Abstract
Melanoma of the oral cavity is a rare malignant disease. On account of the presence at relatively obscure areas in the oral cavity, most of oral malignant melanomas are diagnosed at a late stage. Early diagnosis is essential for successful treatment and perhaps is the key factor in improving the prognosis of oral malignant melanoma. However, no large clinical series exist, and in fact, clinical cases are the sole key source of information. We hereby present a series of four cases of primary oral malignant melanoma of South-East Asian ethnic origin, with long-term, regular follow-up. The age of the patients ranged between 40 and 70 years, with equal sex predilection, and the gingiva was found to be the most common site of its occurrence. Based on clinical and histological parameters, all the cases were diagnosed as primary malignant melanoma, which were further confirmed by using immunohistochemical markers.
Keywords: Malignancy, malignant, melanin, melanocytes
INTRODUCTION
Primary melanoma of the oral cavity (POM) is an infrequent neoplasia of very aggressive characteristics originating from the malignant transformation of the melanocytes of the mucosa.[1] The World Health Organization (WHO) has defined mucosal malignant melanoma as a malignant neoplasm of melanocytes or of melanocyte precursors. It is characterized by the proliferation of atypical melanocytes at the epithelial-connective tissue interface, associated with upward migration into the epithelium and by invasion of the underlying connective tissues. Although usually seen in the skin, melanomas may also arise from melanocytes in the mucosae.[2] The incidence of melanoma has been steadily increasing in the past several decades and is of the order of 3–8% in a year. The lifetime risk of developing invasive melanoma was only one in 500; in 1960, one in 600; in 1992, one in 105; in 1996, One in 88; in 1998 and the lifetime risk would be one in 75 by the year 2000.[3]
It represents 0.2 to 8% of the total cases of melanoma from the other localizations of the body and 0.5% of all oral neoplasia. It occurs between 30 and 90 years of age, with higher incidence in the sixth decade, with slightly more male predilection. The common sites of occurrence are the palate and gingiva, with the maxillary arch being affected 80% of the time The incidence of primary melanoma located in the oral cavity is rare, even more exceptional is the secondary form (metastasis in the oral cavity of primitive distant melanomas). When it is secondary or metastatic, the localization is more frequent in the tongue, parotid, and tonsils.[1] Mucosal melanoma tends to present at an advanced stage with more aggressiveness, and is present in vertical growth (nodular) phase of disease. Histologically, oral mucosal melanomas are classified as in situ, invasive, and combined in situ and invasive. Most oral melanoma lesions (85.0%) are invasive or have both an invasive and in situ pattern.[4]
No specific etiological factors have been identified for oral melanomas. Risk factors have also remained elusive. Like their cutaneous counterparts, primary oral melanomas are believed to arise either from nevus, pre-existing pigmented areas, or de novo (30% cases).[5] We are hereby presenting four rare cases of primary malignant melanomas, at different sites.
CASE REPORTS
Case 1
A 70-year-old female patient reported to the outpatient department with the chief complaint of a rapidly enlarging blackish growth in the front side of her mouth in relation to the upper jaw, since four months. Approximately two months prior to her referral, the patient had noticed a small blackish non-tender growth in the maxillary anterior region, which continuously went on increasing to attain the present size. The patient had a habit of pan chewing since 20 years.
An extraoral examination revealed a localized swelling, with normal overlying skin noted in relation to the upper lip region. A firm, non-tender, swelling present intraorally had raised the upper lip [Figure 1]. Cervical lymphadenopathy was absent.
Figure 1.
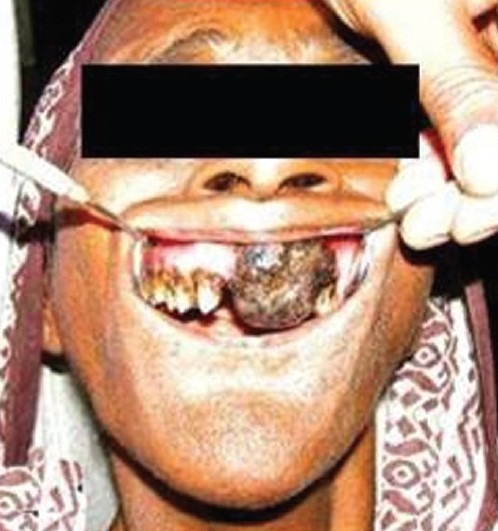
Extraoral and intraoral photograph showing swelling in relation to the upper lip region and blackish lobulated swelling
Intraoral examination revealed a pedunculated, lobulated, blackish swelling, which was firm, fragile, non-tender, non-reducible, non-compressible, non-fluctuant, and non-pulsatile, with well-defined margins occupying the anterior region of the maxilla, which had crossed the midline, extending from the mesial side of 13 to the distal side of 24 [Figure 1].
