Abstract
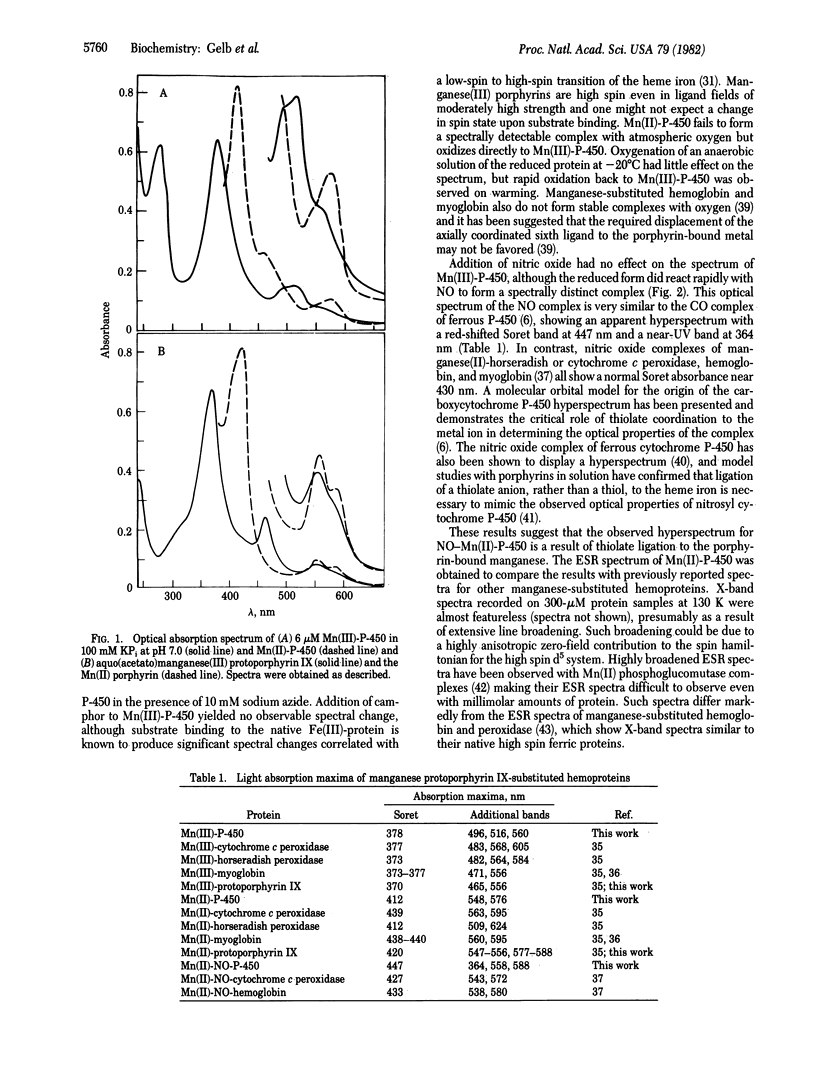
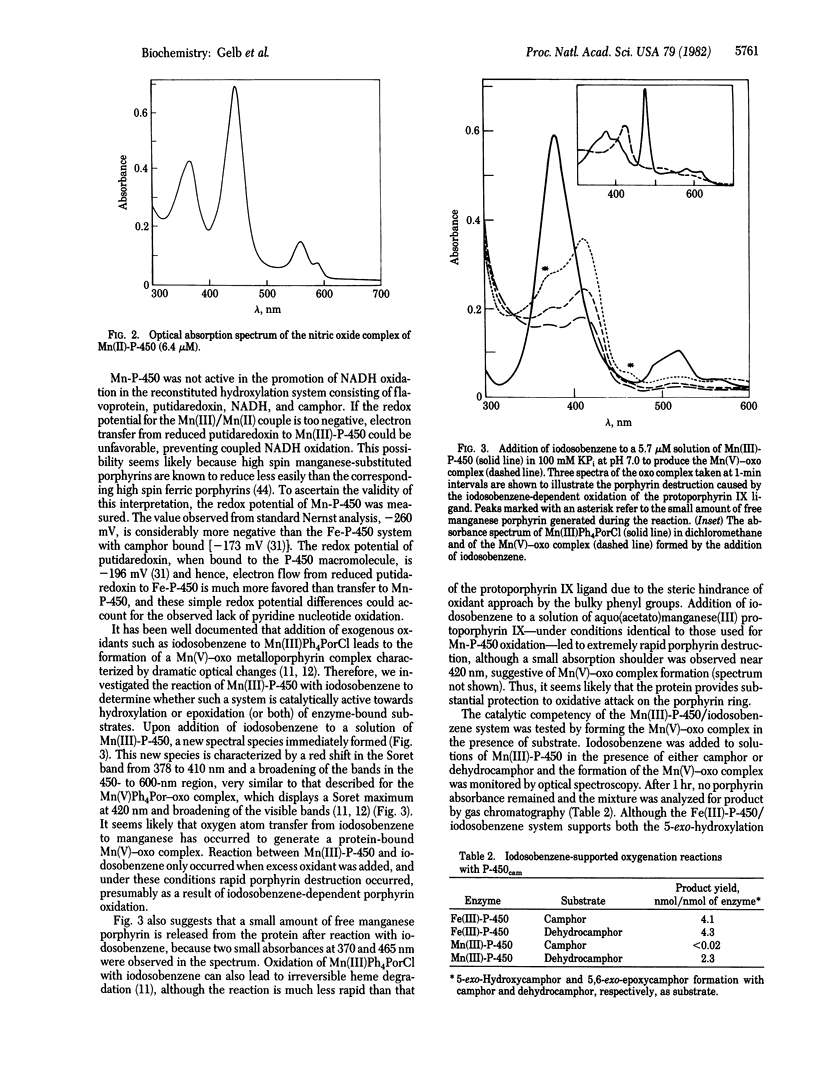
Bacterial cytochrome P-450 induced by camphor (P-450cam) is reconstituted with manganese-protoporphyrin IX, yielding an enzyme that displays unique spectral properties relative to previously characterized manganese-porphyrin systems. The nitric oxide complex of the manganese(II)-protein shows a hyper-metalloporphyrin spectrum suggestive of thiolate ligation to the porphyrin-bound manganese ion. In the presence of iodosobenzene as a source of active oxygen, manganese-substituted cytochrome P-450cam serves as a catalyst for the epoxidation of an enzyme-bound olefin substrate. This reactivity proceeds through a spectrally detectable intermediate that resembles the manganese(V)-oxo complexes that have been well documented with model systems employing artificial manganese-metalloporphyrins in organic solution. Interestingly, manganese-substituted cytochrome-P-450cam shows no hydroxylation activity either in the reconstituted camphor hydroxylase system with pyridine nucleotide or in the presence of iodosobenzene and the Mn(III) form of the protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson J. H., Holm R. H., Trudell J. R., Barth G., Linder R. E., Bunnenberg E., Djerassi C., Tang S. C. Letter: Oxidized cytochrome P-450. Magnetic circular dichroism evidence for thiolate ligation in the substrate-bound form. Implications for the catalytic mechanism. J Am Chem Soc. 1976 Jun 9;98(12):3707–3708. doi: 10.1021/ja00428a054. [DOI] [PubMed] [Google Scholar]
- Ebel R. E., O'Keefe D. H., Peterson J. A. Nitric oxide complexes of cytochrome P-450. FEBS Lett. 1975 Jul 15;55(1):198–201. doi: 10.1016/0014-5793(75)80991-4. [DOI] [PubMed] [Google Scholar]
- Gelb M. H., Malkonen P., Sligar S. G. Cytochrome P450cam catalyzed epoxidation of dehydrocamphor. Biochem Biophys Res Commun. 1982 Feb 11;104(3):853–858. doi: 10.1016/0006-291x(82)91327-4. [DOI] [PubMed] [Google Scholar]
