Abstract
Liver transplantation is one of the treatments for many-life threatening liver diseases. Numerous advances in liver transplant surgery, anaesthesia and perioperative care have allowed for an increasing number of these procedures. The purpose of this review is to consider some of the important advances in perioperative care of liver transplant patients such as pre-operative evaluation, intraoperative monitoring and management and early extubation. A PubMed and EMBASE search of terms “Anaesthesia” and “Liver Transplantation” were performed with filters of articles in “English”, “Adult” and relevant recent publications of randomised control trial, editorial, systemic review and non-systemic review were selected and synthesized according to the author's personal and professional perspective in the field of liver transplantation and anaesthesia. The article outlines strategies in organ preservation, training and transplant database for further research.
Keywords: Adult liver transplantation, anaesthesia, hepatopulmonary syndrome, hepatorenal syndrome, liver transplantation, organ preservation, recent advances, reperfusion injury
INTRODUCTION
Liver transplantation remains one of the treatments for many life-threatening liver diseases. The first human liver transplantation was performed in 1963 in Denver, Colorado.[1] Around 350 liver transplants have been performed from 1995 to 2011 in India.[2] Numerous advances in the perioperative management, like expertise in surgical techniques, better pre-operative optimisation, intraoperative monitoring and management, changes in immunosuppression regime and advances in post-operative management not only increased the number of this procedure but also the outcome. One-year survival post-liver transplant has increased from 72% to 79% in 1998 to 85% to 90% in 2008. Ten-year survival has increased from 33% in 1998 to 53% in 2008 and 66% in 2010.[3,4] Anaesthesia for liver transplantation[5,6] and post-operative management of liver transplant recipients[7] have been reviewed recently. The purpose of this review is to consider some of the important advances in perioperative care of liver transplant patients, in particular to cadaveric liver transplantation, as there has been a recent review on live related liver transplantation by one of the authors.[8]
PRE-OPERATIVE EVALUATION FOR LIVER TRANSPLANTATION
Prognostic indices for a 1-year survival without liver transplantation, e.g. Child-Turcotte-Pugh or Revised Model for end-stage liver disease (MELD) scores, in patients with chronic liver disease determines the urgency for liver transplantation.[9] A thorough assessment of the patient's comorbidities, both associated with and independent of liver failure, actuates the perioperative risks.[10] A summary of the investigations considered in the transplant period is listed in Table 1.
Table 1.
Investigations for a liver transplant recipient
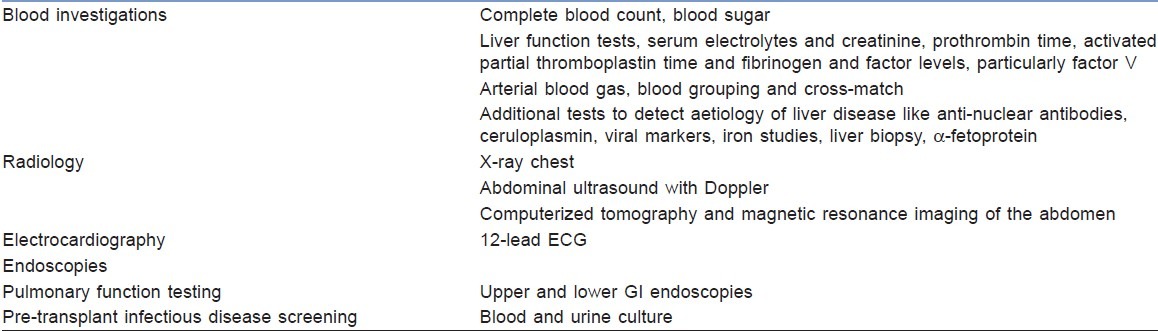
Pre-transplant infections disease screening (dental, ophthalmic and otorhinolaryngological and urogenital) of both donors and recipients is necessary to avoid post-operative infectious complications. A psychosocial assessment of the transplant recipient will help in identifying the negative factors affecting the outcome and to develop interventions to prevent it.
Cardiac evaluation
A transthoracic echocardiography (TTE) on all patients undergoing evaluation for liver transplantation assesses the structural and functional status of the heart.
Coronary artery disease
A 5–26% incidence of Coronary artery disease (CAD) exists in patients undergoing liver transplantation.[11] Patients with risk factors for CAD, such as diabetes, non-alcoholic steato hepatitis (NASH), previous CAD and peripheral vascular disease need further evaluation to rule out CAD. Presence of three out of four cardiac risk factors, i.e. age more than 50 years, hypertension, elevated cholesterol and obesity, requires stress testing. Patients with two or more risk factors could undergo non-invasive computerized tomography to measure their calcium stores and a positive value (more than 100) should undergo stress testing.[11]
Exercise stress tests are difficult to perform in patients with chronic liver disease due to the patient's limited functional capacity. Dobutamine stress myocardial perfusion imaging and dobutamine stress echocardiography can be done for detecting myocardial ischemia in liver disease patients.
Coronary angiography should be followed in patients with abnormal stress test results to rule out CAD. The American College of Cardiology/American Heart Association recommends coronary angiogram in patients more than 40 years of age as part of evaluation for transplantation if they have a known or suspected CAD or have stable angina.[12] While there is no definite guideline as to when patients with CAD should undergo revascularization, there are case reports of combined liver transplant and cardiac surgery, particularly from high-volume centres.[13] Liver transplant in patients with known CAD can be delayed for medical optimization and/or revascularization.[14]
Cirrhotic cardiomyopathy
Cirrhotic cardiomyopathy characterized by abnormal or blunted cardiovascular response to stress, is present in as many as 50% of the patients undergoing liver transplant. These patients have inotropic and chronotropic incompetence, systolic and diastolic dysfunction, prolonged QT and electrical and mechanical dyssynchrony. Yet, these patients demonstrate a normal or increased myocardial contractility at rest.[15]
Rapid haemodynamic changes during clamp release of the inferior vena cava increases filling pressures of the heart. There is an increased risk of congestive heart failure due to the impaired diastolic ventricular relaxation.