An orthopantomogram revealed partially edentulous jaws and a large radiolucent area with ill-defined margins in the maxillary anterior region, extending from 13 to 23 [Figure 2].
Figure 2.
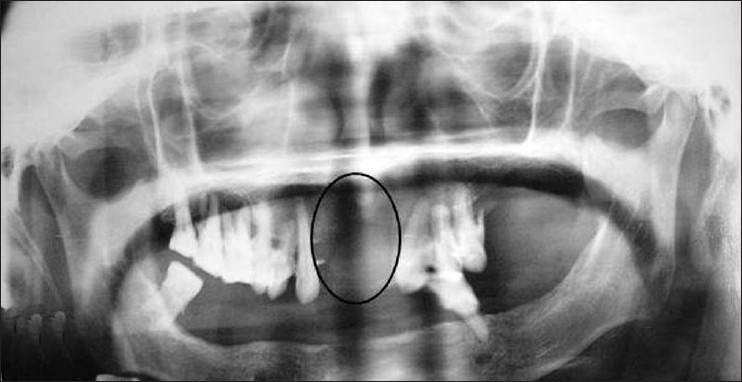
Panoramic view radiograph showing irregular large radiolucent area
Incisional biopsy from the representative site was performed under local anesthesia, with due consent of the patient, and histopathological findings revealed the presence of atrophic stratified squamous epithelium, with large, round-to-oval epitheloid melanocytes, showing both radial and vertical growth phases. The connective tissue stroma showed diffuse proliferation of neoplastic, round-to-oval melanocytes, with a presence of chronic inflammatory cells [Figure 3].
Figure 3.
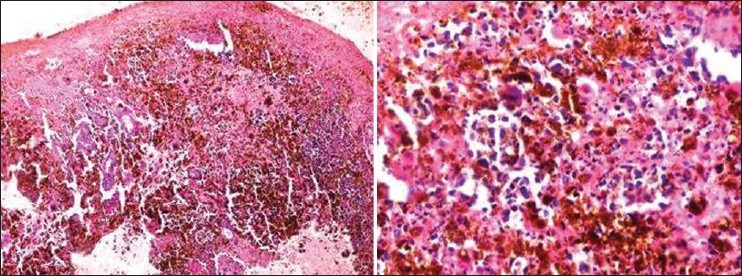
Case 1: Photomicrograph (10× and 40×) showing round-to-oval dysplastic melanocytes and melanin pigmentation interspersed within the connective tissue stroma
The overall clinical, radiological, and histological features were confirmative of invasive malignant melanoma with 0.90 mm tumor thickness, which was further reconfirmed by using an immunohistochemical marker HMB-45 and Melan-A [Figure 4].
Figure 4.
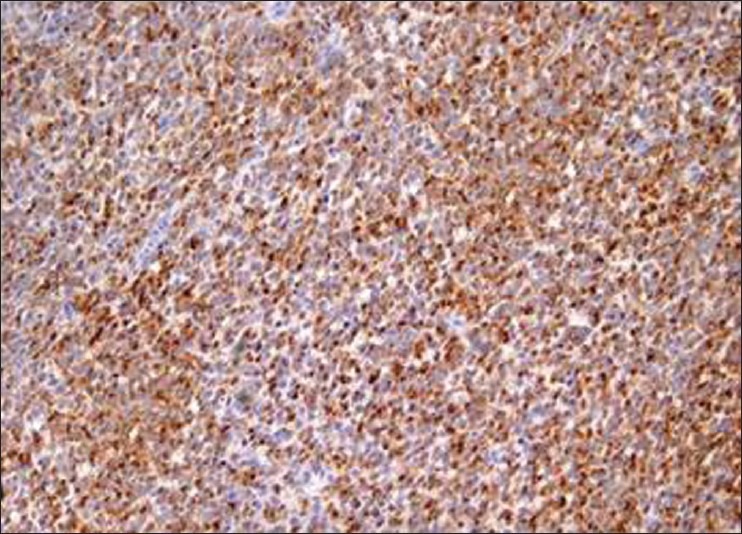
Case 1: Immunohistochemical staining with HMB-45 antibody that stains the cytoplasm of the epithelioid cells
Surgical treatment was not possible because of the large size of the tumor. Radiotherapy for three weeks was initiated. Follow-up showed partial regression of the tumor. Surgery was planned, but the patient never reported back. Ten months later, the patient was hospitalized, but she refused the immunotherapy proposed and accepted only painkillers. Fifteen months later, the patient died. The exact cause of death was not established, because autopsy was not performed.
