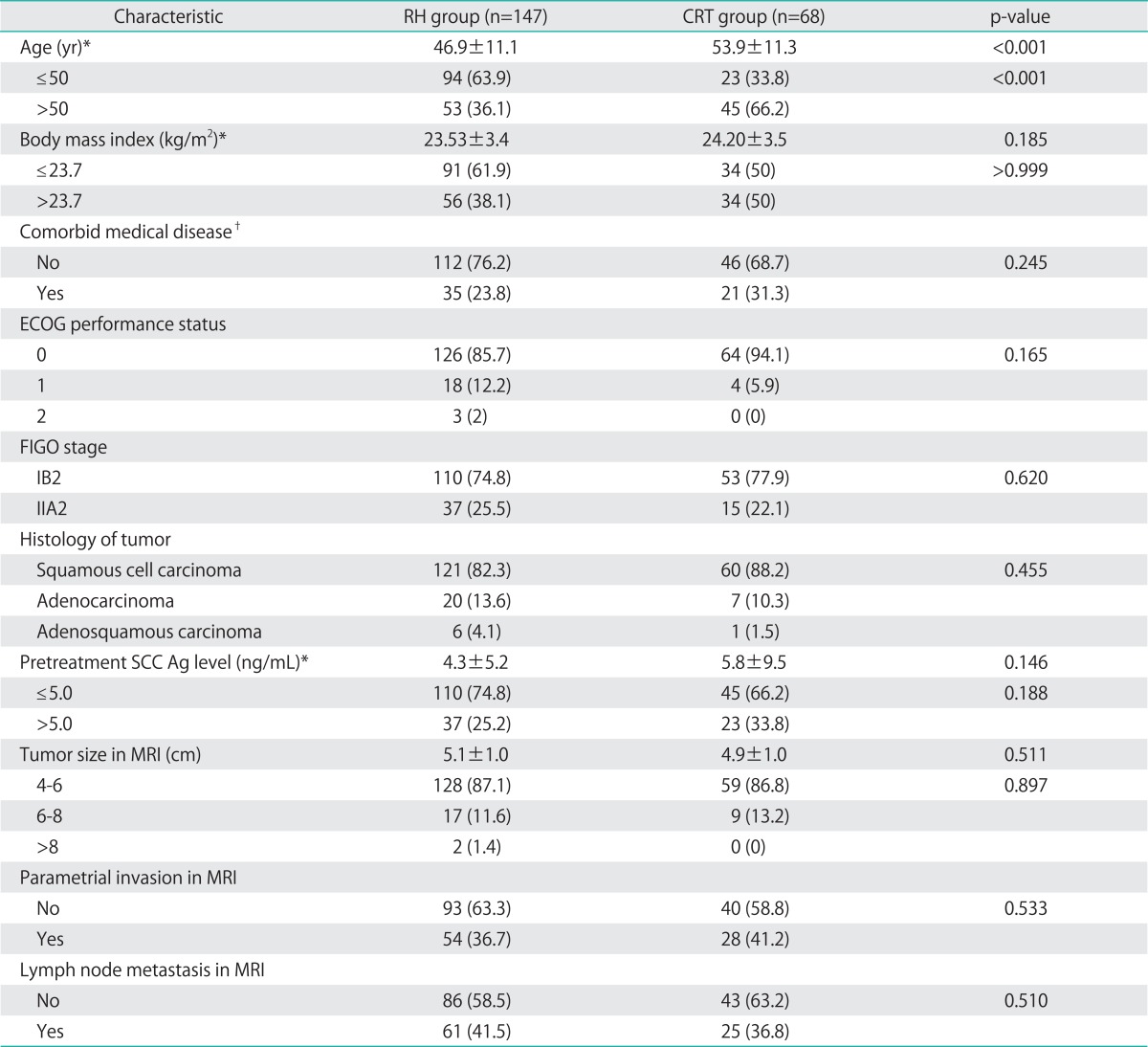
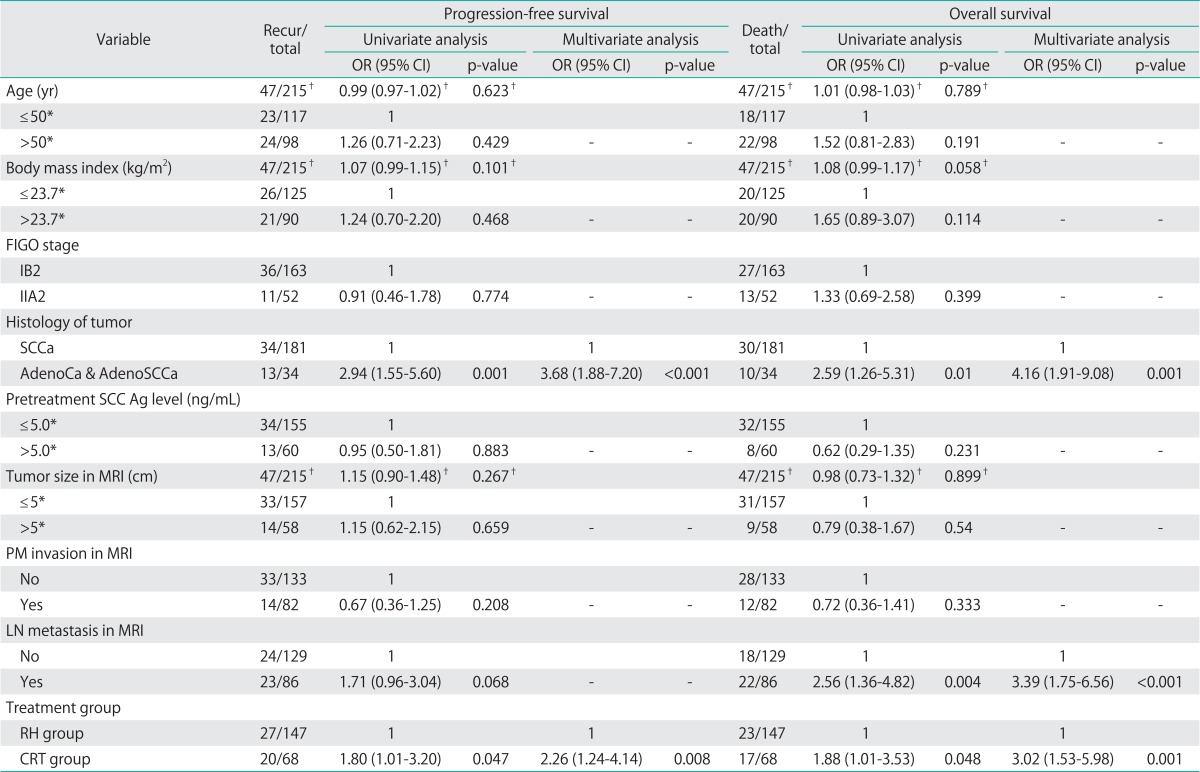
Table 1 shows the demographic and clinical characteristics of the 215 patients. Mean patient age was significantly higher in the CRT group (46.9 vs. 53.9 years, p<0.001), but there was no between-group difference in the body mass index (BMI), the presence of comorbid medical disease, Eastern Cooperative Oncology Group (ECOG) performance status, FIGO stage, tumor histology, pretreatment serum SCC Ag concentration, mean tumor diameter, tumor diameter distribution as assessed using 2 cm intervals, parametrial invasion, or lymph node metastasis on MRI.
Table 1.
Characteristics of patients (n=215)
Values are presented as number (%) or mean±SD.
CRT, chemoradiation therapy; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Obstetrics and Gynecology; MRI, magnetic resonance imaging; RH, radical hysterectomy; SCC Ag, squamous cell carcinoma antigen; SD, standard deviation.
