Abstract
Background:
The possibility of impaired antioxidant status and so increased oxidative damage in periodontal disease is being conjectured. The present randomized controlled study was carried out with the objective of analyzing the activity of superoxide dismutase enzyme and thiol antioxidants in gingival crevicular fluid (GCF) and saliva as indicators of response to periodontal therapy.
Materials and Methods:
Subjects were screened and randomly divided into three groups: 23 periodontally healthy controls, 24 with gingivitis, and 23 with periodontitis. Based on the clinical attachment levels, the periodontitis group was further divided into subgroups, including mild, moderate, and severe periodontitis. GCF and saliva samples were collected for estimation of superoxide dismutase and thiol antioxidant concentrations at baseline and 15 days after nonsurgical treatment. Intragroup comparisons were statistically analyzed using repeated measures analysis of covariance (P value <0.05).
Results:
Superoxide dismutase was present in greater quantities in the GCF compartment (100.32±3.67 U/0.5 mL) than in saliva (39.99±3.52 U/0.5 mL), with elevated levels in mild and moderate subgroups as compared with severe periodontitis. Thiol concentrations were comparable in these media, 14.43±1.57 micromol /L in GCF and 15.09±2.26 micromol/L in saliva. Following treatment, superoxide dismutase and thiol antioxidant concentrations significantly improved in all the patient groups.
Conclusion:
The reduction of the inflammatory response following therapy resulted in improved antioxidant profiles in both the GCF and salivary compartments.
Keywords: Antioxidants, gingival crevicular fluid, gingivitis, periodontitis, saliva, superoxide dismutase
INTRODUCTION
Periodontal disease is widely believed to be initiated by microbial interaction, which triggers a host response by setting off an inflammatory reaction. Neutrophils are the key cells involved in the series of events, which includes release of inflammatory mediators and reactive oxygen species (ROS). ROS include oxygen-derived free radicals, such as superoxide (O2−), hydroxyl (OH), nitric oxide, hydrogen peroxide, and hypochlorous acid (HOCl).[1] Varieties of these molecules appear in the inflamed tissues and are capable of damaging lipids, proteins, and deoxyribonucleic acid, ultimately leading to tissue destruction. This oxidative stress phenomenon is believed in part to be responsible for the inflammatory conditions affecting the periodontium, manifesting as gingivitis and periodontitis.
Antioxidants are groups of substances that are able to prevent the oxidation of substrate by these ROS, thereby offering protection. Currently, there is growing interest in the linkage between antioxidants and periodontal disease. A significant antioxidant enzyme within mammalian tissues is superoxide dismutase, which catalyzes the dismutation of O2− to H2O2 and O2.[2] Superoxide dismutase has also been localized within the human periodontal ligament and may represent an important defense mechanism within gingival cells against superoxide release.[3] Na et al. found markedly increased levels of Mn–superoxide dismutase in inflamed gingival tissue compared with healthy gingiva indicating superoxide dismutase activity increases with the progression of inflammation.[4]
The thiol antioxidants are reduced glutathione, glutaredoxin, and N-acetyl cysteine. Thiols are part of the intraprotein structure and exist in equilibrium with the disulfide group. They prevent the irreversible unfolding of the protein structure due to oxidative stress. Chapple observed that the detection of a low molecular weight thiol in gingival crevicular fluid (GCF) is an important defense mechanism against unwanted ROS-mediated damage.[5]
However, reports on the relationship between antioxidant status and periodontitis have not been consistent. Moore et al. observed no difference in the amount of total antioxidants in the saliva of periodontally healthy and periodontitis subjects.[6]
The purpose of this study was to investigate the role of intrinsic antioxidants, superoxide dismutase enzyme and thiol, in the periodontal environment, their variation in different stages of periodontal disease and how nonsurgical treatment can have an impact on the antioxidant profile.
MATERIALS AND METHODS
Patients referred to the outpatient department of Periodontics at the Manipal College of Dental Sciences in Manipal, India, for treatment, were considered for participation in the study. An informed consent was obtained from those willing to participate in the study. Ethical clearance for the study was obtained by the Kasturba Hospital Ethical Committee at Manipal in India prior to the commencement of the study.
