Abstract
Epidemiological and animal-based investigations have indicated that the development of skin cancer is in part associated with poor dietary practices. Lipid content and subsequently the derived fatty acid composition of the diet are believed to play a major role in the development of tumorigenesis. Omega 3 (ω3) fatty acids, including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), can effectively reduce the risk of skin cancer whereas omega 6 (ω6) fatty acids such as arachidonic acid (AA) reportedly promote risk. To investigate the effects of fatty acids on tumorigenesis, we performed experiments to examine the effects of the ω3 fatty acids EPA and DHA and of the ω6 fatty acid AA on phorbol 12-tetradecanoate 13-acetate (TPA)-induced or epidermal growth factor (EGF)-induced transcription activator protein 1 (AP-1) transactivation and on the subsequent cellular transformation in a mouse epidermal JB6 cell model. DHA treatment resulted in marked inhibition of TPA- and EGF-induced cell transformation by inhibiting AP-1 transactivation. EPA treatment also inhibited TPA-induced AP-1 transactivation and cell transformation but had no effect on EGF-induced transformation. AA treatment had no effect on either TPA- or EGF-induced AP-1 transactivation or transformation, but did abrogate the inhibitory effects of DHA on TPA- or EGF-induced AP-1 transactivation and cell transformation in a dose-dependent manner. The results of this study demonstrate that the inhibitory effects of ω3 fatty acids on tumorigenesis are more significant for DHA than for EPA and are related to an inhibition of AP-1. Similarly, because AA abrogates the beneficial effects of DHA, the dietary ratio of ω6 to ω3 fatty acids may be a significant factor in mediating tumor development.
Nonmelanoma skin cancer is the most common type of cancer in the United States and is presently a major cause of morbidity and mortality in our society (1). Typically associated with sun and UV radiation exposure, the development of skin cancer has also been reported to be related to dietary factors, including dietary lipids. Epidemiological and experimental studies have shown that different classes of dietary fatty acids have a variety of effects on tumor development. Specifically, omega 3 (ω3) fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the nomenclature of which was developed at our institute (2), are reportedly chemopreventive (3–7) whereas omega 6 (ω6) fatty acids, including linoleic acid (LA) and its in vivo metabolized product, arachidonic acid (AA), are chemopromotive (8–11).
Transcription factor activator protein 1 (AP-1) is an inducible eukaryotic transcription factor composed of the products of the jun and fos oncogene families (12–14). The AP-1 dimers bind to and transactivate promoter regions on DNA that contain cis-acting phorbol 12-tetradecanoate 13-acetate (TPA) response elements to induce transcription of genes involved in cell proliferation, metastasis, and cellular metabolism (15). AP-1 is induced by a variety of stimuli and is implicated in the development of cancer (12). Agonists, such as TPA and epidermal growth factor (EGF), are two of the most common agents used to study the experimental induction of AP-1 and cellular transformation in cellular and animal models of cancer (16). Several reports have established the role of AP-1 activation in cellular transformation and tumor promotion. In JB6 mouse epidermal cell lines, TPA and EGF induce AP-1 transcriptional activity in promotion-sensitive (P+) phenotypes but not in promotion-resistant (P−) phenotypes. Blocking AP-1 induction causes P+ cells to revert to the P− phenotype, indicating the unique requirement for AP-1 activity in TPA- and EGF-induced cell transformation (17). Moreover, various chemopreventive agents long known to have inhibitory effects on cell transformation and tumor promotion (e.g., aspirin, sodium salicylate, and retinoic acid) also suppress AP-1 transactivation (18–20).
In the present study, we investigated the effects of EPA, DHA, and AA on TPA- or EGF-induced AP-1 transactivation and on subsequent cell transformation in the JB6 mouse epidermal cell line. This study provides new insight into the primary mechanisms by which certain dietary essential fatty acids inhibit or promote tumorigenesis and support epidemiological reports that suggest that diets rich in ω3 fatty acids and low in ω6 are chemoprotective.
Materials and Methods
Cell Culture and Reagents.
AP-1 luciferase reporter plasmid stably transfected mouse epidermal JB6 P+ 1-1 cells and the JB6 mouse epidermal cell line, Cl 41, were cultured in monolayers at 37°C, 5% CO2 by using Eagle's minimal essential medium, MEM, containing 5% FBS, 2 mM L-glutamine, and 25 μg of gentamicin per ml. FBS and MEM were from BioWhittaker; TPA, aprotinin, leupeptin, and low-endotoxin albumin (LEA) were from Sigma; EGF was from Clonetics (San Diego); DHA (C22:6, 4,7,10,13,16,19-docosahexaenoic acids), EPA (C20:5, 5,8,11,14,17-eicosapentaenoic acids), and AA (C20:4, 5,8,11,14-arachidonic acids) were from Nu Chek Prep (Elysian, MN); luciferase assay substrate was from Promega; specific antibodies against phosphorylated sites of Erks, p38 kinase, and the c-Jun NH2-terminal kinase (JNK) assay kit were from New England Biolabs.