*Divided by mean values. †Hypertension, diabetes mellitus, hyperthyroidism, hypothyroidism, chronic liver disease.
2. Oncologic outcomes
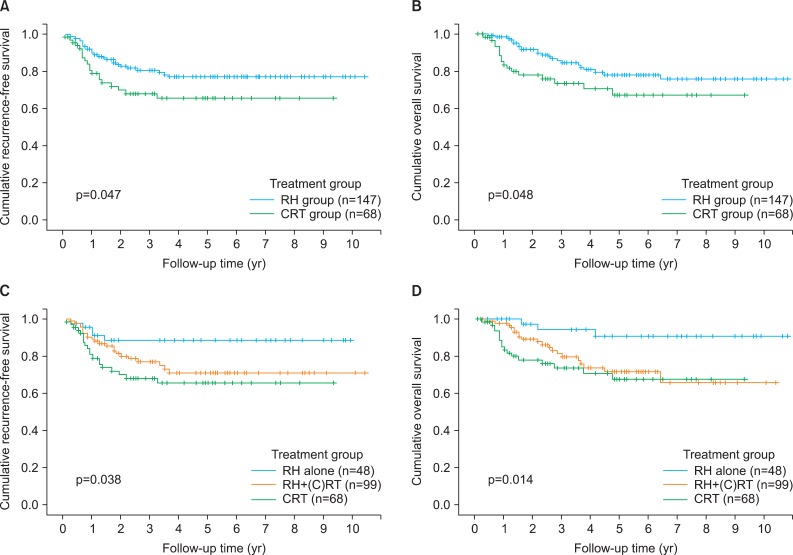
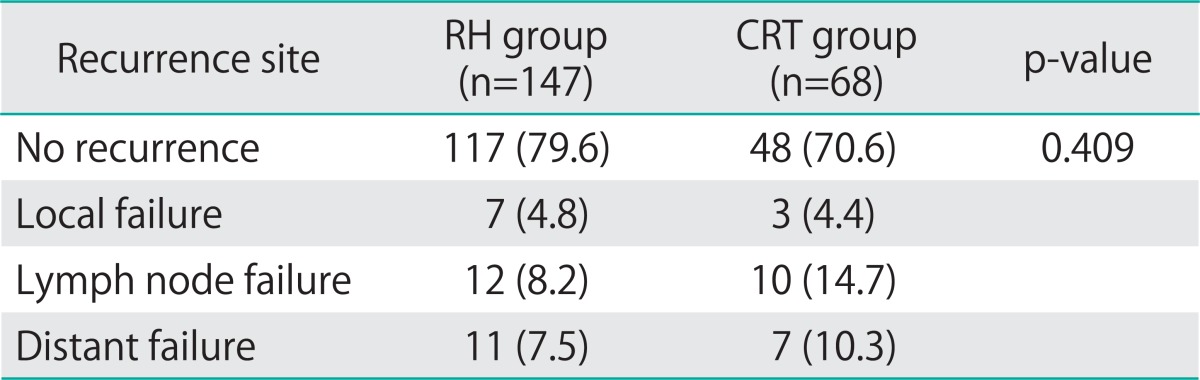
The mean and median follow-up times were 46 and 40 months (range, 3 to 130 months), respectively, for all patients; 47 and 40 months (range, 3 to 130 months), respectively, for patients in the RH group; and 42 and 40 months (range, 3 to 112 months), respectively, for patients in the CRT group (p=0.253). Disease recurrence was observed in 27 RH (18.4%) and 20 CRT (29.4%) patients (p=0.068); 23 (15.6%) and 17 (15.6%) patients, respectively, died of disease (p=0.101). The 5-year RFS rates were 77% in the RH group and 66% in the CRT group (p=0.047) (Fig. 2A); the 5-year OS rates were 78% in the RH group, and 67% in the CRT group (p=0.048) (Fig. 2B). The pattern of recurrence was similar between RH group and CRT group (p=0.409) (Table 2).
