Abstract
Malignant transformation of ameloblastomas arising from an odontogenic cyst or de novo is well-recognized. Malignancies in ameloblastomas may involve metastasis or a local dysplastic change in the tissue. The latter are classified as ameloblastic carcinomas. A 75-year-old male presented with a mandibular cystic swelling, with no evidence of metastasis. Dysplastic ameloblastic cells with spindle-cell transformation were seen arising from a cystic lining with features of a unicystic ameloblastoma. Immunohistochemically the lesion stained positive with cytokeratin 8,19 and alpha smooth muscle actin, but was negative for vimentin. A diagnosis of spindle-cell ameloblastic carcinoma was made. Spindle-cell ameloblastic carcinomas are rare and this is the second case arising from a unicystic ameloblastoma reported in literature. The recognition of this transformation and inclusion of this entity in the classification of ameloblastic carcinomas is stressed.
Keywords: Ameloblastic carcinoma, ameloblastoma, odontogenic carcinoma, spindle-cell transformation, unicystic ameloblastoma
INTRODUCTION
Malignancies in ameloblastomas have been well-recognized and included in the recent classification of odontogenic tumors by the World Health Organization (WHO).[1] Ameloblastic carcinomas are definitive entities that show malignant changes in the ameloblastic tissue with or without evidence of metastases. The changes are generally varied, but well-recognized. Spindle cell change in ameloblastic carcinomas is rare and indicates extreme de-differentiation of the odontogenic tissue. The other commonly described changes include pleomorphism, dysplasia, and keratinization. Use of immunohistochemical markers like cytokeratins and alpha smooth muscle actin have helped in the identification of these changes. We report a case of spindle-cell ameloblastic carcinoma arising from a unicystic ameloblastoma in a 75-year-old male, along with a review of literature, relating to the occurrence and presentation of this rare entity.
CASE REPORT
A 75-year-old socioeconomically compromised male presented to the hospital with complaints of a large swelling in the right mandibular region, extending from the right lower canine to the first molar, causing a disfigurement and difficulty in speech and mastication. The lesion was supposedly present for the past six years.
The patient had a complicated medical history with abdominal distention, uncontrolled diabetes mellitus, and hypertension. Medical referral was sought and a diagnosis of liver cirrhosis and hepatic encephalopathy with secondary ascites was returned. An ultrasonography was done and the fluid in the abdomen tapped under radiographic guidance. No evidence of metastatic foci was detected in the scan.
An orthopantomograph (OPG) of the jaws showed a large unilocular cystic radiolucency extending from 26 to 30 (lower right canine to first molar), with a very thin rim of the border of the mandible being seen [Figure 1]. The patient was edentulous in the affected region and gave a history of tooth extractions approximately three years back. The lesion seemed to have been present at the time, but apparently a diagnosis of tooth-associated infection was made without any attempts to biopsy the same. Clinically, the swelling was quite large, causing expansion of the jaws and cheeks, with adhesion to the vestibular buccal mucosa. No lymphadenopathy was detected. The lesion had a round bluish surface, which was non-ulcerated and caused perforation of the crestal bone, as evidenced by the crackling feel. The patient was otherwise asymptomatic with no signs of pain, nerve paresthesia, or inflammation. A provisional diagnosis of ameloblastoma with a probability of malignancy was made. Computed tomography (CT) scans of the head and neck region corroborated the findings of the OPG, with no evidence of lymphadenopathy.
Figure 1.
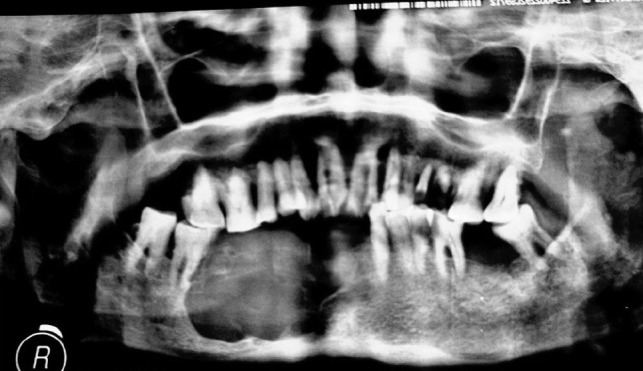
Orthopantomograph showing large destructive lesion in the mandible
Intraoperatively, the lesion was dissected from the mucosa and a hemimandibulectomy was performed extending from 31 to 23 (lower right second molar to the lateral incisor). There was profuse bleeding, which was controlled with electrocautery, Surgicel, and bone wax. A mandibular reconstruction plate was inserted for stability and fixed with titanium screws. Primary closure was attained and an intraoral drain inserted. The drain was removed on the third postoperative day. The excised tissue was sent for histopathological examination.