Pulmonary evaluation
Hepatopulmonary syndrome
In about 8–24% of patients with liver disease, Hepatopulmonary syndrome (HPS) exists. This is characterized by a decreased systemic arterial oxygenation (PaO2 less than 80 mmHg or an alveolar arterial gradient of more than 15 mmHg on room air), pulmonary vascular dilation [contrast-enhanced echocardiography or abnormal uptake in the brain (more than 6%) with radioactive lung perfusion scanning] and liver disease.
Intravenous bubble contrast test can distinguish HPS from intracardiac shunts such as patent foramen ovale.[16]
Portopulmonary hypertension
Portopulmonary hypertension (POPH) is characterized by increased mean pulmonary artery pressure (mPAP) greater than 25 mmHg at rest or greater than 30 mmHg with exercise, elevated pulmonary vascular resistance greater than 240 dyne/s/ cm-5 and normal or decreased pulmonary artery wedge pressure less than 15 mmHg. Among patients awaiting liver transplantation, the incidence of POPH is 2–10%.
A screening TTE uses tricuspid regurgitation to estimate right ventricular systolic pressure (RVSP).If the RVSP is more than 50 mmHg, these patients should undergo right heart catheterization to exclude other causes of pulmonary hypertension, such as high flow state and increased central volume and/or left ventricular dysfunction causing increased mPAP.[16]
Hepatic hydrothorax
In cirrhotic patients, a pleural effusion greater than 500 mL without cardiac, pulmonary or pleural disease is termed hepatic hydrothorax.[17]
Renal evaluation
Renal function is an important predictor of survival with chronic liver failure and liver transplantation.[18]
Hepatorenal syndrome (incidence 18–40%) is a diagnosis of exclusion in patients with chronic liver insufficiency. It is characterized by liver disease, serum creatinine concentration more than 1.5 mg/dL (133 mmol/L) that is not reduced with the administration of albumin after discontinuation of diuretics for 2 days, absence of nephrotoxic drugs, shock and absence of findings suggestive of renal parenchymal disease (urinary protein greater than 500 mg/day, more than 50 red cells/high-power field or abnormal kidneys on ultrasonography).[19]
INTRAOPERATIVE MONITORING AND MANAGEMENT
Haemodynamic monitoring
Haemodynamic monitoring is essential for successful liver transplantation.[20] Additional to the standard cardiovascular monitors (electrocardiogram, pulseoximetry, invasive and non-invasive blood pressure), there is a need for cardiac output monitoring. There are numerous invasive and non-invasive cardiac output monitors available to monitor the haemodynamic change associated with liver transplantation.
Pulmonary artery catheter (PAC) is the gold standard used in haemodynamic monitoring during liver transplant to which other monitors have been compared.[21] However, PAC has its own limitations, including poor real time updating, catheter migration and wedging. Furthermore, wedge pressure used to estimate left ventricular end diastolic pressure (LVEDP) as pre-load may be misleading in patient with liver disease. Table 2 shows the comparison of cardiac output monitors used in liver transplantation.[22–29]
Table 2.
Haemodynamic monitors in liver transplant
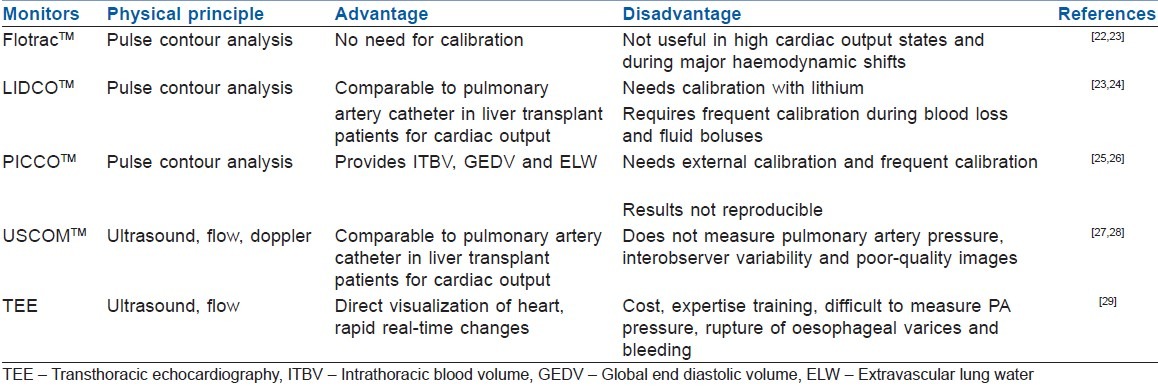
Monitoring of central venous oxygen saturation and mixed venous oxygen saturation during liver transplantation is of little value as they do not reflect changes in cardiac output nor oxygen supply demand mismatch during rapid haemodynamic disturbances.[30,31]
Haemodynamic management
End-stage liver disease is often a high cardiac output state with decreased systemic vascular resistance and depleted intravascular volume. During the different stages of liver transplantation, i.e. pre-anhepatic phase, anhepatic phase and neohepatic phase, there are rapid fluid shifts due to blood loss, inferior vena cava clamping and reperfusion.