Fig. 2.
(A, C) Recurrence-free survival and (B, D) overall survival by treatment group in 215 patients with bulky early-stage cervical cancer. CRT, chemoradiation therapy, (C)RT, (chemo) radiation therapy; RH, radical hysterectomy.
Table 2.
Pattern of recurrence at first recurrence (n=215)
CRT, chemoradiation therapy; RH, radical hysterectomy.
By univariate analysis, all of histologic type, and treatment group, were significantly associated with RFS; whereas none of age, BMI, the presence of comorbid medical disease, FIGO stage, pretreatment serum SCC Ag concentration, tumor size measured on MRI, parametrial invasion, or lymph node metastasis as shown on MRI was so associated (Table 3). All of histologic type, lymph node metastasis as shown on MRI, and treatment group, were significantly associated with OS; whereas none of age, BMI, the presence of comorbid medical disease, FIGO stage, pretreatment serum SCC Ag concentration, tumor size measured on MRI, or parametrial invasion as shown on MRI showed a significant association (Table 3). Multivariate analysis revealed that, after adjusting for histologic type, CRT was associated with a significantly higher risk of recurrence (odds ratio [OR], 2.26; 95% confidence interval [CI], 1.24 to 4.14; p=0.008) (Table 3). Moreover, after adjusting for histologic type and lymph node metastasis as shown on MRI, multivariate analysis showed that CRT was associated with a significantly higher risk of death (OR, 3.02; 95% CI, 1.53 to 5.98; p=0.001) (Table 3).
Table 3.
Univariate and multivariate analysis of progression-free survival and overall survival of patients who received guideline treatment (n=215)
AdenoCa, adenocarcinoma; AdenoSCCa, adenosquamous carcinoma; CRT, chemoradiation therapy; FIGO, International Federation of Obstetrics and Gynecology; LN, lymph node; MRI, magnetic resonance imaging; RH, radical hysterectomy; SCCa, squamous cell carcinoma; SCC Ag, squamous cell carcinoma antigen; PM, parametrium.
*Dichotomous variables were divided by mean values. †As a continuous variable.
When dividing patients into three groups: those who underwent RH alone (n=48), those who underwent RH+(chemo) radiation therapy ((C)RT) (n=99), and those who received primary CRT (n=68), RH alone group had significantly better RFS and OS compared to RH+(C)RT group (p=0.012 for DFS, p=0.003 for OS) and CRT group (p=0.080 for DFS, p=0.020 for OS) (Fig. 2C, D). In RH alone group, 5 (10.4%) of 48 patients had recurrent disease and 3 (6.3%) of them died of disease. Therefore, 43 of 147 patients (29.3%) in RH group were cured by RH alone without RT.
3. Treatment-related complications
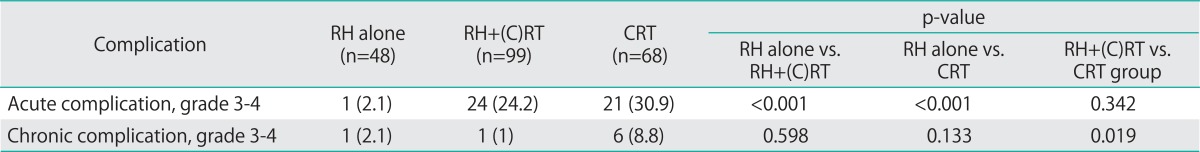
Grade 3-4, early complications were documented in 1 (2.1%), 24 (24.2%), and 21 (30.9%) patients of the RH alone group, RH+RT group, and CRT group, respectively (p=0.001) (Table 4). Grade 3-4, late complications were observed in 1 (2.1%), 1 (1%), and 6 (8.8%) patients of these groups, respectively (p=0.026) (Table 4). Lymphedema of the lower extremities was documented in 6 (12.5%), 9 (9.1%), and 1 (1.5%) patients of these groups, respectively (p=0.058).