Seventy healthy volunteers were selected for the study, which included 39 males and 31 females, within the age range of 20–55 years. The periodontal status of the subjects was determined by measuring the Plaque Index (PI) (Silness and Loe, 1964),[7] Gingival Index (GI) (Loe and Silness, 1963)[8], pocket probing depths, and clinical attachment levels. The subjects were categorized based on the following criteria: periodontally healthy patients had no attachment loss, no probing depths more than 3 mm at any site, and GI score of less than 1. Subjects with gingivitis had a GI score > 1, probing depth < 4 mm, and absence of bone loss radiographically. Periodontitis was considered if at least two nonadjacent sites per quadrant with probing pocket depth > 5 mm were present and demonstrated radiographic bone loss. Following clinical examination, data were recorded and patients were allocated into different groups. Twenty-four patients were categorized as having gingivitis, 23 patients were diagnosed as having periodontitis and 23 patients were periodontally healthy subjects. The periodontitis group was further classified based on the clinical attachment loss as mild (1–2 mm), moderate (3–4 mm), and severe (≥5 mm) subgroups.
Patients who were on anti-inflammatory, antimicrobial therapy, or mouth rinses within the past 3 months, previous or current smokers, pregnant and lactating women, and those on dietary supplements were excluded from the study.
Initially, salivary samples were collected from the patients, followed by sampling of the GCF. Subsequent to sampling, thorough scaling and root planing (wherever necessary) was performed and the patients were given appropriate oral hygiene instructions. Patients were recalled 15 days post-treatment for reassessment of biochemical and clinical parameters. Salivary and GCF samples were obtained and stored till analysis. The antioxidant concentrations were analyzed using spectrophotometric assay.
Sampling of Saliva
Whole saliva was collected in glass beakers and transferred into Eppendorf tubes and centrifuged at 503 × g at 4°C for 5 min. The supernatant was separated and stored at −80°C until analysis.
Gingival crevicular fluid sampling
Sampling was performed between 8:00 and 10:00 am. The area was isolated with cotton rolls with attention to eliminate salivary contamination, and the site gently air dried. The samples were collected by standardized Periopaper strips using Brill's (1962)[9] intrasulcular technique. The strips were inserted into the pockets until a slight resistance was felt and held in the sulci for 30 s with delicate care to avoid irritation of pocket/sulcus epithelium. Any paper contaminated with blood was discarded and collection was repeated in another point. To ensure sufficient assay sensitivity, 12 strips were used for each arch. The GCF strips were pooled in:
1 mL Tris–HCl buffer (pH 6.5) and eluted for 30 min and stored till analysis for superoxide dismutase assay.
500 μL phosphate buffer saline eluted for 30 min and stored till analysis for protein thiol assay.
Superoxide dismutase assay
Superoxide dismutase activity was analyzed by the reduction of nitroblue tetrazolium (NBT) by superoxide, which formed formazan and detected spectrometrically at 560 nm using Genesys 10 UV and expressed in terms of U/mL.[10]
Illumination of riboflavin in the presence of O2 and electron donors, such as methionine generates superoxide radicals, which have been used as the basis for this assay and the reduction of NBT by O2− was followed at 560 nm.
Reagents
Phosphate-buffered saline: 0.01 M phosphate buffer at pH 7.4 to which 0.15 M NaCl is added.
Potassium phosphate buffer: 0.2 M [pH 7.8].
NBT: 1 mg/mL in 0.05 M phosphate buffer.
Riboflavin: 1 mg/mL in 0.05 M phosphate buffer.
Superoxide dismutase substrate: 37.2 mg of methionine and 0.16 mL riboflavin was added to 23.6 mL of 0.05 mL phosphate buffer. This made up the total volume to 23.77 mL. The contents were then equally divided as 2 parts of 11.88 mL. To the first part, 0.62 mL of buffer was added making the volume to 12.5 mL, which would be the superoxide dismutase substrate without NBT. To the second part, 0.62 mL of NBT solution was added making the volume to 12.5 mL, which would serve as superoxide dismutase substrate with NBT. The volume in both the cases was then made up to 100 mL with double distilled water. The superoxide dismutase substrates were freshly prepared every time and during the length of the experiment, kept in a brown bottle to avoid exposure to sunlight.
