Abstract
Background:
Treatment of acute post-thoracotomy pain is particularly important not only to keep the patient comfortable but also to minimize pulmonary complications.
Aim:
This study was designed to test the effect of pre-induction administration of clonidine, given as a single intravenous dose, on post-operative pain scores and fentanyl consumption in patients after thoracic surgery.
Setting and Design:
Tertiary referral centre. Prospective, randomised, double-blind, placebo-controlled trial.
Methods:
Sixty patients were randomly allocated to receive clonidine (3 mcg/kg) or saline pre-operatively before induction of anaesthesia. The primary endpoint was pain on coughing (visual analogue scale (VAS) 0–100 mm) 120 min after surgery, time to first analgesic injection in the post-anaesthesia care unit (PACU) and 24-h fentanyl consumption.
Statistical Analysis:
For between-group comparisons, t-test and U-test were used as appropriate after checking normality of distribution. The incidence of complications between the groups was compared by Fisher's exact test.
Results:
The post-operative VAS for the first 120 min and the fentanyl consumption at 24 h was significantly greater in the placebo group compared with the clonidine group (P<0.05). The sedation score was increased in the clonidine group during study drug infusion, but did not differ significantly on admission to the PACU.
Conclusions:
A single intravenous dose of clonidine (3 mcg/kg) given before induction of anaesthesia significantly reduced the post-operative VAS score in the initial period and fentanyl consumption during 24 h after thoracic surgery.
Keywords: Analgesics, clonidine, non-narcotic, pain, post-operative, surgery, thoracic
INTRODUCTION
The pain that accompanies thoracic surgery is notable for its intensity and duration. Given that the adverse effects of thoracic surgery on pulmonary function can be mitigated by effective perioperative analgesia,[1,2] the most preferred analgesic interventions to limit post-thoracotomy pain would be multimodal analgesia.[3]
Systemic opioids are the main alternative to more invasive techniques like central neuraxial or peripheral nerve block, and become the mainstay of analgesic therapy when invasive approaches are discontinued. However, their side-effects (respiratory depression and inhibition of the cough reflex) are undesirable after thoracotomy.[4,5] Non-steroidal anti-inflammatory drugs (NSAID) can be useful when administered in combination with opioids as a part of multimodal therapeutic strategy to reduce the need for opioids and to produce or ameliorate analgesia, but are not without side-effects of their own.[6]
Clonidine, an α-2 agonist, has been shown to be effective for its analgesic, sedative–hypnotic and sympatholytic properties.[7,8] In addition, clonidine has an opioid-sparing effect and reduces fentanyl requirements by more than 50% without perturbing the haemodynamics.[9] A combination of NSAID and clonidine may act synergistically[10] to reduce the opioid need and individual drug-related side-effects further.
Our aim was to evaluate the effectiveness of a single dose of intravenous (IV) clonidine before induction of anaesthesia for the prevention and treatment of post-operative pain after thoracotomy using a randomized, controlled, double-blind, prospective study.
METHODS
Patients judged to be American Society of Anesthesiology Physical Status (ASA PS) I to III and scheduled for an elective thoracic surgery were studied after obtaining approval from the institutional ethics committee and individual written informed consent. Exclusion criteria included age<18 or >70 years, weight >100 kg, symptomatic coronary artery disease, severe respiratory dysfunction (PaCO2 >45 mmHg and PaO2 <60 mmHg breathing room air, FEV1< 1.2 L), chronic opioid use, abnormal renal or hepatic function, inability to understand and use the visual analogue scale 0–100 mm (VAS), pregnant or breast feeding. The study was conducted in a randomised, double blinded, placebo-controlled manner, and conformed to the CONSORT guidelines.
Patients were randomly assigned in a double-blind fashion to one of the two study group. In the clonidine group (Group C, n=30), patients received IV clonidine 3 mcg/kg diluted with 50 mL normal saline and infused with a syringe pump over 30 min after receiving the patient in the operation theatre lounge. The placebo group (Group P, n=30) patients received a corresponding volume of 0.9% saline. The anaesthesia resident (who was not one of the observers for the study) performed permuted block randomization and blinding of the study medication. Scores for sedation and haemodynamic parameters (systolic arterial pressure (SAP), heart rate (HR) and peripheral oxygen saturation) were recorded by an anaesthetist who was blinded to the patient group, at times 0, 15 and 30 min during infusion of the study drugs.