Table 4.
Acute and chronic complications (n=215)
CRT, chemoradiation therapy; RH, radical hysterectomy.
DISCUSSION
We found that 29.3% of patients with tumors >4 cm in diameter were cured by RH alone without RT, consistent with the rates of 37-51% previously observed in patients with bulky early-stage cervical cancer [6,7,16,17]. Many more patients than expected did well after surgery alone, and such patients experienced the best survival outcomes and the lowest morbidity rates, indicating that RH continues to play a significant role in patients with bulky early-stage cervical cancer. This is one of the best advantages of RH followed by tailored adjuvant therapy because it makes the patients avoid inadvertent radiation therapy.
In our series, multivariate analysis showed that RH was associated with a significantly lower risk of recurrence (OR, 2.26; 95% CI, 1.24 to 4.14; p=0.008) and death (OR, 3.02; 95% CI, 1.53 to 5.98; p=0.001) compared to CRT. Previous studies including a randomized controlled trial suggested that RH afforded survival outcomes similar to those seen after definitive RT or CRT in patients with bulky early-stage cervical cancer [5-7]. However, the cited works included only a small numbers of patients with bulky early-stage cervical cancer, and did not evaluate important prognostic factors in survival analysis, such as lymph node metastasis status or parametrial invasion as seen on MRI. Recently, a relatively large-scaled study suggested that RH would yield better survival outcomes compared to CRT in bulky early-stage cervical cancer [4,9], and a multivariate analysis of Surveillance, Epidemiology, and End Results (SEER) data showed that RH was associated with a 49% improvement in survival compared to CRT in bulky early-stage cervical cancer [8]; an outcome consistent with our findings. This indicates that RH followed by tailored adjuvant therapy is a potentially more effective treatment modality than is CRT for patients with bulky early-stage cervical cancer. In our series, the lymph node failure rate was higher in CRT group than RH group although it was not statistically significant (14.7% vs. 8.2%). This is in good agreement with previous data [18-20]. In addition, several studies suggested that the rate of residual disease on lymph node was 43.8% to 49% after CRT followed by lymphadenectomy in patients with locally advanced cervical cancer [21-23]. We think that this may be one of the reasons for improved survival in RH group.
In our study, the 5-year survival rates differed by about 10 percentage points (78% vs. 66%, p=0.002). Such a difference may be clinically significant and should be further evaluated in randomized controlled trials that include sufficient numbers of study subjects. We have calculated that recruitment of a total of 424 patients (212 patients per group), and the occurrence of 144 events, are required to show that a 10% difference in OS by the fifth year (hazard ratio, 0.627) is statistically significant, with an alpha-value of 0.05 and a beta-value of 0.2 on two-sided tests. Assuming accrual of 85 patients/year, the study would require 10 years, 5 for accrual and 5 for follow-up. Such a trial will be launched soon by the Korean Gynecologic Oncology Group (KGOG 1029). For successful surgical treatment for bulky early-stage cervical cancer in this trial, the radicality of surgery is of paramount importance and surgical procedures should be standardized. To achieve surgical radicality and standardize the surgical procedures, live surgery workshops on laparoscopic radical hysterectomy was held in each center by turns among KGOG affiliated hospitals for the past ten years.