Fatty Acid Preparation.
A 5% LEA solution (16.6 ml) was added to each 10 mg of fatty acid and mixed with a Vortex mixer. The mixture was then bubbled very slowly with N2 for 60 s to remove any oxygen and then incubated in a sealed tube for 4 h at 37°C in a shaking water bath. The fatty acid/albumin complex (0.6 mg of fatty acid per ml) solution was then filter-sterilized, divided into aliquots in single-use vials, and kept frozen at −70°C.
Luciferase Assay for AP-1 Transactivation.
Confluent monolayers of JB6 P+ 1-1 cells were trypsinized, and 8,000 viable cells suspended in 100 μl of 5% FBS MEM were added to each well of a 96-well plate. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2/95% air. Cells were starved for 24 h and then treated for another 48 h by culturing them in 0.1% FBS MEM with different concentrations of the fatty acids indicated. The cells were then exposed to either TPA (20 ng/ml) or EGF (20 ng/ml). After 24 h in culture, the cells were extracted with 100 μl of lysis buffer and luciferase activity was measured by using a luminometer (Monolight 2010; Analytical Luminescence Laboratory, San Diego). The results are expressed as relative AP-1 activity (21).
Anchorage-Independent Transformation Assay.
JB6 C1 41 cells (8 × 103 per ml) were exposed to either TPA or EGF with or without EPA, DHA, or AA at concentrations ranging from 2.5 to 20 μg/ml in 1 ml of 0.33% basal medium of Eagle agar containing 10% FBS over 3.5 ml of 0.5% basal medium Eagle agar containing 10% FBS. Additionally, mixtures of DHA (20 μg/ml) containing increasing concentrations of AA (from 5 μg/ml to 20 μg/ml) were also tested. The cultures were maintained in a 37°C, 5% CO2 incubator for 4 weeks. Cell colonies were then scored as described by Colburn et al. (22). The effects of the fatty acids on cell transformation of JB6 C1 41 cells are presented as a percentage inhibition of cell transformation in soft agar compared with TPA- or EGF-stimulated cells.
AP-1 DNA Binding Studies.
Electrophoretic mobility-shift assays were performed essentially as described (23). Nuclear protein extracts were prepared from JB6 C1 41 cells by the modified method of Monick et al. (24). Briefly, JB6 C1 41 cells were cultured in 10-cm dishes. After 48 h of pretreatment with different concentrations of fatty acids as indicated in low percentage medium (0.1%), the cells were exposed to TPA (20 ng/ml) or EGF (20 ng/ml) and incubated for another 12 h. The cells were then harvested and disrupted in 500 μl of lysis buffer A (25 mM Hepes, pH 7.8/50 mM KCl/0.5% Nonidet P-40/100 μM DTT/10 μg/ml leupeptin/25 μg/ml aprotinin/1 mM PMSF). The pellet containing the nuclei was washed once with 500 μl of buffer B (buffer A without Nonidet P-40), resuspended in 150 μl of extraction buffer (buffer B but with 500 mM KCl and 10% glycerol), and shaken at 4°C for 30 min. The DNA binding reaction (for the electrophoretic mobility-shift assay) was carried out at room temperature for 30 min in a mixture containing 4 μg of nuclear protein, 1 μg of poly(dI⋅dC), and 15,000 cpm of 32P-labeled double-stranded AP-1 oligonucleotide (5′-CGCTTGATGAGTCAGCCGGAA-3′). The samples were fractionated through a 5% polyacrylamide gel. Gels were dried and analyzed by using the Storm 840 Phospho-Image System (Molecular Dynamics).
JNK Kinase Assay.
JB6 Cl 41 cells were pretreated with the various concentrations of fatty acids as indicated and starved for 48 h in 0.1% FBS MEM as described earlier. Cells were then exposed to TPA (20 ng/ml) or EGF (20 ng/ml) followed by culturing for another 30 min. The JNK kinase assay was carried out according to the protocol of New England Biolabs (www.neb.com). c-Jun phosphorylation was selectively measured by Western immunoblotting by using a specific c-Jun antibody against phosphorylated c-Jun at serine-63.
Immunoblotting for Phosphorylated Erks and p38 Kinase.
Immunoblotting for the phosphorylated proteins Erks and p38 kinase was carried out by using specific antibodies against phosphorylated sites on Erks or p38 kinase (19) according to the supplier's recommendations (Cell Signaling Technology, Beverly, MA). Antibody-bound proteins were detected by chemiluminescence and analyzed by using the Storm 840 Phospho-Image System (Molecular Dynamics).
Statistical Analysis.
Significant differences in AP-1 activity were determined by using the Student's t test. The results are expressed as means ± SD.