The patient subsequently underwent postoperative complications and died two weeks following the procedure.
Gross examination of the specimen revealed a large cystic tissue 7 cm × 8 cm in dimension with multiple nodular protuberances. Representative sections were sent from the tissue for routine histopathological examination.
Hematoxylin and eosin sections revealed a highly cellular lesion, with cells resembling the inner enamel epithelium and stellate reticulum forming discernible follicles. Cells in the center of the lesion were intensely pleomorphic with spindle cell variation, vesicular nuclei, mitotic figures, and hyperchromatism [Figure 2]. Intra- and intercellular keratin formation was evident. Periodic acid-Schiff stain (PAS) revealed positivity of the keratin-like areas. Imunohistochemically, the cytokeratin 8 and 19 antibodies were positive, indicating a definitive odontogenic origin [Figures 3 and 4]. Antibodies to alpha-smooth muscle actin (alpha SMA) were also evaluated and a positivity in the epithelial islands, with scattering positivity in the stroma, was seen [Figure 5]. Antibodies to vimentin, stained immunohistochemically, were negative. Based on the above, a histological diagnosis of ameloblastic carcinoma was returned.
Figure 2.
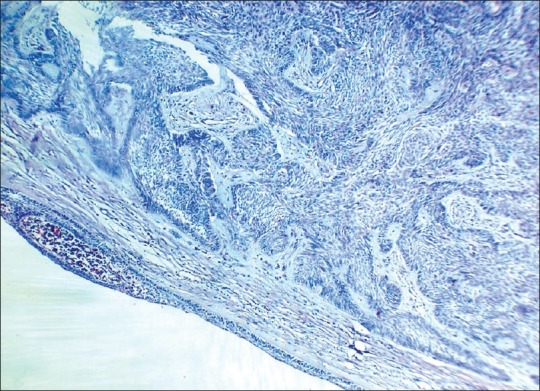
Photomicrograph showing proliferation of spindle-shaped dysplastic odontogenic epithelium arising from unicystic ameloblastoma tissue (H and E ×10)
Figure 3.
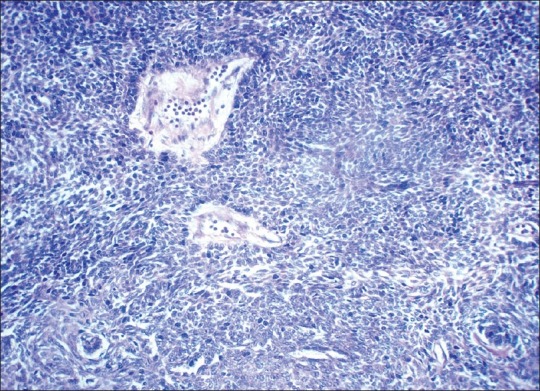
Photomicrograph showing proliferation of highly dysplastic ameloblasts with pleomorphism, hyperchromatic nuclei, mitotic figures and spindle-cell transformation (H and E ×40).
Figure 4.
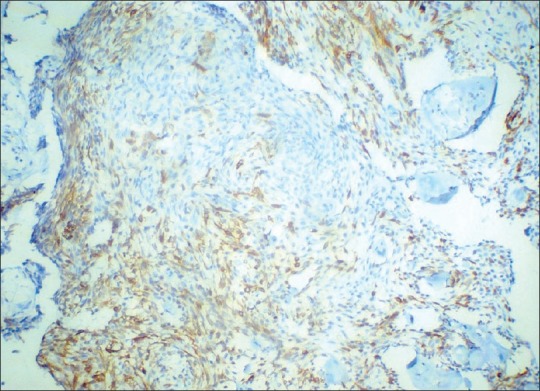
Photomicrograph of lesional tissue showing cytokeratin 19 positivity. Note the intensity of expression of cytokeratin 19 compared with cytokeratin 8 (DAB ×10)
Figure 5.
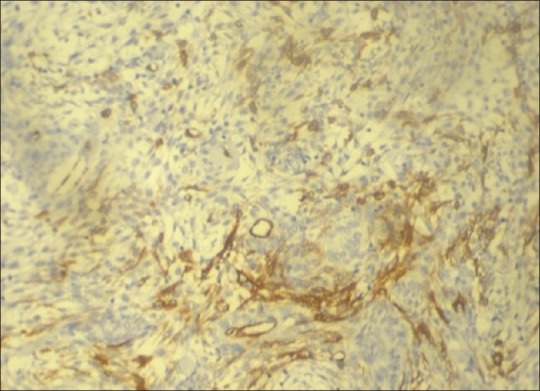
Photomicrograph of lesional tissue showing positivity to alpha-smooth muscle actin antibodies in the epithelial islands. (DAB ×10)
The presence of a definitive cystic lining, the budding of ameloblastic tissue from the lining, and the subsequent dysplastic cells in the center, all pointed to the probability of malignant transformation of a unicystic ameloblastoma. The abundance of pleomorphic spindle cells in the lesion was designative of a spindle-cell variant of ameloblastic carcinoma.