Earlier studies have suggested that use of a combination of RH and RT was associated with the highest morbidity rates compared to RH alone group and CRT group, including serious toxicity frequencies >20% [5,6,24]. Physicians were reluctant to perform RH in patients with bulky early-stage cervical cancer based on these results. However, recent other reports found that the rates of serious toxicity were lower after RH+RT; the rate of toxicity of grades 3-4 was only 7%, being 2% and 3% in terms of gastrointestinal and genitourinary complications, respectively [13,16,25]. When we evaluated the occurrence of grade 3-4 toxicities that required treatment, we found that the rates of grade 3-4 early complications were not different between RH+RT group and CRT group (24.2% vs. 30.9%, p=0.342). Rather, the rates of grade 3-4 late complications was lower in RH+RT group compared to CRT group although the difference was not statistically significant (1% vs. 8.8%, p=0.019). Most complications were associated with radiation therapy per se. Because adjuvant RT in RH+RT group features only external pelvic RT, at doses of 4,140-5,040 cGy, whereas definitive RT in CRT group consists of external pelvic RT followed by intracavitary brachytherapy and parametrial boosting; the radiation dose to the bowel, bladder, and vagina was much higher in the definitive RT group. Therefore, RT-related bladder, rectal, and vaginal complication rates should be greater in this group compared to the adjuvant RT group. Because our study is a retrospective one and included study subjects which is not big enough to confirm the complication rates between the two treatment groups, the complication rates associated with each treatment modality (RH+RT group vs. CRT group) should be re-evaluated in a randomized controlled trial.
Because we assessed patients over a long period of time, during which the selection criteria for initial treatment and adjuvant therapy varied, and this study was a retrospective one, selection bias may have been present. In addition, the treatment strategies for patients with bulky early-stage cervical cancer differed among the two centers, with one favoring RH followed by tailored adjuvant therapy, and the other permitting treatment to be at the discretion of the attending physician. Therefore, this may be a possible bias. However, we tested for associations between different clinicopathological variables that could be potential confounding factors in the two treatment groups. Apart from mean age, there was no significant between-group difference in any clinicopathologic factor or potential prognostic factor, including parametrial invasion status and lymph node metastasis. Further, we employed multivariate analysis after adjusting for all other factors that could significantly impact survival. Therefore, we think that the impact of selection bias on the outcomes was minimized. The strength of this study is that this is the largest one which compared the outcomes of patients with bulky early-stage cervical cancer between RH group and CRT group and which was conducted in the era of CRT.
In conclusion, a significant proportion of patients with bulky early-stage cervical cancer were cured by RH alone. Such patients experienced the best survival outcomes and the lowest morbidity rates. RH followed by tailored adjuvant therapy resulted in a significantly better RFS and OS, and significantly lower treatment-related morbidity rates than were afforded by primary CRT in patients with bulky early-stage cervical cancer. A randomized controlled trial to compare these two treatment modalities is warranted.
Footnotes
No potential conflict of interests relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piver MS, Ghomi A. The twenty-first century role of Piver-Rutledge type III radical hysterectomy and FIGO stage IA, IB1, and IB2 cervical cancer in the era of robotic surgery: a personal perspective. J Gynecol Oncol. 2010;21:219–224. doi: 10.3802/jgo.2010.21.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu HS, Kang SB, Kim KT, Chang KH, Kim JW, Kim JH. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study (KGOG-1005) Int J Gynecol Cancer. 2007;17:132–136. doi: 10.1111/j.1525-1438.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 6.Zivanovic O, Alektiar KM, Sonoda Y, Zhou Q, Iasonos A, Tew WP, et al. Treatment patterns of FIGO Stage IB2 cervical cancer: a single-institution experience of radical hysterectomy with individualized postoperative therapy and definitive radiation therapy. Gynecol Oncol. 2008;111:265–270. doi: 10.1016/j.ygyno.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim WY, Chang SJ, Chang KH, Yoo SC, Chun M, Ryu HS. Treatment patterns and outcomes in bulky stage IB2 cervical cancer patients: a single institution's experience over 14 years. Gynecol Obstet Invest. 2011;71:19–23. doi: 10.1159/000320722. [DOI] [PubMed] [Google Scholar]