Proactive attempts to lower the central venous pressure (CVP) either by phlebotomy or avoiding plasma transfusion during the pre-anhepatic phase have shown to reduce red cell transfusions,[32] but with variable outcomes in post-operative renal status.[33,34] Cywinsky et al. in their retrospective review of data question the reduced graft dysfunction due to lower CVP in neohepatic phase.[35]
Patients with liver disease have been shown to have lower vasopressin levels. Vasopressin is often added intraoperatively to maintain the systemic vascular resistance.[36] Vasopressin reduces portal blood flow by selective splanchnic vasoconstriction and hence may be useful in reducing the intraoperative blood loss.[37] There is no evidence to date on the vasopressor of choice in maintaining the blood pressure during Orthotopic liver transplantation. Epinephrine; norepinephrine and dopamine have all been used.[38] Methylene blue at a dose of 0.5 mg/kg body weight over 10 min can be used as a rescue to treat hypotension due to vasopressor-resistant vasoplegic shock.[39] Phenylephrine is often used to tide over acute hypotensive episodes; however, bear in mind that it increases the pulmonary vascular resistance.
Pulmonary vasodilators [intravenous prostacyclin (epoprostenol) and inhaled iloprost] have shown improvement in POPH, but the optimal management strategy has not yet been defined due to lack of randomized control trials.[40]
Fluid management
Liver transplant surgery is associated with massive fluid shifts both from the perspective of intravascular volume depletion and large surgical blood loss. Albumin can be used in liver transplants as the patients are often hypoalbuminaemic and hypovolaemic;[41] however, the cost factor may factor in the decision of its use. The use of hydroxyl ethyl starches (modern low-molecular weight) and Gelatins in liver transplant has not been supported by convincing evidence. Furthermore, these may affect the coagulation profile and possesses a risk to increased renal injury.[42]
The selection of various crystalloids during OLT should be based on their pH, electrolyte composition, osmolarity and metabolism. There is no ideal crystalloid solution and all have limitations; normal saline (0.9% NS) causes hyperchloremic acidosis while the lactate in Ringers Lactate (RL) requires liver metabolism for its elimination. Further, RL is a hypotonic solution and may increase the intracellular fluid. Plasmalyte has a pH near normal, electrolyte and osmolarity similar to plasma and acetate, which is metabolised extrahepatically to bicarbonate, but it is proinflammatory and potentially cardiotoxic.[43]
Haemostasis and liver disease
Patients for liver transplant present with a wide range of coagulation profiles ranging from normal coagulation (hepatocellular carcinoma) to gross coagulopathy (fulminant hepatic failure).
Some consider the coagulopathy of liver failure to be a balanced coagulopathy having an equal decrease in pro- and anti-coagulant factors.[44] The new state of coagulation lies in a narrow balance and hence can be tipped to either side by the complications of liver disease such as renal failure or infection resulting in bleeding, thrombosis or both.[44]
Coagulation monitors
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) have been found to have a limited role in OLT as they measure only the procoagulant pathway without considerations for platelets function or fibrinolysis.[45] Thromboelastogram (TEG), rotational thromboelastometry (ROTEM) and Sonoclot measure the viscoelastic properties of blood during all stages of thrombus formation, tests stability, firmness of the clot and fibrinolysis. Hence, they provide a detailed assessment of both pro- and anti-coagulant status of the blood.[46] Use of TEG and ROTEM has been found to reduce the transfusion requirements in OLT,[47] but it is important that these systems are well validated and supported by a coagulation laboratory.[48]
Blood component management
Pre-anhepatic phase is associated with blood loss due to dissection of the native liver, particularly in patients with end-stage liver disease. Haemodilution and hypothermia, resulting in altered coagulation status, add on to the pre-existing anaemia and coagulopathy. Aggressive correction of the coagulation status may lead to hypervolaemia and increasing blood loss in addition to the effect on ionized hypocalcaemia. The aim at this stage is to avoid large volume of transfusion and dilutional coagulopathy.[5] During the anhepatic phase, there is ionized hypocalcaemia, failure of clearance of thromboplastin and a rapid rise in tissue-type plasminogen activator with increase in plasmin activity and a hyperfibrinolytic state in addition to the pre-existing coagulopathy.[5]
Antifibrinolytics are used in the liver transplantation to prevent the hyperfibrinolytic state that may occur during the anhepatic and neohepatic phases due to increased tissue plasminogen activator in the absence of plasminogen activator inhibitor and alpha 2 antiplasmin. Use of antifibrinolytics has been questioned by the occurrence thromboembolic complications,[49] but a recent metaanalysis fails to reveal any such association.[50] Antifibrinolytics are used if there is evidence of fibrinolysis and there are no contraindications for its use, such as Budd-Chiari syndrome or hypercoagulable states. In Donation after Cardiac Death (DCD) donors where tissue plasminogen activator (tPA) has been given to prevent biliary complications, the use of antifibrinolytics may be concerning.[50]
The neohepatic phase is associated with a multifactorial coagulopathy of hyperfibrinolysis, dilutional coagulopathy, heparin-like effect, platelet dysfunction, hypothermia and hypocalcaemia.[5]
There is no evidence on which to base cut-off values for haemoglobin or coagulation parameters need to be corrected prior to blood product transfusion. However, there is evidence to support the association between red cell, fresh frozen plasma or platelet transfusion and an increased morbidity and mortality,[51] but considerations should be made in interpreting this data as massive transfusion is a result of either massive blood loss or coagulopathy.