After giving a rest period of 15 min, general anaesthesia was induced with fentanyl 3 mcg/kg, midazolam 0.05 mg/kg and thiopentone 3–5 mg/kg until loss of eyelid reflex. Orotracheal intubation was facilitated by 0.1 mg/kg vecuronium. Routine airway and ventilator management were used as appropriate for the type of surgery. Anaesthesia was maintained with nitrous oxide, oxygen and isoflurane (1–1.2% end-tidal concentration). Neuromuscular relaxation was maintained by bolus injections of vecuronium 0.03 mg/kg at 30-min intervals and fentanyl 1 mcg/ kg at every hour. Increases of 20% in mean arterial pressure (MAP) or heart rate from the baseline or appearance of lacrimation were treated by an additional 1 mcg/kg of fentanyl. The total amount of fentanyl administered during each operation was also recorded. About 20 min before the end of surgery (at the start of skin closure), 2 mcg/kg fentanyl was given IV. At the completion of surgery, residual neuromuscular block was antagonised with neostigmine and atropine, and each patient was extubated when he/she was able to execute simple verbal commands. All patients were transferred pain-free to the post-anaesthesia care unit (PACU). In the PACU, diclofenac (75 mg IM) was administered at 12-h intervals for the first day (first 24 h after receiving the patient in the PACU) and at 8-h intervals (50 mg oral tablet) on the second day. The first dose was given after receiving the patient in the PACU and, if pain relief was considered insufficient (VAS >40 mm at rest) and the patient indicated that he was not satisfied with the level of analgesia that was reached, fentanyl was administered at a dose of 1 mcg/kg IV (rounded off to the nearest integer of 10) and was repeated every 10 min as long as the VAS score remained >40 mm (first analgesic injection, FAI). All the patients received supplemental oxygen by face mask for the first 8 h of the study.
In the PACU, pain intensity to cough was assessed at 30, 60, 120, 180 and 240 min after the end of surgery using a VAS (0–100). At the same time intervals, fentanyl consumption was noted and the patients were asked whether they suffered from post-operative nausea and vomiting (PONV). In case of PONV, 4 mg IV ondansetron was given and, if symptoms persisted, 10 mg IV metoclopramide was administered. The patients were tested at the same registration time-points regarding degree of sedation using the Ramsay sedation scale. From the sedation score data, the maximum scores during the assessment period for each patient were considered for statistical analysis. Pain intensity (VAS) with cough as well as fentanyl consumption was assessed at 24 h.
Primary outcomes were severity of post-operative pain during cough 120 min after the surgery was completed, time to first analgesic request in the PACU and 24-h post-operative fentanyl requirement. Safety and tolerability were evaluated by reporting adverse events like respiratory depression, haemodynamic instability and occurrence of PONV during the study period.
Calculation of sample size was based on the presumption that post-operative VAS scores after pre-induction administration of clonidine would be 30 mm when compared with 45 mm in the placebo group with a standard deviation of 20 mm at all time points. For the results to be of statistical significance with α=0.05 and β=0.80, one needed to recruit 25 patients in each group. To increase the power of the study and to compensate for any possible dropouts, we enrolled 30 patients in each group. Demographic data were analysed with Student's t test for continuous variables and Chi-square test for categorical variables. Post-operative fentanyl consumption was analysed with Student's t-test. The VAS pain scores were analysed with Mann–Whitney U-test; the incidences of side-effects were analysed with Fisher's exact test. The package SPSS 14.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P<0.05 was considered significant.
RESULTS
A total of 60 of 68 patients met the inclusion criteria and consented to participate at the pre-operative anaesthesia visit. Two patients required post-operative mechanical ventilation because of haemorrhagic complications and were dropped out after randomisation [Figure 1]. There were no differences between groups with regard to age, body weight, proportions of ASA I/II/III patients, induction dose of fentanyl and duration of anaesthesia [Table 1].
Figure 1.