Results
Varied Effects of Fatty Acids on AP-1 Activity.
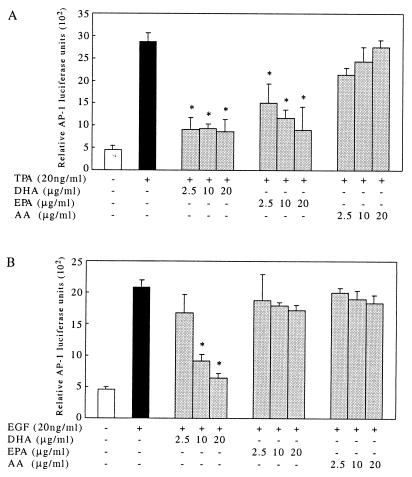
No significant changes were observed in AP-1 activity when stably transfected mouse epidermal JB6 P+ 1-1 cells were cultured with EPA, DHA, or AA alone compared with the LEA control (data not shown). DHA markedly inhibited TPA-induced AP-1 activity (83%, P < 0.01) from the lowest concentration, 2.5 μg/ml, to the highest concentration of 20 μg/ml (Fig. 1A). Increasing the DHA concentration had no further inhibitory effect on AP-1 activity induced by TPA. EPA treatment moderately inhibited TPA-induced AP-1 activity (48% inhibition at 2.5 μg/ml, P < 0.05; Fig. 1A). DHA treatment also resulted in a significant inhibition of EGF-induced AP-1 activity in an apparent concentration-dependent manner, whereas EPA had no effect (Fig. 1B). AA treatment had no effect on either TPA- or EGF-induced AP-1 activity (Fig. 1). To confirm that the observed effects were not due to cytotoxicity, we investigated [3H]thymidine incorporation into the cells under the exact conditions of fatty acid treatment, and no significant effects on growth inhibition or cellular viability were found (data not shown).
Figure 1.
DHA suppresses both TPA- and EGF-induced AP-1 activity, but EPA suppresses only TPA-induced AP-1 activity. AP-1 luciferase reporter plasmid stably transfected JB6 P+ 1-1 cells were cultured and treated as described in Materials and Methods. After a 48-h treatment with fatty acids at the concentrations indicated, 20 ng/ml TPA or EGF was added and the cells were cultured for another 24 h before harvest. (A) DHA at 2.5 μg/ml significantly inhibits TPA-induced AP-1 activity (67% inhibition, P < 0.01; mean ± SD of triplicate experiments, six wells each); increased concentrations of DHA did not further inhibit AP-1 activity. EPA inhibited TPA-induced AP-1 activity in a concentration-dependent manner (P < 0.01; mean ± SD of triplicate experiments, six wells each). AA failed to inhibit AP-1 activity at the concentrations indicated (P > 0.05; mean ± SD of triplicate experiments, six wells each). (B) DHA inhibited EGF-induced AP-1 activity in a concentration-dependent manner (P < 0.01 at 10 or 20 μg/ml; mean ± SD of triplicate experiments, six wells each). Neither EPA nor AA affected EGF-induced AP-1 activity (P > 0.05; mean ± SD of triplicate experiments, six wells each).
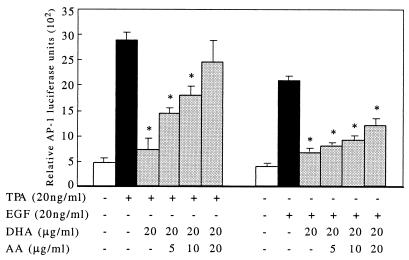
AA Abrogates the Inhibitory Effect of DHA on TPA-induced AP-1 Activity.
Although AA, a direct substrate of cyclooxygenase-2, can be converted to prostglandin E2 (PGE2), which is reported to induce AP-1 activation (25, 26), in our experiments AA did not induce AP-1 activity. To investigate the potential interactive effects of AA and DHA on AP-1 activation, we combined increasing concentrations of AA (Fig. 2) with a fixed amount of DHA (20 μg/ml) that was found to inhibit AP-1 activation in the TPA- or EGF-JB6 model as reported herein. Surprisingly, the DHA inhibitory effect on TPA-induced AP-1 activation was significantly (P < 0.01) abrogated by AA in a concentration-dependent manner (Fig. 2). Similar results were observed in the EGF-stimulated cells, but to a much lesser extent (Fig. 2).
Figure 2.
AA abrogates the inhibitory effect of DHA on TPA- and EGF-induced AP-1 activity. Luciferase reporter plasmid stably transfected JB6 P+ 1-1 cells were cultured as described in Materials and Methods. The cells were treated with a mixture of 20 μg/ml DHA and increasing concentrations of AA as indicated. After 48 h of culture, 20 ng/ml TPA or EGF was added and the cells were harvested after another 24-h incubation. AA significantly reduced the inhibitory effect of DHA on TPA-induced AP-1 activity in a concentration-dependent manner (P < 0.01; mean ± SD of triplicate experiments, six wells each).