Procedure
Pretreatment of sample: 0.5 mL of gingival fluid/saliva was mixed with 0.5 mL of water and 0.25 mL of ethanol was added followed by the addition of 0.15 mL of chloroform. The resultant mixture was mixed in the vortex and kept in the ice chest for 15 min. Then 0.1 mL of water was added to make up the volume to 1.5 mL. The resultant mixture was centrifuged at 224 × g for 15 min using Baxter Heraeus Sepatech (Biofuge A model).
Since the exact amount of superoxide dismutase in the biologic sample is unknown and there are no normal limits defined, two suspensions were produced by varying the sample concentrations using the supernatant formed after centrifugation.
Suspension 1 was made by taking 0.1 mL supernatant and making the volume to 2 mL by adding 1.9 mL of water. From this suspension 1, Test 1 was prepared by taking 0.1 mL of suspension 1 and adding 2.9 mL superoxide dismutase substrate with NBT.
Suspension 2 was made by taking 0.5 mL of supernatant and making up the volume to 2 mL by adding 1.5 mL of water. From this suspension, Test 2 and Test 3 were made. Test 2 was made by taking 0.5 mL of suspension 2 and adding 2.5 mL of superoxide dismutase substrate with NBT. Test 3 was made by taking 0.1 mL of suspension 2 and adding 2.9 mL of superoxide dismutase substrate with NBT.
Test blanks were made by using 0.1 mL of both suspensions 1 and 2 and adding 2.9 mL of superoxide dismutase substrate without NBT.
Control and control blank were made by using 0.1 mL superoxide dismutase buffer and adding 2.9 mL of superoxide dismutase substrate with and without NBT.
Tests, test blanks, controls, and control blanks were illuminated under blue light for 10 min and then measured at 560 nm. The superoxide dismutase levels were expressed as superoxide dismutase units per 0.5 mL of biologic fluid, that is, saliva or GCF.
Thiol assay
GCF and salivary fluid protein thiol is measured by a spectrophotometric method using dithio nitrobenzoic acid (DTNB), which reacts with accessible thiol groups in proteins, reduces them to stable intermediate compounds of mixed disulphide, protein S–S compound, that is, 5-mercapto 2-nitrobenzoate (MNB) that is measured at the end of 5 min at 412 nm using Spectronic 10 UV and compared with a glutathione standard and expressed in terms of micromoles per liter.[11]
RESULTS
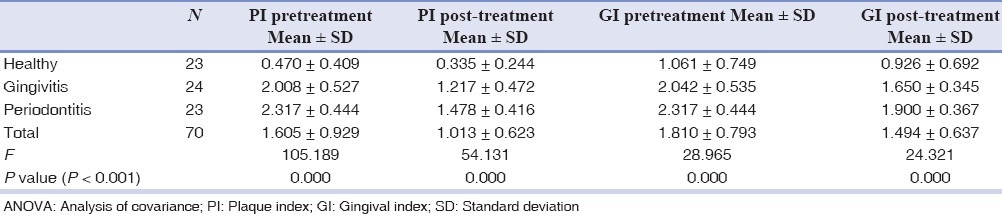
The pre- and post-treatment plaque and gingival indices of all the groups of patients showed statistically significant change using analysis of covariance (ANOVA), suggesting reduction of inflammation and maintenance of oral hygiene after treatment. P value less than 0.001 was considered significant [Table 1].
Table 1.
Variation in plaque and gingival index pre- and post-treatment (ANOVA)
Since the data was skewed, we had used logarithmic transformation to stabilize variance. The geometric mean and geometric standard deviation was used to describe the data. Furthermore, “repeated measures ANOVA” in SPSS version 16.0 was used to compare the antioxidant concentrations, that is, superoxide dismutase and thiol between healthy, gingivitis, and periodontitis groups at two time periods. Mild, moderate, and severe subgroups of periodontitis were also compared at two different time points (pre- and post-treatment) in the saliva and GCF media. P value of less than 0.05 was considered to be statistically significant.
Superoxide dismutase concentration in GCF post-treatment group showed 75.2% improvement in the healthy group, 143.1% improvement in the gingivitis group, and 69.9% improvement in the periodontitis group. Thiol concentration in GCF post-treatment showed 50.4% improvement in the healthy group, 76.3% in the gingivitis group, and 100.9% improvement in the periodontitis group [Table 2].