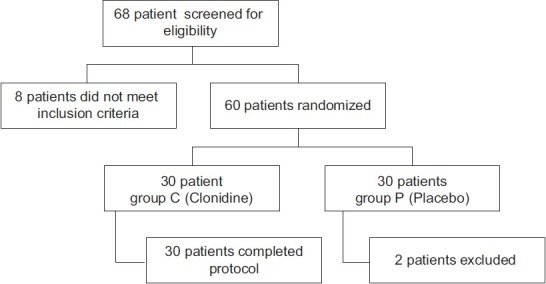
Patient flow (according to CONSORT guidelines)
Table 1.
Patient characteristics, intra-operative data, recovery time, requirement for supplementary fentanyl, time to first analgesic injection and pain score at first analgesic injection in the post-anaesthesia care unit
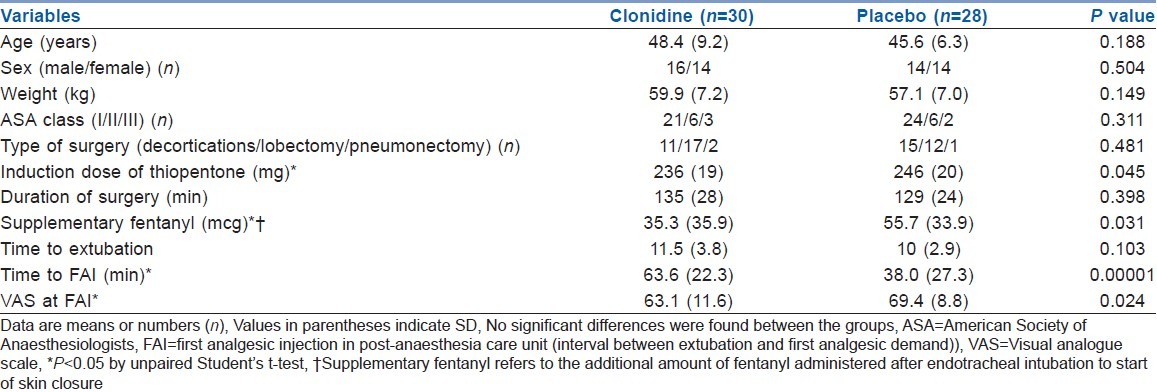
Pre-induction administration of clonidine not only decreased the initial post-operative VAS score but also decreased the induction dose of thiopentone, demand for intra-operative fentanyl supplementation after the induction dose (P=0.03) and increased the time to FAI demand in the PACU (P=0.0002) [Table 1]. The pain scores at cough (VAS) were significantly lower in the clonidine group at FAI, and the same was true for 30, 60 and 120 min after surgery, but thereafter VAS was comparable till 24 h after surgery [Table 2]. The post-operative cumulative fentanyl consumption did differ significantly at demand for FAI and 180 and 240 min after surgery. The fentanyl consumption also tended to be significant at 30, 60 and 120 min (P=0.06, 0.05 and 0.05, respectively) after surgery. There was a difference in the cumulative dose of fentanyl given during the study period between the two study groups [Table 2].
Table 2.
Post-operative pain and cumulative fentanyl consumption

Haemodynamic variables were similar between the two groups in each study period during study drug infusion [Figure 2] and in the PACU. Patients in the clonidine group had a higher mean sedation score at 15 min (P=0.019) and 30 min (P=0.018) after infusion of the study drugs [Figure 2]. All patients were haemodynamically stable and either awake or easily aroused before induction of anaesthesia (15 min after infusion of study drugs). Emergence after the end of surgery was rapid and similar in both groups [Table 1]. Both groups were alert and co-operative in the PACU.
Figure 2.