Case 2
A 42-year-old male patient reported to the Outpatient Department with the chief complaint of a rapidly growing exophytic mass on the left side of his cheek.
The patient was apparently asymptomatic three months back, and then he noticed ulceration over the left buccal mucosa, which was the size of a coin, and then the growth appeared and rapidly increased in size.
An extraoral examination revealed a single, large, firm, nontender, palpable left submandibular lymph node on the left side, which was not fixed to the underlying tissues. A diffuse, firm, tender swelling extending from the left side of the angle of the mouth to the left cheek region and from the inferior border of the mandible to the cheek region was seen. Deviation of the angle of the mouth was also observed [Figure 5].
Figure 5.
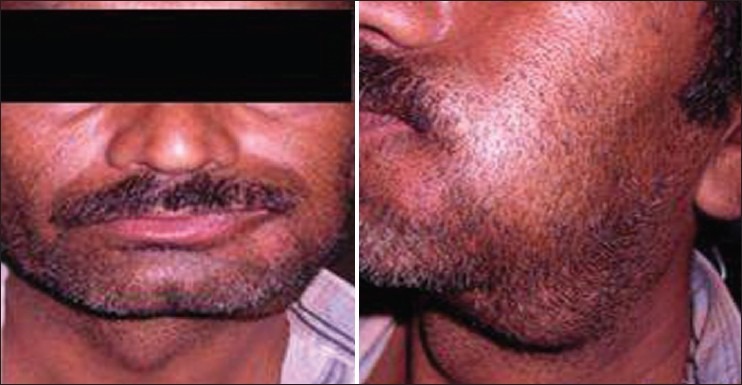
Extraoral photograph showing deviation of face toward the right side and enlarged submandibular lymph nodes
An intraoral examination revealed two brownish black exophytic growths, approximately 3 × 4 cm and 2 × 2 cm in size, with a pebbled surface and firm in consistency, in the left buccal mucosa, extending anteroposteriorly from the commissural area to the 38 region, and superoinferiorly from the buccal vestibule to about 1 cm above the occlusal level. A hyperpigmented area in the retro-molar area on the left side was noticed [Figure 6].
Figure 6.
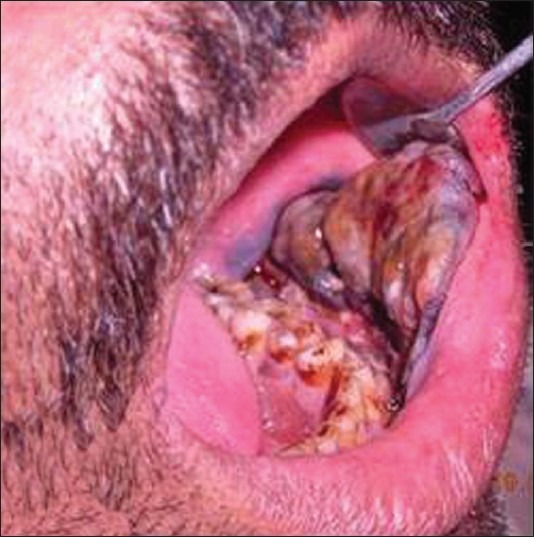
Intraoral photograph showing exophytic growth extending from the commissural area to the 38 region
Incisional biopsy was performed and a histopathological examination revealed dysplastic, oval-to-spindle melanocytes in the lamina propria, interspersed with melanin pigmentation [Figure 7]. The overall clinical, radiological, and histopathological features were confirmative of combined invasive and in situ malignant melanomas, which were further reconfirmed by using an immunohistochemical marker HMB-45 and Melan-A. Wider excision of the lesion was carried out as a treatment approach. A histopathological examination confirmed malignant melanoma, which infiltrated the superficial layer of the corion with a maximum thickness of 1.10. mm, along with regional lymph node metastasis.