- Groves J. T., McClusky G. A. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. Evidence for a carbon radical intermediate. Biochem Biophys Res Commun. 1978 Mar 15;81(1):154–160. doi: 10.1016/0006-291x(78)91643-1. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Rondahl L., Bergman J. Iodosylbenzene derivatives as oxygen donors in cytochrome P-450 catalyzed steroid hydroxylations. Biochemistry. 1979 Mar 6;18(5):865–870. doi: 10.1021/bi00572a020. [DOI] [PubMed] [Google Scholar]
- Hanson L. K., Eaton W. A., Sligar S. G., Gunsalus I. C., Gouterman M., Connell C. R. Letter: Origin of the anomalous Soret spectra of carboxycytochrome P-450. J Am Chem Soc. 1976 Apr 28;98(9):2672–2674. doi: 10.1021/ja00425a050. [DOI] [PubMed] [Google Scholar]
- Heimbrook D. C., Sligar S. G. Multiple mechanisms of cytochrome P450-catalyzed substrate hydroxylations. Biochem Biophys Res Commun. 1981 Mar 31;99(2):530–535. doi: 10.1016/0006-291x(81)91777-0. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Gibson Q. H. Anomalous azide binding to metmanganomyoglobin. Biochemistry. 1976 Aug 10;15(16):3405–3410. doi: 10.1021/bi00661a002. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Gibson Q. H., Bull C., Crepeau R. H., Edelstein S. J., Fisher R. G., McDonald M. J. Manganese-substituted hemoglobin and myoglobin. Ann N Y Acad Sci. 1975 Apr 15;244:174–186. doi: 10.1111/j.1749-6632.1975.tb41530.x. [DOI] [PubMed] [Google Scholar]
- Lichtenberger F., Nastainczyk W., Ullrich V. Cytochrome P450 as an oxene transferase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):939–946. doi: 10.1016/0006-291x(76)90682-3. [DOI] [PubMed] [Google Scholar]
- Lipscomb J. D., Sligar S. G., Namtvedt M. J., Gunsalus I. C. Autooxidation and hydroxylation reactions of oxygenated cytochrome P-450cam. J Biol Chem. 1976 Feb 25;251(4):1116–1124. [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J. Hydroperoxide catalyzed liver microsomal aromatic hydroxylation reactions involving cytochrome P-450. Biochem Biophys Res Commun. 1974 Sep 9;60(1):440–447. doi: 10.1016/0006-291x(74)90223-x. [DOI] [PubMed] [Google Scholar]
- Reed G. H., Ray W. J., Jr Electron paramagnetic resonance studies of manganese (II) coordination in the phosphoglucomutase system. Biochemistry. 1971 Aug 17;10(17):3190–3197. doi: 10.1021/bi00793a005. [DOI] [PubMed] [Google Scholar]
- Sligar S. G. A thermoelectric cuvette cooler for optical studies in cryobiochemistry. Cryobiology. 1979 Oct;16(5):506–508. doi: 10.1016/0011-2240(79)90066-x. [DOI] [PubMed] [Google Scholar]