Table 2.
Geometric mean and standard deviation values of superoxide dismutase and thiol in the three subject groups pre- and post-treatment in GCF

In saliva, the superoxide dismutase concentration improved post-treatment in all the three groups with 22.1% increase in the healthy group, 51.2% increase in the gingivitis group, and an increase of 53.6% in the periodontitis group. Thiol concentrations in saliva also showed significant improvement post-treatment within each of the groups, 45.8% in the healthy group, 52% in the gingivitis group, and 81.6% in the periodontitis group [Table 3]. Comparison between the three groups post-treatment did not show any significant difference in improvement of superoxide dismutase or thiol concentrations.
Table 3.
Geometric mean and standard deviation values of superoxide dismutase and thiol in the three subject groups pre- and post-treatment in saliva

Periodontitis group was further categorized as mild, moderate, and severe subgroups to assess the severity of periodontitis. For analysis, the mild and moderate groups were considered together as there were only two patients in the moderate subgroup.
Following treatment, superoxide dismutase showed 100.8% improvement in the mild and moderate groups and 53.6% improvement in the severe subgroup in GCF. Comparison of GCF thiol concentration showed 74.2% increase post-treatment in the mild and moderate subgroups and 120.2% increase in the severe subgroup [Table 4].
Table 4.
Geometric mean and standard deviation values of superoxide dismutase and thiol in the three subgroups of periodontitis group pre- and post-treatment in GCF

Salivary superoxide dismutase concentrations post-treatment increased by 24.5% in the mild and moderate subgroups and by 75.7% in the severe subgroup of periodontitis. Thiol concentrations in saliva post-treatment showed 54% increase in the mild and moderate subgroups as compared with severe group, which showed about 101.7 % improvement [Table 5]. No statistically significant difference between subgroups was evident for improvement in either antioxidant concentration in GCF or saliva.
Table 5.
Geometric mean and standard deviation values of superoxide dismutase and thiol in the three subgroups of periodontitis group pre- and post-treatment in saliva

DISCUSSION
The present clinical trial was carried out with the objective of analyzing the activity of superoxide dismutase enzyme and thiol antioxidants in the GCF and saliva.
Observation of antioxidant concentrations pretreatment showed reduced quantities of both antioxidants in all groups, that is, healthy, gingivitis, and periodontitis and also with increasing severity of periodontal disease. In a previous study, patients with chronic periodontitis were reported to show high levels of lipid peroxidation in the GCF, indicating destruction of tissue by ROS.[12]
It was observed that the superoxide dismutase activity and thiol antioxidant concentration significantly improved following therapy in all the patient groups, suggesting a positive response to nonsurgical therapy. Therefore, treatment of periodontal disease reduces oxidative stress by a concomitant reduction in inflammatory load by enhancing antioxidant levels, irrespective of the medium, GCF or saliva.[5,13]
The healthy control group also showed improvement indicating that a minimal level of inflammation must have been present since it is rare that an individual will have “pristine” gingiva. There was an increase of the antioxidants’ concentration post-treatment showing a definite link between even minor levels of inflammation and sensitivity of the antioxidant system. According to Ghezzi et al.,[14] even minimal levels of oxidative stress are perceived and the protective antioxidant mechanism is set into action, which is essential for the maintenance of the structural integrity of proteins. This may explain the increased concentration observed post-treatment in the present study.
In the present study, superoxide dismutase presented higher levels in the GCF compartment than in saliva indicating its protective role in this environment. Previous reports indicate that the activity of this enzyme is very low in the extracellular compartments compared with the tissue-related environments and has very less biologic relevance[15,16] but in spite of this, saliva still showed variation in periodontal disease.[17] The varied response of superoxide dismutase in the GCF and saliva may also be attributed to the fact that superoxide dismutase has differential expression and distribution of its isoforms in tissues.[4] Hence, superoxide dismutase activity in GCF may therefore be representative of inflammatory changes in the periodontal environment. GCF has previously been suggested to be the most appropriate fluid to sample when investigating periodontal status, because it passes through the tissues and accumulates biomarkers of tissue events.[18] The levels of thiol antioxidant, in contrast, were similar in GCF and salivary compartments.