- 8.Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol. 2009;201:485.e1–485.e9. doi: 10.1016/j.ajog.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Rungruang B, Courtney-Brooks M, Beriwal S, Zorn KK, Richard SD, Olawaiye AB, et al. Surgery versus radiation therapy for stage IB2 cervical carcinoma: a population-based analysis. Int J Gynecol Cancer. 2012;22:484–489. doi: 10.1097/IGC.0b013e31823f890f. [DOI] [PubMed] [Google Scholar]
- 10.Rocconi RP, Estes JM, Leath CA, 3rd, Kilgore LC, Huh WK, Straughn JM., Jr Management strategies for stage IB2 cervical cancer: a cost-effectiveness analysis. Gynecol Oncol. 2005;97:387–394. doi: 10.1016/j.ygyno.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Jewell EL, Kulasingam S, Myers ER, Alvarez Secord A, Havrilesky LJ. Primary surgery versus chemoradiation in the treatment of IB2 cervical carcinoma: a cost effectiveness analysis. Gynecol Oncol. 2007;107:532–540. doi: 10.1016/j.ygyno.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 12.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 14.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 15.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 16.Havrilesky LJ, Leath CA, Huh W, Calingaert B, Bentley RC, Soper JT, et al. Radical hysterectomy and pelvic lymphadenectomy for stage IB2 cervical cancer. Gynecol Oncol. 2004;93:429–434. doi: 10.1016/j.ygyno.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy according to tumor size divided by 2-cm interval in patients with early cervical cancer. Ann Oncol. 2011;22:59–67. doi: 10.1093/annonc/mdq321. [DOI] [PubMed] [Google Scholar]
- 18.Beadle BM, Jhingran A, Yom SS, Ramirez PT, Eifel PJ. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76:1396–1403. doi: 10.1016/j.ijrobp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 20.Rotman M, Choi K, Guse C, Marcial V, Hornback N, John M. Prophylactic irradiation of the para-aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. Int J Radiat Oncol Biol Phys. 1990;19:513–521. doi: 10.1016/0360-3016(90)90475-y. [DOI] [PubMed] [Google Scholar]
- 21.Delpech Y, Haie-Meder C, Rey A, Zafrani Y, Uzan C, Gouy S, et al. Para-aortic involvement and interest of para-aortic lymphadenectomy after chemoradiation therapy in patients with stage IB2 and II cervical carcinoma radiologically confined to the pelvic cavity. Ann Surg Oncol. 2007;14:3223–3231. doi: 10.1245/s10434-007-9526-1. [DOI] [PubMed] [Google Scholar]
- 22.Ferrandina G, Distefano M, Ludovisi M, Morganti A, Smaniotto D, D'Agostino G, et al. Lymph node involvement in locally advanced cervical cancer patients administered preoperative chemoradiation versus chemotherapy. Ann Surg Oncol. 2007;14:1129–1135. doi: 10.1245/s10434-006-9252-0. [DOI] [PubMed] [Google Scholar]
- 23.Houvenaeghel G, Lelievre L, Rigouard AL, Buttarelli M, Jacquemier J, Viens P, et al. Residual pelvic lymph node involvement after concomitant chemoradiation for locally advanced cervical cancer. Gynecol Oncol. 2006;102:74–79. doi: 10.1016/j.ygyno.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Berveling MJ, Langendijk JA, Beukema JC, Mourits MJ, Reyners AK, Pras E. Health-related quality of life and late morbidity in concurrent chemoradiation and radiotherapy alone in patients with locally advanced cervical carcinoma. J Gynecol Oncol. 2011;22:152–160. doi: 10.3802/jgo.2011.22.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruen A, Musik T, Kohler C, Fuller J, Wendt T, Stromberger C, et al. Adjuvant chemoradiation after laparoscopically assisted vaginal radical hysterectomy (LARVH) in patients with cervical cancer: oncologic outcome and morbidity. Strahlenther Onkol. 2011;187:344–349. doi: 10.1007/s00066-011-2197-7. [DOI] [PubMed] [Google Scholar]