- Sligar S. G., Gunsalus I. C. A thermodynamic model of regulation: modulation of redox equilibria in camphor monoxygenase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1078–1082. doi: 10.1073/pnas.73.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. O., Peisach J. A model compound for nitrosyl cytochrome P-450; further evidence for mercaptide sulfur ligation to heme. FEBS Lett. 1976 Mar 1;62(3):364–368. doi: 10.1016/0014-5793(76)80095-6. [DOI] [PubMed] [Google Scholar]
- Stern J. O., Peisach J. A model compound study of the CO-adduct of cytochrome P-450. J Biol Chem. 1974 Dec 10;249(23):7495–7498. [PubMed] [Google Scholar]
- Wagner G. C., Perez M., Toscano W. A., Jr, Gunsalus I. C. Apoprotein formation and heme reconstitution of cytochrome P-450cam. J Biol Chem. 1981 Jun 25;256(12):6262–6265. [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Asakura T. Studies on cytochrome c peroxidase. XI. A crystalline enzyme reconstituted from apoenzyme and manganese protoporphyrin IX. J Biol Chem. 1968 Jul 25;243(14):3996–3998. [PubMed] [Google Scholar]
- Yonetani T., Asakura T. Studies on cytochrome c peroxidase. XV. Comparison of manganese porphyrin-containing cytochrome c peroxidase, horseradish peroxidase, and myoglobin. J Biol Chem. 1969 Sep 10;244(17):4580–4588. [PubMed] [Google Scholar]
- Yonetani T., Drott H. R., Leigh J. S., Jr, Reed G. H., Waterman M. R., Asakura T. Electromagnetic properties of hemoproteins. 3. Electron paramagnetic resonance characteristics of iron (III) and manganese (II) protoporphyrins IX and their apohemoprotein complexes in high spin states. J Biol Chem. 1970 Jun 10;245(11):2998–3003. [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr, Reed G. H. Electromagnetic properties of hemoproteins. V. Optical and electron paramagnetic resonance characteristics of nitric oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972 Apr 25;247(8):2447–2455. [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Cytochrome P-450cam. III. Removal and replacement of ferriprotoporphyrin IX. J Biol Chem. 1974 Jan 10;249(1):107–110. [PubMed] [Google Scholar]