Figure 7.
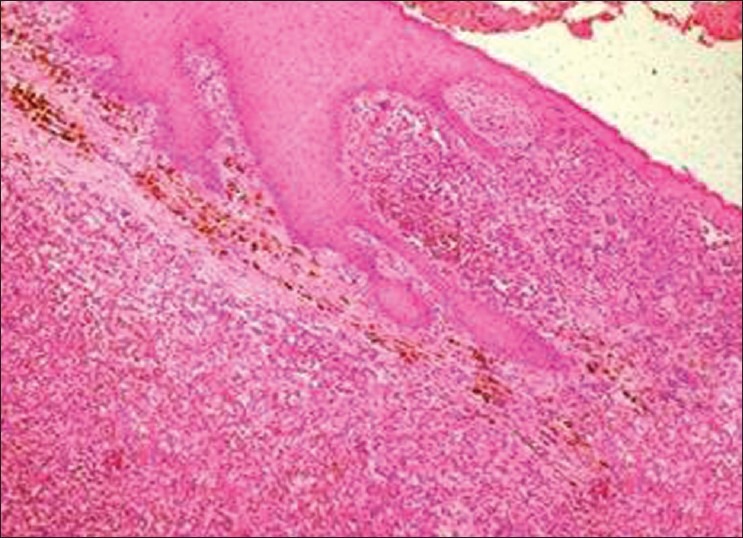
Case 2: Photomicrograph (10×) showing invasive growth pattern and atypical melanocytes and melanophages
Case 3
A 65-year-old-male patient reported to the Outpatient Department with the chief complaint of painful swelling in the left side of the mouth, since 15 days. The patient was apparently asymptomatic 15 days prior, and then he noticed a small, nontender, firm swelling over the upper front gums on the left side.
A radiographic examination was carried out, but no significant finding was observed. An extraoral examination revealed palpable bilateral submandibular lymph nodes, 2 × 2 cm in size, firm, mobile. and nontender. and fixed to the inferior border of mandible. Intraoral examination revealed a blackish, pedunculated, firm tender swelling in the upper gingiva in relation to 21, 22, 23, and 24 of size 0.5 × 1.5 cm. The blackish discoloration of the gingiva was seen in relation to 21 to 28, and from the palatal side it extended in relation to 21, 22, 23. and 26, 27, and 28. Diffuse melanotic areas were observed bilaterally on the buccal mucosa and palate.
Incisional biopsy was performed and histopathological examination revealed atypical melanocytes, interspersed with melanin pigmentation, in the deep connective tissue stroma. The overall clinical, radiological, and histopathological features were confirmative of invasive malignant melanoma, which was further reconfirmed by using the immunohistochemical marker HMB-45 and Melan-A [Figure 8]. Partial maxillectomy along with selective neck dissection was chosen as a treatment approach. Histopathological examination confirmed 3.20 mm thickness gingival melanoma, with level II lymph node involvement.
Figure 8.

Case 3: Immunohistochemical staining with Melan-A antibody showing cytoplasmic staining
Case 4
A 40-year-old female patient reported to the Outpatient Department (OPD) with the chief complaint of painful swelling in the upper right front region of the gums and blackish discoloration over the palatal side. The patient was apparently asymptomatic four to five months back, when she noticed a swelling in the upper right front gums, for which she went to a private practitioner for treatment and was told about blackish discoloration over the right palatal side. A radiographic examination was carried out, but no significant finding was observed. An extraoral examination revealed a single, firm, nontender, palpable, submandibular lymph node of size 5 × 5 cm on the right side. An intraoral examination revealed a blackish discoloration over the interdental area of 21, 22, 23, and an area of blackish discoloration and a nontender, firm, sessile swelling on the palatal aspect of 11 and 12, of size 1 × 1 cm, with irregular margins [Figure 9].
Figure 9.