Intraoperative blood salvage and retransfusion of the autologous blood is a cost-effective method to reduce the need for allogenic red blood cells in OLT provided there are no contraindications to cell savage[52] and the centre is a high turnover (with a minimum of 80 cases of cell savage per year).[53]
There is no evidence to support the routine use of recombinant factor VIIa in liver transplantation. However, there are several case reports where it has been used in massive bleeding.[54] Analysis of randomized controlled trials for the safety of off -label use of factor VIIa found an increased incidence of arterial thrombosis.[55]
Neuro monitoring
Patients with liver disease undergoing transplantation are at risk of a wide range of neurological complications, including cerebral oedema, encephalopathy, seizures, hypoxia and central pontine myelinolysis.[56] Conventional intracranial pressure (ICP) monitoring in liver transplant patients is complicated by bleeding and infection without any survival advantage.[57] In acute liver failure patients, the use of transcranial Doppler, which measures cerebral perfusion pressures, has proven advantages. However, its use in the perioperative period has yet to be defined.[58]
The inhalational anaesthetic requirement is less for patients with liver disease compared with healthy patients.[59] Anaesthetic requirements vary along with their MELD scores in different phases of liver transplantation.[60] Bispectral index (BIS) monitoring has been used in the peritransplant period to monitor consciousness and hence can be used intraoperatively to titrate the anaesthetic requirement.[61] The routine use of BIS monitoring in OLT is of limited value because of its cost and minimal clinical utility in decision making.[62]
Neurological management
Patients with elevated ICP should be placed with head elevated by 30°. Osmotic diuresis with IV mannitol is effective in reducing cerebral oedema, but runs the risk of fluid overload and pulmonary oedema in patients with hepatorenal syndrome.
In the setting of intracranial hypertension refractory to mannitol therapy, therapeutic hypernatraemia (serum sodium 145–155 mmol/L) may be used. Moderate hypothermia (32°–34°C) may also prevent or control elevated ICP. Induction of a barbiturate coma should be considered if osmotic therapy and hypothermia fail.[63] Mechanical hyperventilation to reduce pCO2, except for temporary reduction in raised ICPs, is no longer considered as an optimum intervention.[64]
ORGAN PRESERVATION
The aim of organ preservation is to maintain the viability of the donor graft and to allow for the restoration of normal function of the organ.
Cold storage and perfusate
Static cold storage (SCS) is the standard method of liver preservation. It is based on the principle that hypothermia reduces the metabolism and enzymatic activity and slows down cell death, but during the process it causes cellular oedema. Cellular oedema is prevented by addition of various substances to the preservative fluid. Euro Collins, University of Wisconsin (UW), Histidine-Tryptophan-Ketoglutarate (HTK), Marshall's hypertonic citrate (HOC) and Celsior are few of the perfusates being used in liver preservation.[65,66]
UW and HTK are the most common perfusate fluids used in liver transplantation. UW being expensive with increased viscosity and a high potassium/low sodium ratio (120 mM/L, 25 mM/L) has been its major disadvantages. HTK is inexpensive, less viscous and has a low potassium/high sodium ratio (9 and 15 mM/L) and has found a larger acceptance in liver transplantation.[67] HTK contains low potassium and therefore does not need to be flushed before reperfusion.[66]
Feng et al. in their metaanalysis of studies comparing UW and HTK did not find any major differences in outcome in liver transplants from live donor or from heart beating donors, but found that HTK is better at preventing biliary complications and more bile production in patients with marginal non-heart beating donors. This is probably due to better flushing as a result of low viscosity.[68]
Machine perfusion
Hypothermic machine perfusion (HMP) combines the advantage of protecting the organ with hypothermia and continuous perfusion to restoration of energy and oxygenation.[69] Although extensively used in renal and in lung transplantation, the evidence for HMP in liver transplantation is based mainly on animal data. There is a paucity of publications on the benefits in human subjects. Guarrera et al.[70] show that in 20 liver transplant patients there was a benefit of HMP over SCS.
The use of normothermic machine perfusion (NMP) permits continuous machine perfusion while avoiding the complications of hypothermia, such as cold preservation injury and ischaemic rewarming during reperfusion, and provides nutrition at physiological temperatures.[71] However, there are no human studies till date. The advantage of HMP and NMP has to be tested in large randomized human clinical trials before being put into practice.
Ischemia reperfusion injury
During the periods of ischemia and reperfusion of the liver, there is a microcirculatory failure, activation of Kupffer cells and production of reactive oxygen species, inflammatory responses and apoptosis of the hepatocytes.[72]
Ischemia reperfusion injury (IRI) is associated with primary graft dysfunction and delayed graft function. Strategies of prevention of IRI include ischaemic preconditioning (IP) and pharmacological management.
A recent review on the effect of ischaemic preconditioning on liver transplant (live and deceased donor) shows evidence of IP on decreasing the IRI in liver transplant patients. But, most of the studies use small sample size and the periods of IP are not uniform.[73]
Some of the pharmacological manipulations aiming at reduction in IRI include antioxidants, free radical scavengers and preconditioning.
N-acetyl cysteine (NAC) is being used in liver transplantation patients to prevent renal failure and IRI of the new liver. NAC, in addition to its direct antioxidant property, replenishes glutathione and acts as a free radical scavenger.[74] Systemic review of the use of NAC in liver transplantation for the purpose of preventing IRI of the graft liver is inconclusive.[75] A recent RCT with use of NAC for preventing hepatorenal IRI did not show any improvement.[76]
Inhalational anaesthetics, especially sevoflurane, have been shown to offer protection against IRI in the myocardium in cardiac patients.[77] There is some evidence to suggest that sevoflurane possess the same properties in liver surgery patients by reduced transaminase levels.[78] Infusion of remifentanil before induction of ischaemia has been shown to reduce ischaemic injury in rat liver model, and its clinical use has yet to be determined.[79]
FAST TRACKING AND EARLY EXTUBATION
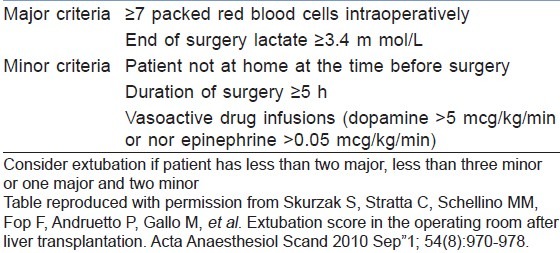
Early extubation in selected patients has shown to improve early graft function and reduce duration of stay in the Intensive Care Unit and nosocomial infections. However, selection of patients for early extubation depends on duration of the surgery, amount of blood and products transfused, patients’ pre-operative status (MELD score), ischaemia time and status of the graft.[80,81] A safe operating room extubation after liver transplantation (SORELT) prediction rule may be used to select patients for early extubation, but requires validation[82] [Table 3].