DISCUSSION
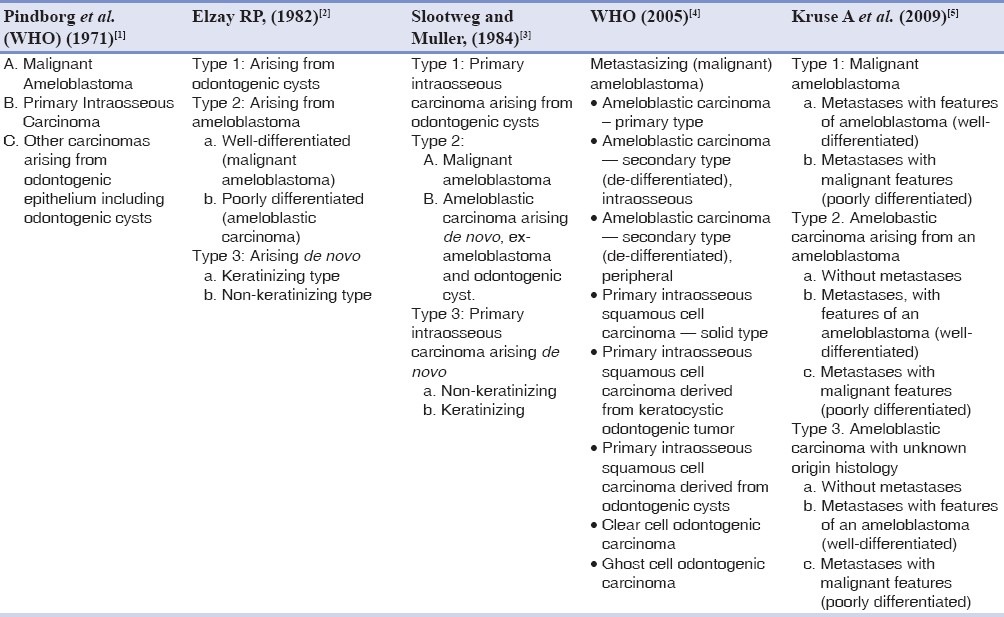
Malignant transformation of ameloblastomas have been variously classified by Elzay (1982),[2] Slootweg and Muller (1984),[3] the World Health Organization (WHO) (1971, 2005),[1,4] and recently by Kruse A (2009)[5] [Table 1]. Ameloblastic carcinomas are defined as lesions that exhibit histological features of an ameloblastoma with cytological atypia.[4] Even as most classifications dwell on the histogenesis of the lesion, the variations in histological atypia have not been used as the basis for subclassifications of the entity. Occurrence of atypia may take the form of cellular pleomorphism of the basaloid cells, large polygonal squamoid cells, with pale vesicular nuclei, increased and abnormal mitotic figures, presence of granular cells and clear cells, and spindle-shaped de-differentiation.[1,4] The latter has not been widely reported and very few cases are forthcoming in literature, with this type of presentation. The earliest mention of the term, ‘spindle-shaped ameloblastic carcinoma’ was by Slater in 1999.[6] Describing a group of odontogenic carcinomas that showed a spindle-cell sarcomatoid change, he advocated differentiation of these lesions from odontogenic sarcomas by the absence of ameloblastic fibrosarcoma-like features in the spindle-cell variant of ameloblastic carcinoma. In addition, the use of immunohistochemical markers served to highlight the non-sarcomatous origin of the spindle cells.
Table 1.
A comparison of the classifications for ameloblastic carcinomas
The present case showed predominant features of ameloblastoma, with a distinct evidence of origin from a unicystic ameloblastoma. The histological presentation of the latter was retained in many areas of the lesion, especially at the periphery. Interestingly, a similar case has been presented earlier by Jindal et al.,[7] of a spindle-cell differentiation of ameloblastic carcinoma arising from a unicystic ameloblastoma. To the best of our knowledge, a review of literature lists this as the second case of its category. Table 2 presents a collection of cases of ameloblastic carcinoma with spindle-cell differentiation.
Table 2.