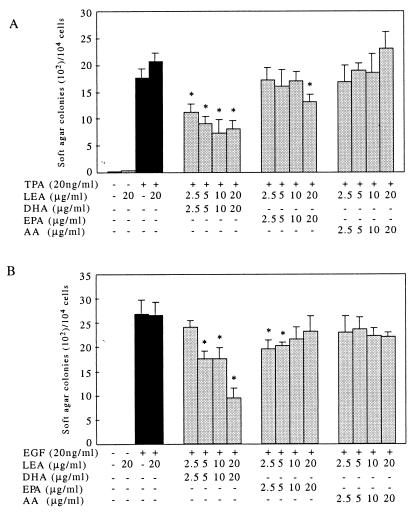
DHA Suppresses JB6 Cell Anchorage-Independent Transformation.
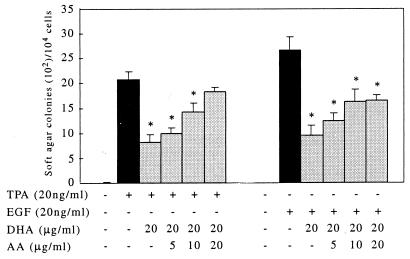
Cell transformation was determined by a cell anchorage-independent growth assay on soft agar. In the absence of TPA or EGF, no Cl 41 colony formation in soft agar was observed. However, treatment by TPA or EGF promoted significant Cl 41 transformation and colony formation on soft agar. EGF treatment resulted in larger and more robust cell colony formation when compared with cells treated with TPA. DHA significantly repressed TPA- and EGF-induced cell transformation and colony formation on soft agar (Fig. 3 A and B). However, treatment with EPA suppressed TPA-induced cell transformation only at the highest dose (20 μg/ml, Fig. 3A) but had no effect on EGF-induced transformation (Fig. 3B). AA did not significantly effect cell transformation induced by TPA or EGF on soft agar. AA treatment did, however, reduce the inhibitory effects of DHA in both TPA- and EGF-induced cell transformation (Fig. 4). These findings are in agreement with the effects of EPA, DHA, and AA treatment on AP-1 activity as reported herein.
Figure 3.
DHA suppresses both TPA- and EGF-stimulated cell transformation on soft agar, but EPA only slightly inhibits TPA-induced cell transformation on soft agar. JB6 Cl 41 cells were seeded into triplicate wells and exposed to 20 ng/ml TPA or EGF with or without fatty acids at the different concentrations as indicated in Materials and Methods. The cell colonies were scored after a 4-week incubation in a 37°C, 95% air/5% CO2 atmosphere. (A) TPA-induced cell transformation on soft agar was inhibited 61% by 10 μg/ml DHA (P < 0.01, n = 3), but was inhibited by only 21% with 20 μg/ml EPA. AA had no effect on TPA-induced cell transformation (P > 0.05, n = 3). (B) Cell transformation on soft agar was inhibited 61% by 20 μg/ml of EGF, but neither EPA nor AA had an effect on TPA-induced cell transformation.
Figure 4.
AA abrogates the DHA inhibitory effect on TPA- or EGF-induced cell transformation. JB6 Cl 41 cells from triplicate wells were exposed to 20 ng/ml TPA or EGF with 20 μg/ml DHA and increasing concentrations of AA as indicated. Cells were incubated for another 4 weeks as described in the legend of Fig. 3. AA effectively abrogated the inhibitory effects of DHA on both TPA- and EGF-induced cell transformation on soft agar (P < 0.01; n = 3).
DHA Represses AP-1 DNA Binding.