Superoxide dismutase in GCF was observed in reduced quantities in the gingivitis and periodontitis groups compared with the healthy group but improves considerably after treatment in all the groups. It was also observed that thiol concentration was lesser in the gingivitis and periodontitis groups compared with the healthy group. These findings are in accordance with the reported literature.[17,19] The lower concentrations of thiol antioxidants in the periodontitis group can be attributed to the presence of periodontopathic pathogens that readily degrade them to form hydrogen sulfide, which can be toxic.[20,21]
In the GCF, superoxide dismutase concentration was seen to be considerably elevated in mild and moderate periodontitis patients as compared to sites with severe periodontitis. The state of disease activity within the pockets in these subjects may be a contributory factor in the increased utilization of superoxide leading to an exhaustion of local antioxidants. It may be construed that in deep pockets, nonsurgical treatment alone may not reduce the inflammatory load sufficiently to enhance the antioxidant status. Deeper pockets will require surgical intervention to completely eliminate the reservoirs of inflammation. This has also been recognized by Ellis et al. in 1998 who observed that pockets with probing depths more than 6 mm had a significant depletion of superoxide dismutase.[22]
In the periodontitis group, the thiol antioxidants in GCF showed improvement in mild and moderate as well as severe periodontitis subgroups after treatment. This may be due to thiol antioxidants coordinating many biologic responses to inflammation and immunity. Huang et al. found that within periodontitis subjects, glutathione peroxidase levels correlated negatively with pocket depth and attachment loss and increased post-therapy.[23] It has also been stated that while therapy does not fully restore reduced glutathione (GSH) concentrations in GCF, it does restore the redox balance [Reduced glutathione or gamma-glutamylcysteinylglycine (GSH) to oxidized glutathione or glutathione disulfide (GSSG) ratio], suggesting that the abnormal redox balance arises secondary to oxidative stress resulting from periodontal inflammation.[24]
Reduced amounts of salivary thiol levels were observed in the gingivitis and periodontitis groups compared with the healthy group. A comparable decrease was evident in severe subgroup of periodontitis, which was greater than in the mild and moderate subgroups. However, no differences were noted between groups. Chapple et al. in 1997 noted a similar observation where salivary total antioxidant capacity was reduced in periodontitis cases compared with controls.[25]
Treatment resulted in increase in salivary thiol concentrations in gingivitis and periodontitis groups and the periodontitis subgroups, which was statistically significant within groups but not between groups. Similar observations were made by Tsai et al. who found that salivary glutathione concentrations were significantly reduced in periodontitis subjects relative to controls and that treatment increased glutathione concentrations.[12] The present clinical trial was conducted over a short period of time to assess the effect of nonsurgical therapy alone. However, more definitive results may be obtained with longer periods of follow up as well as by the institution of surgical modality of treatment, in cases of periodontitis.
CONCLUSIONS
Oxidative stress is gaining increased relevance in relation to the inflammatory process and it is highlighted as one of the major reasons for manifestation of disease. Periodontal disease is also thought to be caused due to an imbalance between harmful free radicals and protective antioxidants. Therefore, the present study was designed to evaluate the influence of specific antioxidants in periodontal disease pathogenesis.
The following conclusions may be drawn from the observations in the present study:
Antioxidant concentration was seen to be in progressively decreasing quantities from healthy subjects to patients with gingivitis and periodontitis.
Increased severity of periodontitis was also associated with gradual decrease in antioxidant concentration.
The two groups of antioxidants, superoxide dismutase and thiol showed improvement in “healthy,” gingivitis and periodontitis groups following nonsurgical therapy.
Concentration of the two antioxidants within the mild and moderate as well as severe subgroups also improved post-treatment.
Decrease in inflammatory component was associated with an increase in the antioxidant profile.
Superoxide dismutase was seen to be in higher concentrations in GCF than in saliva.
Thiol antioxidant showed similar profiles in the GCF and saliva.