Reported cases of spindle-cell ameloblastic carcinomas with demographic data and immunohistochemical investigations
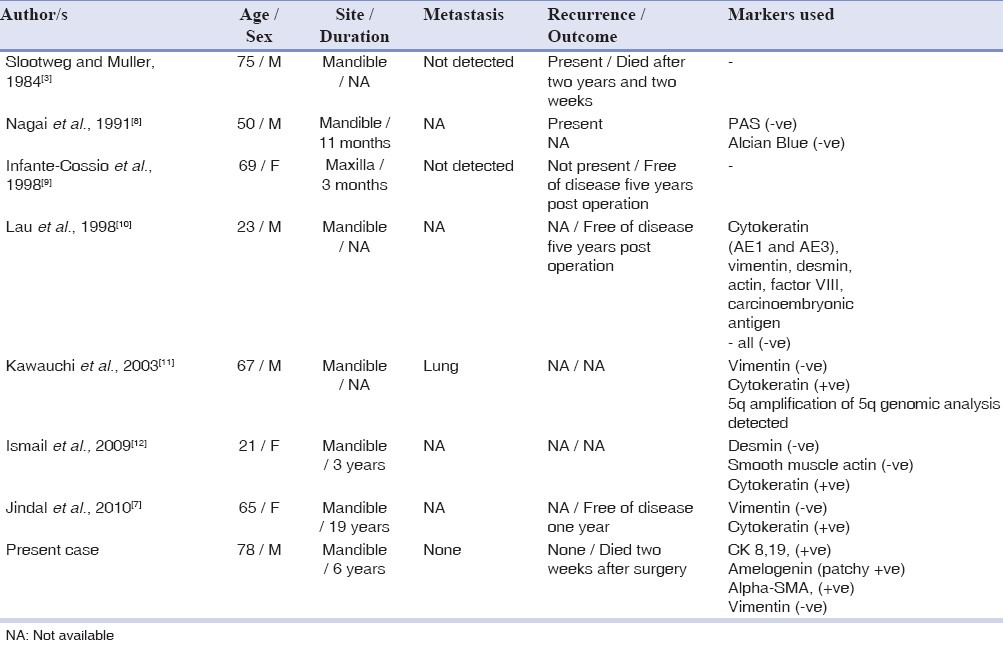
Immunohistochemistry of reported cases of a spindle-cell variant of ameloblastic carcinoma have shown conflicting results [Table 2]. Cytokeratin positivity was seen in two of the seven cases reported. All the cases, including the present one, were negative for vimentin. Smooth muscle actin demonstration was negative in the only case reported.[12]
Spindle-shaped cells in a malignant lesion can be characterized as malignant fibroblasts (MF) or cancer-associated fibroblasts (CAF). Although many are utilized, presently there are no diagnostic markers for any of the above-mentioned cells. Alpha smooth muscle actin (alpha-SMA), fibroblast-activated protein (FAP), fibroblasts-specific-protein-1 (FSP-1/S100A4), PDGF-beta, and neuron-glial antigen (NG2) are most common markers currently used.[13] De Wever et al.,[14] have defined a set of criteria for establishing the identity of spindle-cell proliferation in malignant lesions. According to this criteria, a spindle cell is characterized as stromal MF if it is positive to alpha-SMA, and to at least three other markers from a list of positive markers such as paladin 4Ig, podoplanin, vimentin / desmin, endosialin, cadherin 11, prolyl-4 hydroxylase (P4H), as well as, negative to markers such as cytokeratin, CD14, CD31, CD34, and smoothelin. It is regarded as CAF if this criterion is not met. Bello et al.,[15] have stated that expression of alpha SMA in the epithelial odontogenic islands is virtually diagnostic of an ameloblastic carcinoma. Based on the above, it is safe to assume that the spindle-cell proliferation in the present case is not of fibroblastic origin, and is a variant of the manifestation of the dysplastic epithelial odontogenic tissue.
Only one other case originated from a unicystic ameloblastoma.[7] Metastasis was detected in only one out of the seven cases, and that was to the lung.[11] In the present case there was no evidence of regional lymphadenopathy or metastases, and the fatal outcome postoperatively was listed as due to hepatocellular failure and hepatic encephalopathy. Only one other reported case had a fatal outcome, almost two years after the detection.