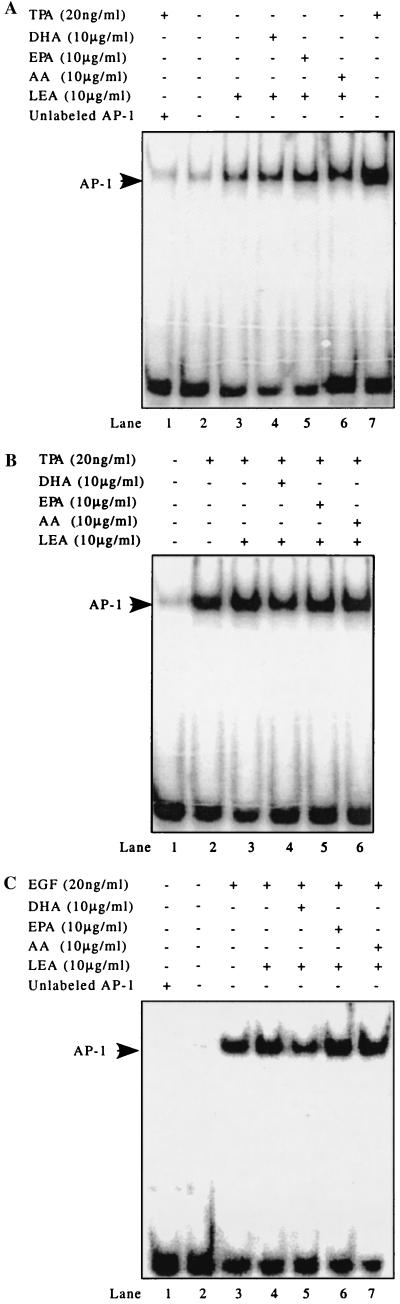
As a transcription factor, AP-1 mediates gene transcription induced by TPA, growth factors, cytokines, and many other stimuli by binding to the TPA response element site in the promoter region of a specific target gene (27). AP-1 DNA binding activity as an index of activation was therefore measured by electrophoretic mobility-shift assay after pretreatment of cells with EPA, DHA, or AA. Our findings indicated that LEA alone (10 μg/ml) enhanced AP-1 DNA binding (Fig. 5A, lane 3). This result, however, may be due to the positive effects of albumin on cell growth. In comparison to LEA alone and in the absence of an agonist, DHA did not effect AP-1 DNA binding, whereas EPA and AA slightly increased DNA binding of AP-1 (Fig. 5A, lanes 5 and 6). Treatment with TPA (20 ng/ml) resulted in a significant increase in AP-1 DNA binding (Fig. 5A, lane 7). This increased binding could be completely eliminated by adding a 10-fold excess of unlabeled AP-1 oligonucleotide, confirming that the electrophoretic mobility-shift band was specific for AP-1 binding (Fig. 5A, lane 1). DHA (10 μg/ml) treatment inhibited AP-1 DNA binding induced by either TPA or EGF (Fig. 5 B and C). However, EPA had no effect on AP-1 DNA binding.
Figure 5.
DHA blocks TPA- or EGF-induced AP-1 DNA binding. JB6 Cl 41 cells were treated, nuclear proteins were extracted, and electrophoretic mobility-shift assays were carried out as described in Materials and Methods. (A) TPA-induced AP-1 binding is totally eliminated by a 10-fold excess of unlabeled AP-1 oligonucleotide (lane 1 and lane 7). LEA increased AP-1 binding. EPA and AA, but not DHA, induced a slightly higher AP-1 binding compared with LEA alone. (B and C) DHA suppressed either TPA- or EGF-induced AP-1 DNA binding (lane 4 of B and lane 5 of C). EPA or AA had no effect on either TPA- or EGF-induced AP-1 DNA binding (lane 5 and 6 of B and lane 6 and 7 of C).
Mitogen-Activated Protein (MAP) Kinases Are Not Involved in Fatty Acid-Mediated AP-1 Activity Induced by TPA or EGF.
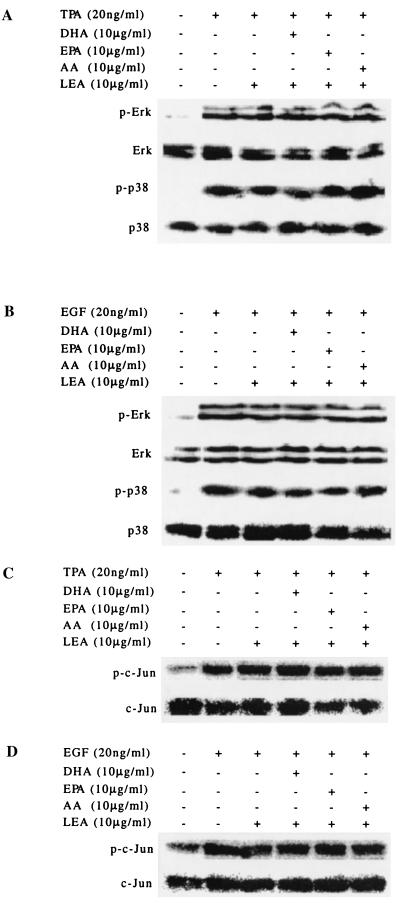
MAP kinases contribute to the induction of AP-1 activity in response to a broad range of extracellular stimuli (27). In our experiments, both TPA and EGF induced phosphorylation of Erks and p38 kinase (Fig. 6 A and B). Neither TPA nor EGF induced detectable phosphorylation of JNKs as assessed by Western blot analysis (data not shown). TPA and EGF did, however, induce the phosphorylation of a direct substrate of JNKs, c-Jun at serine-63 (Fig. 6 C and D). None of the fatty acids tested, however, affected TPA- or EGF-induced phosphorylation of any of the MAP kinases measured (Fig. 6).
Figure 6.