Table 3.
Safe operating room extubation after liver transplantation score
OUTCOMES OF LIVER TRANSPLANTATION
Graft dysfunction is one of the dreaded complications of liver transplantation. Various factors predict the occurrence of graft dysfunction[83] [Table 4]. A simple model of donor age and pre-operative MELD (D-MELD) defined a subgroup of donor–recipient matches with significantly poorer short- and long-term outcomes (D-MELD>1600).[84]
Table 4.
Predictors of graft dysfunction

COST-EFFECTIVENESS IN PERIOPERATIVE CARE FOR LIVER TRANSPLANTATION
The perioperative management of liver transplant is not uniform across the institutions and countries. Cost of the individual procedures plays a major role in the decision making during the perioperative care. Recently, a cost-effective analysis of liver transplantation perioperative care modalities in low- and medium-income countries has been published, suggesting the best way to maximize cost-effectiveness in a resource-limited environment[85] [Table 5].
Table 5.
Cost-effective modalities in perioperative care

CENTRAL DATABASE, RESEARCH AND TRAINING
At present, the number of centres performing liver transplantation in India is limited, but the number is steadily increasing.
An electronic central database of organ transplants (including liver), like the United Network for Organ Sharing (UNOS) database of the United States, has been established by the Indian Society of Organ Transplantation to collect the information, to help in long-term follow-up and in research studies that can improve future patient care.[2] Simulation and porcine model can be used as a teaching tool for training in management of liver transplantation.[86,87]
CONCLUSION
Liver transplantation has evolved over the years in terms of pre-operative preparation, perioperative monitoring and management, organ perfusion and fast tracking. Studies are needed in the areas of haemodynamic monitoring, blood and coagulation management and fast tracking to prove the efficacy of one technique over the other. Training anaesthesia residents with simulation and animal models will prepare them to face the challenge of managing these complicated patients. Creating a national database will help in future research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Starzl TE, VonKaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantaion of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Indian society of organ transplantation. Home page. [Last cited in 2012 April 16]. Available from: http://www.transplantindia.com/index.asp .
- 3.The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Home page. [Last cited in 2012 April 16]. Available from: http://www.srtr.org/annual_reports/2010/survival_rates.htm .
- 4.Åberg F, Isoniemi H, Höckerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- 5.Hannaman MJ, Hevesi ZG. Anesthesia care for liver transplantation. Transplant Rev (Orlando) 2011;25:36–43. doi: 10.1016/j.trre.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu LL, Niemann CU. Intraoperative management of liver transplant patients. Transplant Rev (Orlando) 2011;25:124–9. doi: 10.1016/j.trre.2010.10.006. Epub 2011 Apr 21. [DOI] [PubMed] [Google Scholar]
- 7.Gopal PB, Kapoor D, Raya R, Subrahmanyam M, Juneja D, Sukanya B. Critical care issues in adult liver transplantation. Indian J Crit Care Med. 2009;13:113–9. doi: 10.4103/0972-5229.58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang GS, McCluskey SA. Anesthesia and outcome after partial hepatectomy for adult-to-adult donor transplantation. Curr Opin Organ Transplant. 2010;15:377–82. doi: 10.1097/MOT.0b013e3283387f75. [DOI] [PubMed] [Google Scholar]
- 9.Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952–60. doi: 10.1053/j.gastro.2011.02.017. Epub 2011 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keeffe EB, Ahmed A. In: Zakim & Boyer's Hepatology. 6th ed. Philadelphia: Elsevier; 2006. Section VIII: Liver transplantation; pretransplant evaluation and care; pp. 933–45. [Google Scholar]
- 11.Mandell MS, Lindenfeld J, Tsou MY, Zimmerman M. Cardiac evaluation of liver transplant candidates. World J Gastroenterol. 2008;14:3445–51. doi: 10.3748/wjg.14.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod D, Koffron A, Dewolf A, Baker A, Fryer J, Baker T, et al. Safety and efficacy of combined orthotopic liver transplantation and coronary artery bypass grafting. Liver Transpl. 2004;10:1386–90. doi: 10.1002/lt.20244. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Palli G, Cárdenas A. Pre operative cardio pulmonary assessment of the liver transplant candidate. Ann Hepatol. 2011;10:421–33. [PubMed] [Google Scholar]
- 15.Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539–49. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 16.Yeshua H, Blendis LM, Oren R. Pulmonary manifestations of liver diseases. Semin Cardiothorac Vasc Anesth. 2009;13:60–9. doi: 10.1177/1089253209334615. [DOI] [PubMed] [Google Scholar]
- 17.Singh C, Sager JS. Pulmonary complications of cirrhosis. Med Clin North Am. 2009;93:871–83. doi: 10.1016/j.mcna.2009.03.006. viii. [DOI] [PubMed] [Google Scholar]
- 18.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–85. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. Epub 2010 Jun 1. Review. [DOI] [PubMed] [Google Scholar]