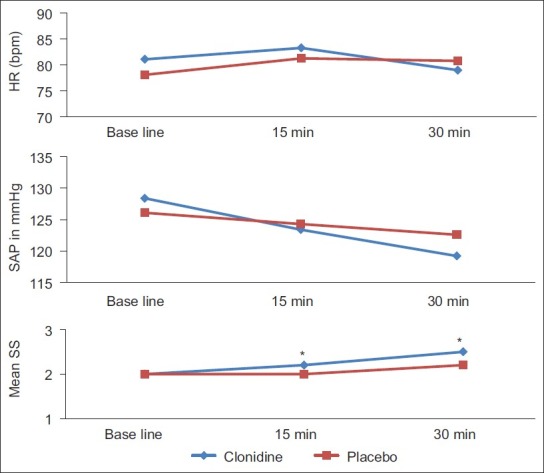
Changes in haemodynamic variables and sedation score after infusion of study drugs during the pre-operative period. Data are expressed as mean (SD). *P<0.05, between groups by t-test
There was no difference in sedation levels after arrival in the PACU, but the overall sedation score was higher in clonidine group, without attaining any statistical significance compared with the placebo group (P=0.742) [Table 3].
Table 3.
Incidence of side-effects
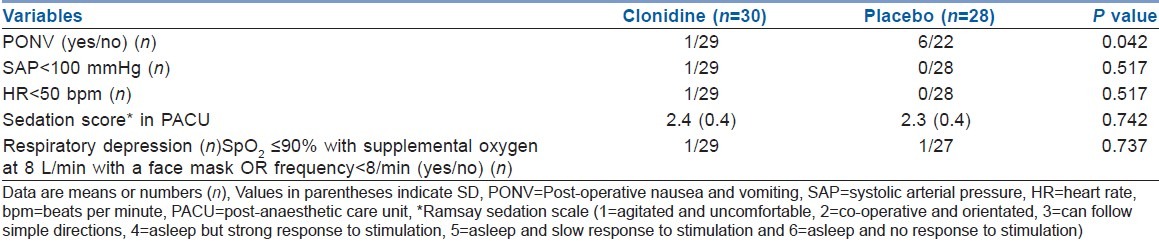
There was no difference in the occurrence of side-effects post-operatively, except for occurrences of PONV (P=0.042) [Table 3]. One patient from each group had respiratory depression (after study drug infusion in the clonidine group and post-operative period in the placebo group), but this was accompanied by hypotension and bradycardia in the clonidine group. The bradycardia and hypotension was treated with Atropine 0.6 mg IV and by increasing the fluid infusion rate. Respiratory depression was treated successfully with aggressive nursing care.
DISCUSSION
The main findings in this study were that patients who received a single dose of IV clonidine before induction of anaesthesia had lower pain scores in the early post-operative hours and consumed less fentanyl in the peri-operative period. Moreover, this group of patients had less occurrences of PONV in the post-operative period. Possible mechanisms for this include an analgesic-sparing effect, anxiolytic effect, pre-emptive analgesic effect, synergistic effect with NSAIDs[9] or a residual additive effect of clonidine.
Both currently available α-2-agonists, clonidine and dexmedetomidine, induce significant sedation, and the clinical analgesic effectiveness of these compounds as adjuvant has consistently shown beneficial effects in reducing post-operative pain. These drugs can be useful when administered in combination with opioids as part of a multimodal therapeutic strategy to reduce the need for opioid, which may be controversial in patients undergoing thoracic surgery due to their possible respiratory depressant effect,[4,11] influence on cough mechanism[12] and PONV in the post-operative period.[13] Our study confirms the analgesic effect of clonidine after thoracic surgery as described by other authors in different types of surgeries in various age groups.[14–17]
Another benefit of using clonidine in the pre-operative period is its ability to provide excellent anxiolysis comparable to midazolam.[8,18] Reduction in pre-operative anxiety is good for the patient's pre-operative well-being and is associated with decreased levels of post-operative pain per se, independent of drug effect.[19] In our study, although we did not measure the anxiety level, we assumed that this could have played a role along with its spinal and supra-spinal-mediated analgesic activities[20] in reducing the post-operative fentanyl requirement and delay in FAI demand.
Pre-induction administration of clonidine may have a pre-emptive analgesic effect,[21] possibly emerging post-operatively, after the effect of the intra-operatively administered analgesic dose wears off. Further studies are needed to reveal this relationship. There may also have been a residual additive effect of clonidine owing to its long elimination half-life.
Patients in the clonidine group tended to be more sedated in the pre-induction period [Figure 2], but we did not find significant differences between the groups’ mean sedation scores at each time point in the PACU. However, we observed clinically important sedation (sedation score ≥ 4) in one patient from the clonidine group. Sedation in all other patients in either group was easily overcome by calling the patient's name in a normal or loud voice.