Fatty acids do not block TPA- or EGF-induced phosphorylation of Erks or p38 kinase and JNK kinase activity. JB6 Cl 41 cells were treated and Western blot analysis was carried out as described in Materials and Methods. (A and B) Erks and p38 kinase were phosphorylated after treatment with either TPA or EGF. None of the fatty acids were capable of blocking the phosphorylation. p-Erk and p-p38 indicate the phosphorylated proteins. (C and D) JNK activity was assessed by determining the phosphorylation of JNK's direct substrate, c-Jun. Western blotting was carried out by using a specific c-Jun antibody against c-Jun phosphorylated at serine-63. Both TPA and EGF induced an increase in JNK activity, and none of the fatty acids blocked the increase.
Discussion
Epidemiological studies have suggested that ω3 fatty acids, such as DHA and EPA, are effective chemopreventive agents (13, 28–30). In animal experiments, fish oil, rich in the long-chain ω3 fatty acids EPA and DHA, significantly suppressed the incidence of colon cancer and average tumor volume (31). In contrast, maintaining animals on diets high in ω6 fatty acids, such as LA from vegetable oil, significantly increased cancer incidence (31). In hairless mice, 2 weeks of fish oil feeding resulted in a significant reduction in the inflammatory response in skin and increased skin repair after ultraviolet A exposure, compared with animals raised on corn oil high in LA (32). In mice, the photocarcinogenic response increased with increasing amounts of LA in the diet, compared with a diet high in saturated fat (33). These results agreed with data from other groups that reported that diets high in ω6 fatty acids, such as LA or AA, significantly elevated risk of developing cancer (34–37). In the present study, we report that DHA significantly inhibits TPA- or EGF-induced cell transformation in a mouse epidermal JB6 cell model. Based on the findings that DHA suppresses TPA- and EGF-induced AP-1 DNA binding and AP-1 transactivation, we hypothesize that the inhibitory effects of DHA on cancer development are due, at least in part, to the suppression of AP-1 activity. EPA, however, was not as effective as DHA in suppressing AP-1 transactivation, a result that may argue against an eicosanoid-mediated event. Topical treatment with EPA, however, was reported to protect against ultraviolet B-induced damage by preventing immunosuppression (38). In the present study, when AA was tested no direct induction of AP-1 activity was observed. AA treatment in conjunction with DHA, however, strongly abrogated the DHA-mediated inhibition of AP-1 activity. In addition, the effects of fatty acids on AP-1 transactivation are consistent with their observed effects on cell transformation in soft agar anchorage-independent colony formation, further demonstrating that the reported cell transformation effects are due to interaction with AP-1.
The precise mechanisms that explain the effects of various fatty acids on tumor development are largely unclear. Epidermal cells lack Δ6 desaturase activity, which is the rate-limiting enzyme for the conversion of LA into AA and α-linolenic acid into DHA. In addition, many unique eicosanoid and lipoxygenase metabolites are present in the skin, where they may also play a central role in cell signaling related to skin cancer development (39). The role that diet and, specifically, fatty acids play in photocarcinogenesis remains largely unknown but is reportedly related to eicosanoid signaling, peroxidation effects, and modulation of the immune system (40). Other suggestions include an eicosanoid-mediated mechanism whereby formation of PGE2, a major prostaglandin of the mammalian epidermis derived from AA, is either stimulated or inhibited by ω3 or ω6 fatty acids (41). Cyclooxygenase-2 activity and PGE2 are reportedly involved in tumor development (42, 43). Increased proportions of AA in cellular membrane lipids (the eicosanoid precursor pool), resulting from diets high in ω6 and low in ω3, provide an increased PGE2 level. EPA and DHA compete with AA and thus strongly inhibit the formation of PGE2 from AA (44–46). Several studies (47–49) have suggested that PGE2 is signaling through AP-1 and thus may play a definitive role in tumor development. Additionally, the significance of eicosanoids in mediation of skin cancer development has been demonstrated by the inhibition of photocarcinogenesis in the hairless mouse treated with the cyclooxygenase inhibitor indomethacin (50). These observations may explain the effects of fatty acids on tumor development. Moreover, the different effects of EPA and DHA on AP-1 transactivation observed in our experiments may be related to their differential effects on tumor suppression.
Many mechanisms are involved in the up- and down-regulation of AP-1 activity (12). MAP kinases are the most common signaling pathways known to mediate AP-1 function (27), and the blocking of MAP kinases leads to the inhibition of AP-1 transactivation and subsequent cell transformation (51–57). In the present study, however, we found no effect of EPA, DHA, or AA on TPA- or EGF-induced activation of JNKs, Erks, or p38 kinases, the three members of the MAP kinase family. These results indicate that the effects of EPA and DHA on AP-1 activity may not be mediated by MAP kinase pathways, but rather by other mechanisms (27).