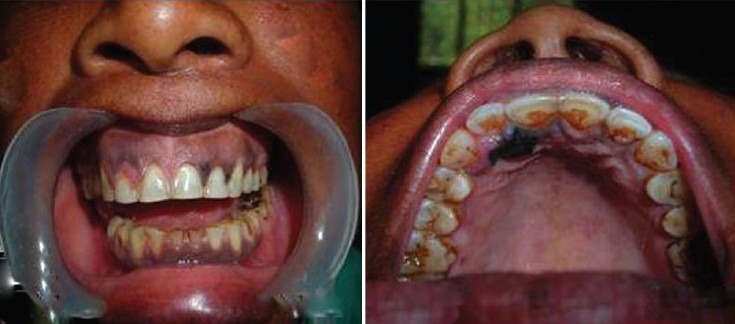
Intraoral photograph showing blackish discoloration of the gingiva and palate
Incisional biopsy was performed and histopathological examination showed oval-to-spindle neoplastic melanocytes in the connective tissue stroma [Figure 10]. The overall clinical, radiological, and histopathological features were confirmative of invasive malignant melanoma, which was further reconfirmed by using immunohistochemical markers.
Figure 10.
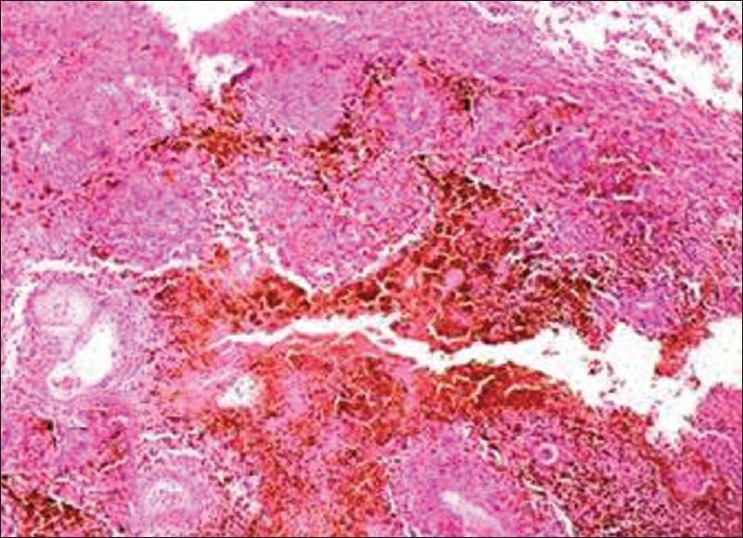
Case 4: Photomicrograph (10×) showing atypical melanocytes in the connective tissue stroma
With the diagnosis of primary malignant melanoma of the gingiva, surgical intervention was performed and the gingival lesion along with the left maxillary alveolar process was resected with macroscopic free margins. Reconstruction of the defect was done with buccal fat pad flap and postoperative recovery was found to be uneventful. Histopathology confirmed melanoma with 1.50 mm tumor thickness with level I lymph node involvement. The patient was under follow-up without any recurrence.
DISCUSSION
Oral malignant melanoma may demonstrate significant heterogeneity in the morphological features, developmental process, and biological behavior.[6]
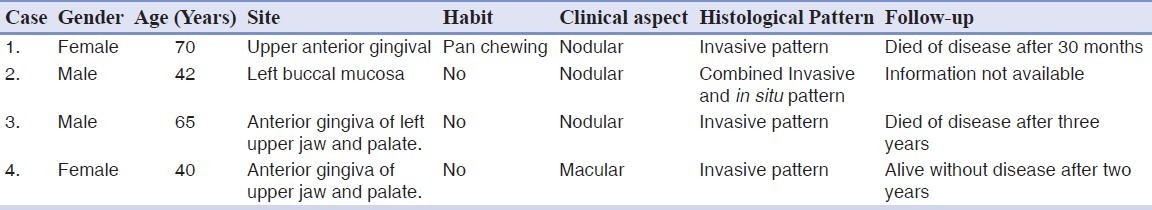
Indian studies showed that 20.41 to 34.4% of all melanomas were on the mucosal surface, and among them, 16% of the tumors were intraoral. Hashemi Pour reported an age range between 56 and 77 years for occurrence of malignant melanoma. The mean age of patients was 69.2 years. From different studies, the male-to-female ratio ranged between 1 / 1 and 2 / 1.[5] Oral melanoma was generally included in the category of acral-lentiginous melanoma. It occurred most frequently in the maxilla, with the palate as a common site (32% incidence) followed by maxillary gingiva (16%) and other affected regions, in descending order of incidence are the buccal mucosa, mandibular gingiva, lips, tongue, and buccal floor.[7] In our studied cases, three cases were found on the upper gingiva whereas, one case was found on the buccal mucosa, with a male to female ratio of 1 : 1, which fell well within the data given in the literature [Table 1].
Table 1.