It can therefore be construed that nonsurgical treatment by itself can bring about a significant improvement in periodontal health as demonstrated by heightened antioxidant levels in the gingival crevice and salivary compartments. The findings of the study restate the importance of continued monitoring of patients with supportive periodontal therapy as enhanced antioxidant status was evident in the apparently “healthy” group as well. There are limited studies focusing on the antioxidant profile following nonsurgical treatment. Hence, the information obtained from this study may provide scope for use of external antioxidant therapy in the management of periodontitis, especially among those patients refractory to conventional treatment.
ACKNOWLEDGEMENTS
We would like to acknowledge the postgraduates and faculty of the department of Biochemistry, Kasturba Medical College, Manipal, India for their help throughout the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Canacki CF, Cicek Y, Canacki V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc) 2005;70:619–28. doi: 10.1007/s10541-005-0161-9. [DOI] [PubMed] [Google Scholar]
- 2.Fridovich I. Superoxide anion radical (O2-), Superoxide dismutases and related matters. J Biol Chem. 1997;272:515–8. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby BH, Davis LW. The electron microscopic immunolocalization of a copper–zinc superoxide dismutase in association with collagen fibers of periodontal soft tissues. J Periodontol. 1991;62:413–20. doi: 10.1902/jop.1991.62.7.413. [DOI] [PubMed] [Google Scholar]
- 4.Na HJ, Kim OS, Park BJ. Expression of Superoxide dismutase isoforms in inflamed gingiva. J Korean Acad Periodontol. 2006;36:97–112. [Google Scholar]
- 5.Chapple IL. The role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Mol Pathol. 1996;49:247–55. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994;21:417–25. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 7.Silness J, Loe H. Periodontal Disease in Pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 8.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 9.Brill N. The Gingival Pocket Fluid. Acta Odontol Scand. 1962;20:1–115. doi: 10.3109/00016356209026102. [DOI] [PubMed] [Google Scholar]
- 10.Beanchamp C, Frodovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 11.Mochnik PA, Frei B, Ames BN. Measurement of antioxidants in human blood plasma. In: Packer L, editor. Methods in Enzymology. Vol. 234. San Diego: Academic Press; 1994. pp. 269–79. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–84. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 13.Bartold PM, Page RC. The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. J Oral Pathol. 1986;15:367–74. doi: 10.1111/j.1600-0714.1986.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghezzi P. Oxidoreduction of protein thiols in redox regulation. Biochem Soc Trans. 2005;33:1378–81. doi: 10.1042/BST0331378. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Antioxidant defense mechanisms: From the beginning to the end (of the beginning) Free Radic Res. 1999;31:261–72. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 16.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 17.Diab-Ladki R, Pellat B, Chahine R. Decrease in the total antioxidant activity of saliva in patients with periodontal diseases. Clin Oral Investig. 2003;7:103–7. doi: 10.1007/s00784-003-0208-5. [DOI] [PubMed] [Google Scholar]
- 18.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000;2007(43):160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 19.Hojo Y. Selenium and glutathione peroxidase in human saliva and other human body fluids. Sci Total Environ. 1987;65:85–94. doi: 10.1016/0048-9697(87)90163-x. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson J, Larsen JT, Edlund MB. Peptostreptococcus micros has a uniquely high capacity to form hydrogen peroxide from glutathione. Oral Microbiol Immunol. 1993;8:42–5. doi: 10.1111/j.1399-302x.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 21.Chu L, Dong Z, Xu X, Cochran DL, Ebersole JL. Role of glutathione metabolism of Treponema denticola in bacterial growth and virulence expression. Infect Immun. 2002;70:1113–20. doi: 10.1128/IAI.70.3.1113-1120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998;27:101–5. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang P, Su T, Wang H. The relationship between GPx activity in gingival fluid and clinical parameters of adult periodontitis. Hua Xi Kou Qiang Yi Xue Za Zhi. 2000;18:106–8. [PubMed] [Google Scholar]
- 24.Grant MM, Brock GR, Matthews JB, Chapple IL. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J Clin Periodontol. 2010;37:17–23. doi: 10.1111/j.1600-051X.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 25.Chapple IL, Mason GM, Matthews JB, Thorpe GHG, Maxwell SRJ, Whitehead T. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem. 1997;34:412–21. doi: 10.1177/000456329703400413. [DOI] [PubMed] [Google Scholar]