The differential diagnosis of spindle cells in an odontogenic carcinoma should include malignancies like ameloblastic fibrosarcoma, odontogenic sarcomas, carcinosarcomas, and fibrosarcomas. Negative staining for vimentin possibly excludes the fibrosarcomas, the carcinosarcomas, and the odontogenic sarcomas. The differentiation from ameloblastic fibrosarcomas is the presence of ameloblastic islands surrounded by spindle-shaped fibroblasts of odontogenic mesenchyme, which exhibit pleomorphism and atypia. Presence of odontogenic hard tissue-like dentinoid and cementoid is a characteristic feature of odontosarcomas.[6]
Radiologically, the radiolucency was indicative of an osteolytic lesion and entities like odontogenic cysts, odontogenic tumors, primary intraosseous carcinoma, and bone lesions were considered. The rather smooth outline of the radiolucency, with a longstanding period of duration (six years), inclined toward an odontogenic lesion rather than an aggressive non-odontogenic entity.
The different histological patterns exhibited by ameloblastic carcinoma based on literature reports prompt a subclassification:
Ameloblastic carcinoma (subtypes)
Ameloblastic type — the most common presentation with atypical and pleomorphic ameloblasts
Granular cell type — where a majority of cells are of the granular cell variety
Clear-cell variant — where a majority of cells are of the clear cell variety
Spindle-cell variant — where spindle-cell differentiation predominates the histology
Spindle-cell differentiation of an ameloblastic carcinoma is relatively rare and till date only seven cases have been reported in literature. The import of this occurrence, in our opinion, should be equated with the presence of clear cells and granular cells, both of which convey an aggressive nature. The few reported cases do not convey adequate data on the biological behavior of this lesion and more cases need to be studied to support this opinion.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Pindborg JJ, Kramer IR, Torloni H. Histological Typing of Odontogenic Tumors, Jaw Cysts and Allied Lesions. Geneva: World Health Organization; 1971. pp. 35–6. [Google Scholar]
- 2.Elzay RP. Primary intraosseous carcinomas of the jaws. Review and update of odontogenic carcinomas. Oral Surg Oral Med Oral Pathol. 1982;54:299–303. doi: 10.1016/0030-4220(82)90099-8. [DOI] [PubMed] [Google Scholar]
- 3.Slootweg PJ, Muüller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984;57:168–76. doi: 10.1016/0030-4220(84)90207-x. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumors No. 9. Lyon, France: IARC Press; 2005. Pathology and Genetics of Head and Neck Tumors; p. 287. [Google Scholar]
- 5.Kruse AL, Zwahlen RA, Gratz KW. New classification of maxillary ameloblastic carcinoma based on an evidence-based literature review over the last 60 years. Head Neck Oncol. 2009;1:31–4. doi: 10.1186/1758-3284-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slater LJ. Odontogenic sarcoma and carcinosarcoma. Semin Diag Pathol. 1999;16:325–32. [PubMed] [Google Scholar]
- 7.Jindal C, Palaskar S, Kaur H, Shankari M. Low-grade spindle-cell ameloblastic carcinoma: report of an unusual case with immunohistochemical findings and review of the literature. Curr Oncol. 2010;17:52–7. doi: 10.3747/co.v17i5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai N, Takeshita N, Nagatsuka H, Inoue M, Nishijima K, Nojima T, et al. Ameloblastic carcinoma: Case report and review. J Oral Pathol Med. 1991;20:460–3. doi: 10.1111/j.1600-0714.1991.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 9.Infante–Cossio P, Hernandez–Guisado JM, Fernandez–Machin P, Garcia–Perla A, Rollon–Mayordomo A, Gutierrez–Perez JL. Ameloblastic carcinoma of the maxilla: report of 3 cases. J Craniomaxillofac Surg. 1998;26:159–62. doi: 10.1016/s1010-5182(98)80006-1. [DOI] [PubMed] [Google Scholar]
- 10.Lau SK, Tideman H, Wu PC. Ameloblastic carcinoma of the jaws.A report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;85:78–81. doi: 10.1016/s1079-2104(98)90402-4. [DOI] [PubMed] [Google Scholar]
- 11.Kawauchi S, Hayatsu Y, Takahashi M, Furuya T, Oga A, Niwa S, et al. Spindle-cell ameloblastic carcinoma: a case report with immunohistochemical, ultrastructural, and comparative genomic hybridization analyses. Oncol Rep. 2003;10:31–4. [PubMed] [Google Scholar]
- 12.Ismail SB, Zain RB, Yaacob HB, Abraham MT. Ameloblastic carcinoma (spindle cell variant) Pathology. 2009;41:292–5. doi: 10.1080/00313020902756345. [DOI] [PubMed] [Google Scholar]
- 13.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 14.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 15.Bello IO, Alanen K, Slootweg PJ, Salo T. Alpha smooth actin within epithelial islands is predictive of ameloblastic carcinoma. Oral Oncol. 2009;45:760–5. doi: 10.1016/j.oraloncology.2008.11.011. [DOI] [PubMed] [Google Scholar]