- 20.Luo CF, Hei ZQ, Luo GJ, Li SR, Ma WH, Chi XJ. Significance of hemodynamic changes and monitoring value of patients with severe hepatitis during perioperative orthotopic liver transplantation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:727–9. [PubMed] [Google Scholar]
- 21.De Wolf AM. Pulmonary artery catheter: Rest in peace? Not just quite yet… Liver Transpl. 2008;14:917–8. doi: 10.1002/lt.21543. [DOI] [PubMed] [Google Scholar]
- 22.Pugsley J, Lerner AB. Cardiac output monitoring: Is there a gold standard and how do the newer technologies compare? Semin Cardiothorac Vasc Anesth. 2010;14:274–82. doi: 10.1177/1089253210386386. Epub 2010 Nov 7. Review. [DOI] [PubMed] [Google Scholar]
- 23.Krejci V, Vannucci A, Abbas A, Chapman W, Kangrga IM. Comparison of calibrated and uncalibrated arterial pressure-based cardiac output monitors during orthotopic liver transplantation. Liver Transpl. 2010;16:773–82. doi: 10.1002/lt.22056. [DOI] [PubMed] [Google Scholar]
- 24.Costa MG, Della Rocca G, Chiarandini P, Mattelig S, Pompei L, Barriga MS, et al. Continuous and intermittent cardiac output measurement in hyperdynamic conditions: Pulmonary artery catheter vs. lithium dilution technique. Intensive Care Med. 2008;34:257–63. doi: 10.1007/s00134-007-0878-6. Epub 2007 Oct 6. [DOI] [PubMed] [Google Scholar]
- 25.Grigorov Tzenkov I, Arnal Velasco D, Perez Peña JM, Olmedilla Arnal L, Garutti Martínez I, Sanz Fernández J. Cardiac output by femoral arterial thermodilution-calibrated pulse contour analysis during liver transplantation: Comparison with pulmonary artery thermodilution. Transplant Proc. 2003;35:1920–2. doi: 10.1016/s0041-1345(03)00602-x. [DOI] [PubMed] [Google Scholar]
- 26.Della Rocca G, Costa MG, Pompei L, Coccia C, Pietropaoli P. Continuous and intermittent cardiac output measurement: Pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth. 2002;88:350–6. doi: 10.1093/bja/88.3.350. [DOI] [PubMed] [Google Scholar]
- 27.Su BC, Yu HP, Yang MW, Lin CC, Kao MC, Chang CH, et al. Reliability of a new ultrasonic cardiac output monitor in recipients of living donor liver transplantation. Liver Transpl. 2008;14:1029–37. doi: 10.1002/lt.21461. [DOI] [PubMed] [Google Scholar]
- 28.Wong LS, Yong BH, Young KK, Lau LS, Cheng KL, Man JS, et al. Comparison of the USCOM ultrasound cardiac output monitor with pulmonary artery catheter thermodilution in patients undergoing liver transplantation. Liver Transpl. 2008;14:1038–43. doi: 10.1002/lt.21483. [DOI] [PubMed] [Google Scholar]
- 29.Burtenshaw AJ, Isaac JL. The role of trans-oesophageal echocardiography for perioperative cardiovascular monitoring during orthotopic liver transplantation. Liver Transpl. 2006;12:1577–83. doi: 10.1002/lt.20929. [DOI] [PubMed] [Google Scholar]
- 30.El-Masry A, Mukhtar AM, El-Sherbeny AM, Fathy M, El-Meteini M. Comparison of central venous oxygen saturation and mixed venous oxygen saturation during liver transplantation. Anaesthesia. 2009;64:378–82. doi: 10.1111/j.1365-2044.2008.05793.x. [DOI] [PubMed] [Google Scholar]
- 31.Acosta F, Sansano T, Palenciano CG, Roqués V, Clavel N, González P, et al. Does mixed venous oxygen saturation reflect the changes in cardiac output during liver transplantation? Transplant Proc. 2002;34:277. doi: 10.1016/s0041-1345(01)02762-2. [DOI] [PubMed] [Google Scholar]
- 32.Massicotte L, Lenis S, Thibeault L, Sassine MP, Seal RF, Roy A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006;12:117–23. doi: 10.1002/lt.20559. [DOI] [PubMed] [Google Scholar]
- 33.Feng ZY, Xu X, Zhu SM, Bein B, Zheng SS. Effects of low central venous pressure during preanhepatic phase on blood loss and liver and renal function in liver transplantation. World J Surg. 2010;34:1864–73. doi: 10.1007/s00268-010-0544-y. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder RA, Collins BH, Tuttle-Newhall E, Robertson K, Plotkin J, Johnson LB, et al. Intraoperative fluid management during orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2004;18:438–41. doi: 10.1053/j.jvca.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Cywinski JB, Mascha E, You J, Argalious M, Kapural L, Christiansen E, et al. Central venous pressure during the post-anhepatic phase is not associated with early postoperative outcomes following orthotopic liver transplantation. Minerva Anestesiol. 2010;76:795–804. [PubMed] [Google Scholar]
- 36.Wagener G, Kovalevskaya G, Minhaz M, Mattis F, Emond JC, Landry DW. Vasopressin deficiency and vasodilatory state in end-stage liver disease. J Cardiothorac Vasc Anesth. 2011;25:665–70. doi: 10.1053/j.jvca.2010.09.018. Epub 2010 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagener G, Gubitosa G, Renz J, Kinkhabwala M, Brentjens T, Guarrera JV, et al. Vasopressin decreases portal vein pressure and flow in the native liver during liver transplantation. Liver Transpl. 2008;14:1664–70. doi: 10.1002/lt.21602. [DOI] [PubMed] [Google Scholar]
- 38.Skagen CL, Said A. Vasoconstrictor use in liver transplantation: Is there evidence for rational use? Minerva Gastroenterol Dietol. 2010;56:279–96. [PubMed] [Google Scholar]
- 39.Cao Z, Gao Y, Tao G. Vasoplegic syndrome during liver transplantation. Anesth Analg. 2009;108:1941–3. doi: 10.1213/ane.0b013e3181a286fc. [DOI] [PubMed] [Google Scholar]