Clinical–pathological findings in patients with primary oral mucosal melanoma
Primary oral melanoma is considered only when the following criteria, described by GREENE (1953), are fulfilled: Demonstration of melanoma in the oral mucosa, presence of junctional activity, and inability to demonstrate extraoral primary melanoma.[1] Our cases described here, justify the criteria to be considered as primary oral melanoma.
A diagnosis of melanoma depends on the identification of the melanin pigment that is confirmed by the Fontana-Masson silver stain and the appropriate immunohistochemical staining pattern that includes HMB-45 antibodies, Melan-A, tyrosinase, and antimicropthalmia transcription factor. S-100 protein is always positive in melanomas.[8] All our cases showed marked positivity toward HMB-45 and Melan-A, confirming the diagnosis of malignant melanoma.
For the POM, there are no geographic differences as occur with its cutaneous homolog, since there is no evidence of the influence of ultraviolet (UV) radiation in the development. The POM is very aggressive and as in its beginning it may pose a stable clinical aspect, differential diagnosis with other entities such as Addison's disease, Peutz Jeghers's syndrome or Kaposi's sarcoma must be performed. Also, with melanin pigmentations, both from the racial or irritative causes, melanocytic nevi, melanoacanthoma, and other pigmentations of exogenous cause, such as, amalgam tattoo, must be considered.[9] Delgado Azañero et al. has presented a practical and technically simple method for the clinical diagnosis of POM, which allows differentiating this neoplasia from other pigmented lesions. The clinical test consists of rubbing the surface of the lesion with gauze, with the objective of verifying if it stains black due to the presence of melanin pigment on its surface. The authors refer that a positive result was obtained in 84.6% of the cases, and that the method possesses an elevated sensibility to anticipate diagnosis, and that a negative result does not exclude the possibility of this neoplasia, as there are cases in which the malignant cells have not invaded the superficial layers of the epithelium.[10] The melanoma that involves the mucosa when presented clinically as nodular, with a vertical growth that invades the submucosa of the region of the head and neck, is more aggressive. The prognosis is poor, based on the histological type, the depth at the microscopic level of the tumor's invasion, and its localization. The literature points out that in the oral cavity it occurs with greater frequency in the mucosa that covers the bone tissue, such as, the hard palate and gingiva. These localizations play a significant role in the early invasion of the adjacent bone, being an additional reason for the poor prognosis presented by this pathology. The precursors are not well identified; some authors point out an atypical melanocytic hyperplasia (increase in the cell number) that proliferate in a previous stage to the apparition of the neoplasia, others refer to a pre-existing melanosis when these precursors will be the initial stage of a prolonged evolution of horizontal growth, before entering into the invasive stage of vertical growth. Other authors also consider these as precursors of the melanocytic nevi, and qualify as interesting, the coincidence that in the oral activity, these are localized with higher frequency in the hard palate as the melanoma. Weber described POM for the first time in 1859, and clear classification criteria have not been incorporated, as they exist for the cutaneous melanomas. Many classifications have been established, but none have been universally accepted.[9]
Oral melanomas should be separated from cutaneous lesions and they can be simply subclassified into in situ and invasive types. It might also be useful, for natural history considerations, to identify invasive melanomas with an in situ component, because these lesions could have originated from an in situ melanoma. In cases of oral melanocytic lesions in which microscopic cytology or architecture was equivocal, the designation ‘atypical melanocytic proliferation’ could be used. This term would be a working diagnosis, and definitive diagnosis would necessitate a clinical–pathological correlation, re-biopsy, and clinical follow-up.[11] ABCDE (asymmetry, border irregularities, color variegations, diameter greater than 6 mm, and elevation on a raised surface) criteria used in detection of cutaneous melanoma could also be helpful in the diagnosis of oral melanomas.[4] In the WESTOP Banff workshop, 50 cases of malignant melanoma were reviewed, and it was found that approximately 15% of the cases were in situ mucosal lesions, 30% were invasive lesions and 55% of the lesions had a combined pattern.[11] In our four studied cases, three cases were found to be invasive melanoma and one case showed a combined pattern [Table 1]. On account of the apparent late stage of these lesions, it could not be determined whether the in situ component occurred before or after the invasive component. However, these combined lesions often had a clinical history of a pre-existing pigmented patch in the site of tumor development.