In summary, our data showed that ω3 fatty acids efficiently inhibit tumor promoter-induced AP-1 transactivation and subsequent cell transformation in mouse epidermal JB6 cells. DHA was a more efficient inhibitor than EPA. The ω6 fatty acid, AA, had no effect on AP-1 activity but abrogated the inhibitory effects of DHA on tumor promoter-induced AP-1 activity and subsequent cell transformation. We conclude that signaling to AP-1 is involved in the observed effects of EPA, DHA and AA on tumor development.
Acknowledgments
We thank Ms. Andria Hansen for her secretarial assistance and review. This work was supported by The Hormel Foundation and by National Institutes of Health Grants CA 77646, CA 81064, and CA 74916
Abbreviations
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- LA
linoleic acid
- AA
arachidonic acid
- AP-1
activator protein 1
- TPA
phorbol 12-tetradecanoate 13-acetate
- EGF
epidermal growth factor
- LEA
low-endotoxin albumin
- JNK
cJun NH2-terminal kinase
- MAP
mitogen-activated kinase
- PGE2
prostaglandin E2
References
- 1.Miller D L, Weinstock M A. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Holman R T, Mohrhauer H. Acta Chem Scand. 1963;17:S84–S90. [Google Scholar]
- 3.Yen A, Black H S, Tschen J. Arch Dermatol Res. 1994;286:331–336. doi: 10.1007/BF00402224. [DOI] [PubMed] [Google Scholar]
- 4.Potter J D, Slattery M L, Bostick R M, Gapstur S M. Epidemiol Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 5.Reddy B S. Cancer Metastasis Rev. 1994;13:285–302. doi: 10.1007/BF00666099. [DOI] [PubMed] [Google Scholar]
- 6.Parkinson A J, Cruz A L, Heyward W L, Bulkow L R, Hall D, Barstaed L, Connor W E. Am J Clin Nutr. 1994;59:384–388. doi: 10.1093/ajcn/59.2.384. [DOI] [PubMed] [Google Scholar]
- 7.Caygill C P J, Charlett A, Hill M J. Br J Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillyard L A, Abraham S. Cancer Res. 1979;39:4430–4437. [PubMed] [Google Scholar]
- 9.Sauer L A, Dauchy R T, Blask D E. Cancer Res. 2000;60:5289–5295. [PubMed] [Google Scholar]
- 10.Rose D P, Connolly J M. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 11.Rose D P. Am J Clin Nutr. 1997;66:998S–1003S. doi: 10.1093/ajcn/66.4.998S. [DOI] [PubMed] [Google Scholar]
- 12.Angel P, Karin M. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 13.Orengo I F, Black H S, Kettler A H, Wolf J E., Jr Photochem Photobiol. 1989;49:71–77. doi: 10.1111/j.1751-1097.1989.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 14.Curran T, Franza B R., Jr Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 15.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Rahmsdorf H J, Jonat C, Herrlich P, Karin M. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 16.Hunter T, Karin M. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Birrer M J, Watts R G, Matrisian L M, Colburn N H. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Z, Huang C, Brown R E, Ma W-Y. J Biol Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Ma W-Y, Hanenberger D, Cleary M P, Bowden G T, Dong Z. J Biol Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- 20.Agadir A, Chen G, Bost F, Li Y, Mercola D, Zhang X. J Biol Chem. 1999;274:29779–29785. doi: 10.1074/jbc.274.42.29779. [DOI] [PubMed] [Google Scholar]
- 21.Ding M, Shi X, Dong Z, Chen F, Lu Y, Castranova V, Vallyathan V. J Biol Chem. 1999;274:30611–30616. doi: 10.1074/jbc.274.43.30611. [DOI] [PubMed] [Google Scholar]
- 22.Colburn N H, Wendel E J, Abruzzo G. Proc Natl Acad Sci USA. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberger S F, Bowden G T. Oncogene. 1996;12:2301–2308. [PubMed] [Google Scholar]
- 24.Monick M M, Carter A B, Hunninghake G W. J Biol Chem. 1999;274:18075–18080. doi: 10.1074/jbc.274.25.18075. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Hughes-Fulford M. Br J Cancer. 2000;82:2000–2006. doi: 10.1054/bjoc.2000.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonson M S, Herman W H, Dunn M J. Exp Cell Res. 1994;215:137–144. doi: 10.1006/excr.1994.1325. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 28.Branden L M, Carroll K K. Lipids. 1986;21:285–286. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- 29.Steinmetz K A, Potter J D. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 30.Hakim I A, Harris R B, Ritenbaugh C. Nutr Cancer. 2000;36:155–162. doi: 10.1207/S15327914NC3602_3. [DOI] [PubMed] [Google Scholar]