- 40.Savale L, O’Callaghan DS, Magnier R, Le Pavec J, Hervé P, Jaïs X, et al. Current management approaches to portopulmonary hypertension. Int J Clin Pract Suppl. 2011;169:11–8. doi: 10.1111/j.1742-1241.2010.02600.x. [DOI] [PubMed] [Google Scholar]
- 41.Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossettias G. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7:216–34. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groeneveld AB, Navickis RJ, Wilkes MM. Update on the comparative safety of colloids: A systematic review of clinical studies. Ann Surg. 2011;253:470–83. doi: 10.1097/SLA.0b013e318202ff00. [DOI] [PubMed] [Google Scholar]
- 43.Davies PG, Venkatesh B, Morgan TJ, Presneill JJ, Kruger PS, Thomas BJ, et al. Plasma acetate, gluconate and interleukin-6 profiles during and after cardiopulmonary bypass: A comparison of plasma-lyte 148 with a bicarbonate-balanced solution. Crit Care. 2011;15:R21. doi: 10.1186/cc9966. Epub 2011 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: Evidence and clinical consequences. Blood. 2010;116:878–85. doi: 10.1182/blood-2010-02-261891. Epub 2010 Apr 16. [DOI] [PubMed] [Google Scholar]
- 45.Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ. Review article: The prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26:141–8. doi: 10.1111/j.1365-2036.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- 46.Bischof D, Dalbert S, Zollinger A, Ganter MT, Hofer CK. Thrombelastography in the surgical patient. Minerva Anestesiol. 2010;76:131–7. Epub 2009 Nov 24. [PubMed] [Google Scholar]
- 47.Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: Randomized clinical trial. Transplant Proc. 2010;42:2590–3. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 48.Dalmau A, Sabaté A, Aparicio I. Hemostasis and coagulation monitoring and management during liver transplantation. Curr Opin Organ Transplant. 2009;14:286–90. doi: 10.1097/MOT.0b013e32832a6b7c. [DOI] [PubMed] [Google Scholar]
- 49.Ramsay MA, Randall HB, Burton EC. Intravascular thrombosis and thromboembolism during liver transplantation: Antifibrinolytic therapy implicated? Liver Transpl. 2004;10:310–4. doi: 10.1002/lt.20064. [DOI] [PubMed] [Google Scholar]
- 50.Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: A systematic review and meta-analysis. Am J Transplant. 2007;7:185–94. doi: 10.1111/j.1600-6143.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 51.De Boer MT, Christensen MC, Asmussen M, van der Hilst CS, Hendriks HG, Slooff MJ, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32–44. doi: 10.1213/01.ane.0000289638.26666.ed. [DOI] [PubMed] [Google Scholar]
- 52.Sankarankutty AK, Teixeira AC, Souza FF, Mente ED, Oliveira GR, Almeida RC, et al. Impact of blood salvage during liver transplantation on reduction in transfusion requirements. Acta Cir Bras. 2006;21(Suppl 1):44–7. doi: 10.1590/s0102-86502006000700011. [DOI] [PubMed] [Google Scholar]
- 53.Phillips SD, Maguire D, Deshpande R, Muiesan P, Bowles MJ, Rela M, et al. A prospective study investigating the cost effectiveness of intraoperative blood salvage during liver transplantation. Transplantation. 2006;81:536–40. doi: 10.1097/01.tp.0000199318.17013.c5. [DOI] [PubMed] [Google Scholar]
- 54.Da Silva Viana J. Recombinant factor VIIa in major abdominal surgery and liver transplantation. Transplant Proc. 2006;38:818–9. doi: 10.1016/j.transproceed.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 55.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 56.Kim BS, Lee SG, Hwang S, Park KM, Kim KH, Ahn CS, et al. Neurologic complications in adult living donor liver transplant recipients. Clin Transplant. 2007;21:544–7. doi: 10.1111/j.1399-0012.2007.00687.x. [DOI] [PubMed] [Google Scholar]
- 57.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–9. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal S, Brooks DM, Kang Y, Linden PK, Patzer JF. Noninvasive monitoring of cerebral perfusion pressure in patients with acute liver failure using transcranial doppler ultrasonography. Liver Transpl. 2008;14:1048–57. doi: 10.1002/lt.21499. [DOI] [PubMed] [Google Scholar]
- 59.Wang CH, Chen CL, Cheng KW, Huang CJ, Chen KH, Wang CC, et al. Bispectral index monitoring in healthy, cirrhotic, and end-stage liver disease patients undergoing hepatic operation. Transplant Proc. 2008;40:2489–91. doi: 10.1016/j.transproceed.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Kang JG, Ko JS, Kim GS, Gwak MS, Kim YR, Lee SK. The relationship between inhalational anesthetic requirements and the severity of liver disease in liver transplant recipients according to three phases of liver transplantation. Transplant Proc. 2010;42:854–7. doi: 10.1016/j.transproceed.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 61.Hwang S, Lee SG, Park JI, Song GW, Ryu JH, Jung DH, et al. Continuous peritransplant assessment of consciousness using bispectral index monitoring for patients with fulminant hepatic failure undergoing urgent liver transplantation. Clin Transplant. 2010;24:91–7. doi: 10.1111/j.1399-0012.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 62.Schumann R, Hudcova J, Bonney I, Cepeda MS. Availability of anesthetic effect monitoring: Utilization, intraoperative management and time to extubation in liver transplantation. Transplant Proc. 2010;42:4564–6. doi: 10.1016/j.transproceed.2010.09.164. [DOI] [PubMed] [Google Scholar]