Clonidine has been investigated extensively for its analgesic and anaesthetic-sparing qualities, but its long elimination half-life makes its use for continuous IV sedation and analgesia difficult, and there is no consensus on appropriate dose regimen. The antihypertensive and bradycardia effect of clonidine[22] at all times was related to the clonidine concentration above 1 ng/mL. Hall et al.[23] demonstrated that 1-h infusion of 4 mcg/kg of clonidine resulted in a plasma concentration of 2 ng/mL. Marinangeli et al.[24] in a dose finding study concluded that 3 mcg/kg bolus dose followed by a continuous infusion of 0.3 mcg/ kg/h is the optimal intravenous dose for post-operative analgesia. However, Bernard et al.[17] cautioned against use of a continuous infusion in the presence of inadequate filling pressure. As we do not use central venous pressure monitoring routinely for thoracic surgery and adopt a conservative fluid approach in the post-operative period, we preferred not to use a continuous infusion in the post-operative period. Owing to the long half-life of clonidine and inclusion of an NSAID (diclofenac, 75 mg) in our post-operative analgesic regimen, unlike that of Marinangeli et al.,[24] we thought a loading dose of clonidine 3 mcg/kg would be sufficient for our patients.
In the current study, except one female patient in the clonidine group who had a fall in SAP to 86 mmHg after the loading dose of clonidine in the pre-induction period, no other patient had any adverse cardiovascular events as defined in the study protocol. This raises concern regarding the use of clonidine in fasting patients with volume deficits in the pre-operative period.
In our study, both the groups were comparable with regard to the risk of suffering PONV (gender distribution and techniques of anaesthesia), except for the amount of opioids (fentanyl) for post-operative pain management. Although in the current study clonidine has been shown to reduce PONV, the low incidence of PONV in the clonidine group appeared to be related to its ability to reduce emetic sequelae by decreasing the need for fentanyl immediately after surgery.[25]
Limitations of the study
We have not used patient-controlled analgesia (PCA) to assess the fentanyl-sparing effects of the single IV pre-induction dose of clonidine as we presumed that reduction in the use of fentanyl is unlikely to have any financial benefits, as it would not reduce the number of unit doses of PCA medication. Furthermore, clonidine was administered as a single dose before surgery, and the beneficial effects regarding post-operative pain and fentanyl consumption were only present in the early post-operative period.
CONCLUSION
Clonidine administered in the pre-induction period was effective in reducing post-operative pain intensity and fentanyl requirement in patients undergoing thoracotomy procedure. Moreover, clonidine resulted in lower occurrence of PONV, without an increased incidence of side-effects. We therefore suggest that pre-induction single-dose intravenous 3 mcg/kg clonidine is an effective adjuvant for reducing post-operative pain and fentanyl consumption in patients undergoing elective thoracic procedures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb. 1990;35:144–50. [PubMed] [Google Scholar]
- 2.Sabanathan S. Has postoperative pain been eradicated? Ann R Coll Surg Engl. 1995;77:202–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Pavelescu D, Mirea L, Paăduraru M, Beuran M, Chiotoroiu A, Grinţescu I. The role of multimodal analgesia in the decrease of postoperative surgical stress response in major neoplastic thoraco-abdominal surgery. Chirurgia (Bucur) 2011;106:723–8. [PubMed] [Google Scholar]
- 4.Lehmann KA, Grond S, Freier J, Zech D. Postoperative pain management and respiratory depression after thoracotomy: A comparison of intramuscular piritramide and intravenous patient-controlled analgesia using fentanyl or buprenorphine. J Clin Anesth. 1991;3:194–201. doi: 10.1016/0952-8180(91)90158-j. [DOI] [PubMed] [Google Scholar]