- 31.Zhou S, Wang G, Chen B, Wang P. J Environ Pathol Toxicol Oncol. 2000;19:81–86. [PubMed] [Google Scholar]
- 32.Yen A, Black H S, Tschen J. Arch Dermatol Res. 1994;286:331–336. doi: 10.1007/BF00402224. [DOI] [PubMed] [Google Scholar]
- 33.Reeve V E, Bosnic M, Boehmwilcox C. Cancer Lett. 1996;108:271–279. doi: 10.1016/s0304-3835(96)04460-6. [DOI] [PubMed] [Google Scholar]
- 34.de Lorgeril M, Salen P, Martin J-L, Monjaud I, Boucher P, Mamelle N. Arch Intern Med. 1998;158:1181–1187. doi: 10.1001/archinte.158.11.1181. [DOI] [PubMed] [Google Scholar]
- 35.Willet W C, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 36.Holman R T. J Nutr. 1998;128:427S–433S. doi: 10.1093/jn/128.2.427S. [DOI] [PubMed] [Google Scholar]
- 37.Okuyama H, Kabayashi T, Watanabe S. Prog Lipid Res. 1997;35:409–457. doi: 10.1016/s0163-7827(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 38.Moison R M W, Steenvoorden D P T, van Henegouwen G M J B. Photochem Photobiol. 2001;73:64–70. doi: 10.1562/0031-8655(2001)073<0064:taeapa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Ziboh V A, Miller C C, Cho Y H. Am J Clin Nutr. 2000;71, Suppl. 1:361S–366S. doi: 10.1093/ajcn/71.1.361s. [DOI] [PubMed] [Google Scholar]
- 40.Ley R D, Reeve V E. Environ Health Perspect. 1997;105,Suppl.:981–984. doi: 10.1289/ehp.97105s4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greeves M W, McDonald-Gibson W. Br J Pharmacol. 1972;46:172–175. doi: 10.1111/j.1476-5381.1972.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attiga F A, Fernandez P M, Weeraratna A T, Manyak M J, Patierno S R. Cancer Res. 2000;60:4629–4637. [PubMed] [Google Scholar]
- 43.Liu X H, Kirschenbaum A, Yao S, Stearns M E, Holland J F, Claffey K, Levine A C. Clin Exp Metastasis. 1999;17:687–694. doi: 10.1023/a:1006728119549. [DOI] [PubMed] [Google Scholar]
- 44.Lands W E M, LeTellier P R, Rome L H, Vanderhoek J Y. Adv Biosci. 1973;9:15–28. [Google Scholar]
- 45.Hamberg M. Biochim Biophys Acta. 1980;618:389–398. doi: 10.1016/0005-2760(80)90257-x. [DOI] [PubMed] [Google Scholar]
- 46.Needleman P, Sprecher H, Whitaker H O, Wyche A. Adv Prostaglandin Thromboxane Res. 1980;6:61–68. [PubMed] [Google Scholar]
- 47.Simonson M S, Herman W H, Dunn M J. Exp Cell Res. 1994;215:137–144. doi: 10.1006/excr.1994.1325. [DOI] [PubMed] [Google Scholar]
- 48.Dendorfer U, Oettgen P, Libermann T A. Mol Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Won J S, Suh H W, Kim Y H, Song D K, Huh S O, Lee J K, Lee K J. Mol Brain Res. 1998;60:203–214. doi: 10.1016/s0169-328x(98)00182-x. [DOI] [PubMed] [Google Scholar]
- 50.Reeve V E, Matheson M J, Bosnic M, Boehmwilcox C. Cancer Lett. 1995;95:213–219. doi: 10.1016/0304-3835(95)03886-2. [DOI] [PubMed] [Google Scholar]
- 51.Dong Z, Ma W, Huang C, Yang C S. Cancer Res. 1997;57:4414–4419. [PubMed] [Google Scholar]
- 52.Bost F, McKay R, Dean N, Mercola D. J Biol Chem. 1997;272:33422–33429. doi: 10.1074/jbc.272.52.33422. [DOI] [PubMed] [Google Scholar]
- 53.Behrens A, Jochum W, Sibilia M, Wagner E F. Oncogene. 2000;19:2657–2663. doi: 10.1038/sj.onc.1203603. [DOI] [PubMed] [Google Scholar]
- 54.Monno S, Newman M V, Cook M, Lowe W L., Jr Endocrinology. 2000;144:544–550. doi: 10.1210/endo.141.2.7307. [DOI] [PubMed] [Google Scholar]
- 55.Tchou W W, Yie T A, Tan T H, Rom W N, Tchou-Wong K M. Oncogene. 1999;18:6974–6980. doi: 10.1038/sj.onc.1203195. [DOI] [PubMed] [Google Scholar]
- 56.Huang C, Li J, Ma W-Y, Dong Z. J Biol Chem. 1999;274:29672–29676. doi: 10.1074/jbc.274.42.29672. [DOI] [PubMed] [Google Scholar]
- 57.Pang L, Sawada T, Decker S J, Saltiel A R. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]