- 63.Ford RM, Sakaria SS, Subramanian RM. Critical care management of patients before liver transplantation. Transplant Rev (Orlando) 2010;24:190–206. doi: 10.1016/j.trre.2010.05.003. Epub 2010 Aug 4. [DOI] [PubMed] [Google Scholar]
- 64.Jalan R. Acute liver failure: Current management and future prospects. J Hepatol. 2005;42(Suppl (1)):S115–23. doi: 10.1016/j.jhep.2004.11.010. Epub 2004 Dec 15. [DOI] [PubMed] [Google Scholar]
- 65.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289–98. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 66.Feng XN, Xu X, Zheng SS. Current status and perspective of liver preservation solutions. Hepatobiliary Pancreat Dis Int. 2006;5:490–4. [PubMed] [Google Scholar]
- 67.Kibondo A, Talbot D, Cullis-Hill D, Maag K, Cunningham AC, Carter NM. Perfusates: Their properties and usage for the maintenance and storage of organs for transplantation. Curr Anaesth Crit Care. 2010;21:216–9. [Google Scholar]
- 68.Feng L, Zhao N, Yao X, Sun X, Du L, Diao X, et al. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: A systematic review. Liver Transpl. 2007;13:1125–36. doi: 10.1002/lt.21208. [DOI] [PubMed] [Google Scholar]
- 69.Monbaliu D, Brassil J. Machine perfusion of the liver: Past, present and future. Curr Opin Organ Transplant. 2010;15:160–6. doi: 10.1097/MOT.0b013e328337342b. [DOI] [PubMed] [Google Scholar]
- 70.Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant. 2010;10:372–81. doi: 10.1111/j.1600-6143.2009.02932.x. Epub 2009 Dec 2. [DOI] [PubMed] [Google Scholar]
- 71.Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 72.Bahde R, Spiegel HU. Hepatic ischaemia-reperfusion injury from bench to bedside. Br J Surg. 2010;97:1461–75. doi: 10.1002/bjs.7176. [DOI] [PubMed] [Google Scholar]
- 73.Yan S, Jin LM, Liu YX, Zhou L, Xie HY, Zheng SS. Outcomes and mechanisms of ischemic preconditioning in liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:346–54. [PubMed] [Google Scholar]
- 74.Hoffmann K, Büchler MW, Schemmer P. Supplementation of amino acids to prevent reperfusion injury after liver surgery and transplantation--where do we stand today? Clin Nutr. 2011;30:143–7. doi: 10.1016/j.clnu.2010.09.006. Epub 2010 Oct 20. [DOI] [PubMed] [Google Scholar]
- 75.McKay A, Cassidy D, Sutherland F, Dixon E. Clinical results of N-acetylcysteine after major hepatic surgery: A review. J Hepatobiliary Pancreat Surg. 2008;15:473–8. doi: 10.1007/s00534-007-1306-6. Epub 2008 Oct 4. [DOI] [PubMed] [Google Scholar]
- 76.Hilmi IA, Peng Z, Planinsic RM, Damian D, Dai F, Tyurina YY, et al. N-acetylcysteine does not prevent hepatorenal ischaemia-reperfusion injury in patients undergoing orthotopic liver transplantation. Nephrol Dial Transplant. 2010;25:2328–33. doi: 10.1093/ndt/gfq077. [DOI] [PubMed] [Google Scholar]
- 77.Lango R, Mroziński P. Clinical importance of anaesthetic preconditioning. Anestezjol Intens Ter. 2010;42:206–12. [PubMed] [Google Scholar]
- 78.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248:909–18. doi: 10.1097/SLA.0b013e31818f3dda. [DOI] [PubMed] [Google Scholar]
- 79.Yang LQ, Tao KM, Liu YT, Cheung CW, Irwin MG, Wong GT, et al. Remifentanil preconditioning reduces hepatic ischemia-reperfusion injury in rats via inducible nitric oxide synthase expression. Anesthesiology. 2011;114:1036–47. doi: 10.1097/ALN.0b013e3182104956. [DOI] [PubMed] [Google Scholar]
- 80.Mandell MS, Stoner TJ, Barnett R, Shaked A, Bellamy M, Biancofiore G, et al. A multicenter evaluation of safety of early extubation in liver transplant recipients. Liver Transpl. 2007;13:1557–63. doi: 10.1002/lt.21263. [DOI] [PubMed] [Google Scholar]
- 81.Mandell MS, Campsen J, Zimmerman M, Biancofiore G, Tsou MY. The clinical value of early extubation. Curr Opin Organ Transplant. 2009;14:297–302. doi: 10.1097/MOT.0b013e32832b2f6c. [DOI] [PubMed] [Google Scholar]
- 82.Skurzak S, Stratta C, Schellino MM, Fop F, Andruetto P, Gallo M, et al. Extubation score in the operating room after liver transplantation. Acta Anaesthesiol Scand. 2010;54:970–8. doi: 10.1111/j.1399-6576.2010.02274.x. Epub 2010 Jul 12. [DOI] [PubMed] [Google Scholar]
- 83.Briceño J, Ciria R. Early graft dysfunction after liver transplantation. Transplant Proc. 2010;42:631–3. doi: 10.1016/j.transproceed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–26. doi: 10.1111/j.1600-6143.2008.02491.x. [DOI] [PubMed] [Google Scholar]
- 85.Rando K, Niemann CU, Taura P, Klinck J. Optimizing cost-effectiveness in perioperative care for liver transplantation: A model for low- to medium-income countries. Liver Transpl. 2011;17:1247–78. doi: 10.1002/lt.22405. [DOI] [PubMed] [Google Scholar]
- 86.Aggarwal S, Bane BC, Boucek CD, Planinsic RM, Lutz JW, Metro DG. Simulation: A teaching tool for liver transplantation anesthesiology. Clin Transplant. 2011 doi: 10.1111/j.1399-0012.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- 87.Martín-Cancho MF, Calles C, Celdrán D, Sánchez-Margallo FM. Experimental training in anaesthesia management during hepatic transplant. Med Educ. 2011;45:1147. doi: 10.1111/j.1365-2923.2011.04121.x. Epub 2011 Sep 21. [DOI] [PubMed] [Google Scholar]