- 5.Baxter AD, Laganière S, Samson B, Stewart J, Hull K, Goernert L. A comparison of lumbar epidural and intravenous fentanyl infusions for post-thoracotomy analgesia. Can J Anaesth. 1994;41:184–91. doi: 10.1007/BF03009829. [DOI] [PubMed] [Google Scholar]
- 6.Hyllested M, Jones S, Pedersen JL, Kehlet H. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: A qualitative review. Br J Anaesth. 2002;88:199–214. doi: 10.1093/bja/88.2.199. [DOI] [PubMed] [Google Scholar]
- 7.Segal IS, Jarvis DJ, Duncan SR, White PF, Maze M. Clinical efficacy of oral-transdermal clonidine combinations during the perioperative period. Anesthesiology. 1991;74:220–5. doi: 10.1097/00000542-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiol Scand. 2010;54:397–402. doi: 10.1111/j.1399-6576.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 9.Viggiano M, Badetti C, Roux F, Mendizabal H, Bernini V, Manelli JC. Controlled analgesia in a burn patient: Fentanyl sparing effect of clonidine. Ann Fr Anesth Reanim. 1998;17:19–26. doi: 10.1016/s0750-7658(97)80177-3. [DOI] [PubMed] [Google Scholar]
- 10.Miranda HF, Pinardi G. Isobolographic analysis of the antinociceptive interactions of clonidine with nonsteroidal anti-inflammatory drugs. Pharmacol Res. 2004;50:273–8. doi: 10.1016/j.phrs.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Sato C, Tanaka H, Kawamoto M, Yuge O, Ogawa R. Low oxygen saturation during early postoperative period in adult patients receiving opioids by intravenous patient-controlled analgesia. Masui. 2004;53:659–63. [PubMed] [Google Scholar]
- 12.Yoo YC, Na S, Jeong JJ, Choi EM, Moon BE, Lee JR. Dose-dependent attenuation by fentanyl on cough during emergence from general anesthesia. Acta Anaesthesiol Scand. 2011;55:1215–20. doi: 10.1111/j.1399-6576.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 13.Pizzi LT, Toner R, Foley K, Thomson E, Chow W, Kim M, et al. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–14. doi: 10.1002/j.1875-9114.2012.01101.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Goyagi T, Tanaka M, Nishikawa T. Oral clonidine premedication enhances postoperative analgesia by epidural morphine. Anesth Analg. 1999;89:1487–91. doi: 10.1097/00000539-199912000-00032. [DOI] [PubMed] [Google Scholar]
- 15.De Kock MF, Pichon G, Scholtes JL. Intraoperative clonidine enhances postoperative morphine patient-controlled analgesia. Can J Anaesth. 1992;39:537–44. doi: 10.1007/BF03008314. [DOI] [PubMed] [Google Scholar]
- 16.Bernard JM, Kick O, Bonnet F. Comparison of intravenous and epidural clonidine for postoperative patient-controlled analgesia. Anesth Analg. 1995;81:706–12. doi: 10.1097/00000539-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bernard JM, Hommeril JL, Passuti N, Pinaud M. Postoperative analgesia by intravenous clonidine. Anesthesiology. 1991;75:577–82. doi: 10.1097/00000542-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Shi X, Miao X, Xu J. Effects of premedication of midazolam or clonidine on perioperative anxiety and pain in children. Biosci Trends. 2009;3:115–8. [PubMed] [Google Scholar]
- 19.Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–23. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 20.Millan MJ. Evidence that an alpha2 A-adrenoceptor subtype mediates antinociception in mice. Eur J Pharmacol. 1992;215:355–6. doi: 10.1016/0014-2999(92)90059-d. [DOI] [PubMed] [Google Scholar]
- 21.Persec J, Persec Z, Buković D, Husedzinović I, Buković N, Pavelić L. Effects of clonidine preemptive analgesia on acute postoperative pain in abdominal surgery. Coll Antropol. 2007;31:1071–5. [PubMed] [Google Scholar]
- 22.Frisk-Holmberg M. Clinical pharmacology of clonidine. Chest. 1983;83:395–7. doi: 10.1378/chest.83.2_supplement.395. [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Marinangeli F, Ciccozzi A, Donatelli F, Di Pietro A, Iovinelli G, Rawal N, et al. Clonidine for treatment of postoperative pain: A dose-finding study. Eur J Pain. 2002;6:35–42. doi: 10.1053/eujp.2001.0270. [DOI] [PubMed] [Google Scholar]
- 25.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]