The relative inaccessibility of the mucosa to self-examination often delays diagnosis, leading to late detection and poor survival. At presentation, approximately 13 to 19% of the patients have lymph node metastases and another 16 to 20% are likely to develop metastases subsequently. The aggressive behavior of oral malignant melanoma is particularly problematic. Malignant melanoma in the oral cavity accounts for 0.2 to 8.0% of all malignant melanomas, and it has a much poorer prognosis than its counterpart on the skin.[4] Prognosis, although poor, is highly variable. After five years the survival rate for patients with oral melanomas has been reported to vary between 5.2 and 20%, with a steady decline in survival after the traditional measure of five years.[6] On the other hand, good results have been reported and it has been emphasized that the disease is potentially curable if diagnosed and treated at an early stage.[6]
Surgery remains the most effective treatment for malignant melanoma and aggressive surgical control of the local disease may result in prolonged disease-free survival.[6] Although wide resection with a surgical margin of 20 to 50 mm from a cutaneous melanoma is considered satisfactory, this kind of resection is not always possible for oral malignant melanoma.[12]
CONCLUSION
It is essential to include oral examination in full body skin examinations. For prevention of oral mucosal melanomas, any solitary pigmentation having no obvious explanation should always be biopsied. The ‘chameleonic’ presentation of a mainly asymptomatic condition, the rarity of these lesions, the poor prognosis, and the necessity of a highly specialized treatment, are factors that could influence the diagnostic and management process of these malignancies. Vigilant comprehensive analysis of published cases and recognition of new ones may be helpful in establishing definite classification and proposing clinical features that would facilitate its early diagnosis, as a prerequisite for timely treatment and better prognosis of this rare pathology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aguas SC, Quarracino MC, Lence AN, Lanfranchi-Tizeira HE. Primary melanoma of the oral cavity: Ten cases and review of 177 cases from literature. Med Oral Patol Oral Cir Bucal. 2009;14:E265–71. [PubMed] [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics. Lyon: IARC Press; 2005. World Health Organization classification of head and neck tumours. [Google Scholar]
- 3.Barker BF, Carpenter WM, Daniels TE, Kahn MA, Leider AS, Lozada-Nur F. Oral mucosal melanomas: the WESTOP Banff workshop proceedings. Western Society of Teachers of Oral Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:672–9. doi: 10.1016/s1079-2104(97)90318-8. [DOI] [PubMed] [Google Scholar]
- 4.Delgado Azañero WA, Mosqueda Taylor A. A practical method for clinical diagnosis of oral mucosal melanomas. Med Oral. 2003;8:348–52. [PubMed] [Google Scholar]
- 5.Dimitrakopoulos I, Lazaridis N, Skordalaki A. Primary malignant melanoma of the oral cavity-Report of an unusual case. Aust Dent J. 1998;43:379–81. [PubMed] [Google Scholar]
- 6.Freedberg IM, Wolff K, Austen KF. Dermatology in general medicine. 5th ed. United States: Mc Graw-Hill; 1999. pp. 981–1097. [Google Scholar]
- 7.Gondivkar SM, Indurkar A, Degwekar S, Bhowate R. Primary oral melanoma- A case report and review of literature. Quintessence Int. 2009;40:41–6. doi: 10.3290/j.qi.a14113. [DOI] [PubMed] [Google Scholar]
- 8.Norhafizah M, Mustafa WM, Sabariah AR, Shiran MS, Pathmanathan R. Mucosal malignant melanoma of the maxillary sinus. Med J Malaysia. 2010;65:211–3. [PubMed] [Google Scholar]
- 9.Hashemi Pour MS. Malignant melanoma of the oral cavity: A review of literature. Indian J Dent Res. 2008;19:47–51. doi: 10.4103/0970-9290.38932. [DOI] [PubMed] [Google Scholar]
- 10.Leesa NL, Moleri AB, Merly AF, Moreira LC, Moreira MJ, Antunes HS. Oral melanoma: An unusual presentation. Dermatol Online J. 2008;14:17. [PubMed] [Google Scholar]
- 11.Regezi JA, Sciubba J. Oral Pathology. 2nd ed. Philadelphia: WB Saunders; 1993. pp. 165–71. [Google Scholar]
- 12.Tanaka N, Amagasa T, Jwaki H. Oral malignant melanoma in Japan. Oral Surg Oral Med Oral Pathol. 1994;78:81–90. doi: 10.1016/0030-4220(94)90121-x. [DOI] [PubMed] [Google Scholar]


