Abstract
Objectives:
The highly lipid-soluble opioids, sufentanil and fentanyl, are used in combination with low-concentration bupivacaine to provide combined spinal epidural (CSE) analgesia during labour. We designed a prospective, randomized, single-blind study to compare the efficacy of these two opioids with bupivacaine in terms of the quality of analgesia, side-effects and maternal and foetal outcome.
Methods:
Sixty parturients requesting labour analgesia were divided into two groups randomly. Group S (n=30) received bupivacaine heavy (2.5 mg) and sufentanil (5 mcg) intrathecally and 10 mL intermittent bolus of sufentanil 0.30 mcg/mL in bupivacaine 0.125% as epidural top-ups. Group F (n=30) received bupivacaine heavy (2.5 mg) and fentanyl (25 mcg) intrathecally and 10 mL intermittent bolus of fentanyl 2.5 mcg/mL in bupivacaine 0.125% as epidural top-ups. Duration of intrathecal and epidural analgesia, mean duration between epidural top-ups and total analgesic requirements were noted. Pain and overall satisfaction scores were assessed with a 10-point visual scale. Mode of delivery and neonatal Apgar scores were recorded.
Results:
Maternal demographic characteristics were comparable between the groups. Although CSE provided satisfactory analgesia in both the groups, parturients of group S had a significant prolongation of analgesia through the intrathecal route compared with parturients of group F. Incidence of caesarean, instrumental delivery did not differ between the groups. No difference in the incidence of motor blockade or cephalad extent of sensory analgesia was observed. Neonatal outcome and incidence of side-effects were similar in both the groups.
Conclusion:
We conclude that combined spinal epidural using sufentanil and fentanyl achieved high patient satisfaction and excellent labour analgesia without serious maternal or neonatal side-effects. Sufentanil provided a significantly longer duration of labour analgesia compared with fentanyl.
Keywords: Combined spinal epidural, fentanyl, labour analgesia, sufentanil
INTRODUCTION
Pain is the single most predominant sentinel of the beginning of labour. Labour pain is excruciating and a significant contributor of stress and anxiety. Painful uterine contractions cause maternal hyperventilation and increased catecholamine concentration resulting in maternal and foetal hypoxaemia.[1] An effective analgesia takes away the disadvantages and results in better maternal and foetal outcome. Hence, the control of pain should form an integral part of labour management at any level.
An ideal labour analgesic technique should provide adequate and satisfactory analgesia without any motor blockade or adverse maternal and foetal effects. Among the variety of labour analgesia techniques ranging from parenteral and inhalational agents, regional analgesia has an edge over other methods in achieving the above goals. Combined spinal epidural (CSE) analgesia is increasingly used to provide pain relief during labour. It combines the advantage of rapid onset of spinal analgesia and the flexibility of the epidural catheter.[2] Dose adjustments and frequency of administration of the drug according to parturients’ requirement is possible with the epidural route which can also be extended to provide anaesthesia for caesarean delivery if need arises.
The quality of analgesia is excellent when highly lipid-soluble opioids are added. Addition of opioids to local anaesthetics reduces its requirement by synergistic effect of opioid receptors in the spinal cord. This reduces the chances of motor blockade and the haemodynamic perturbation.[3] Although fentanyl and sufentanil are the most commonly used opioids for epidural labour analgesia, studies comparing CSE technique employing sufentanil and fentanyl are very few and give conflicting results.[4] At the assumed equipotent doses of sufentanil to fentanyl ratio of 6:1, there is some evidence that sufentanil is clinically superior to fentanyl as an adjunct to bupivacaine in labour epidurals.[5]
Hence, we decided to compare the efficacy, quality and duration of analgesia and maternal and foetal effects of adding the two highly lipid-soluble opioids, sufentanil and fentanyl to low-concentration bupivacaine for CSE labour analgesia.
METHODS
A randomized, prospective, single-blinded study was carried out on 60 parturients over a period of 6 months after obtaining clearance from the institutional ethical committee. Parturients belonging to ASA grade I and II with singleton, term pregnancy in spontaneous labour with cervical dilatation of less than 4 cm, with normal foetal heart tracings requesting labour analgesia, were included in the study. Parturients with medical disorders, obstetric complications, malpresentation of foetus and those with contraindication for regional analgesia were excluded from the study. After obtaining informed written consent, parturients were randomly allocated by a computer-generated table of random numbers by a person blinded to the procedure to avoid selection bias into two groups of 30 each as group S (n=30) and group F (n=30).
Before institution of CSE, parturients were administered 1000 mL ringer lactate solution and monitors were connected and baseline readings recorded. Patients were placed in the left lateral position and, under aseptic precautions, CSE analgesia was administered through a midline approach (L2-L3 or L3-L4 level). Lumbar epidural space was identified with an 18G Tuohy needle using loss of resistance to saline technique. After negative aspiration for blood and cerebrospinal fluid, the epidural catheter was threaded on through the Tuohy needle and the needle was carefully withdrawn. Spinal analgesia was instituted using a 25G Whitacre needle one space below. On identification of subarachnoid space, 1 mL of drug containing 0.5 mL 0.5% bupivacaine heavy (2.5 mg) and 0.5 mL sufentanil (5 mcg) for group S or 0.5 mL fentanyl (25 mcg) for group F was injected intrathecally and the epidural catheter was fixed thereafter. The parturient was turned supine immediately after subarachnoid block and the uterus was displaced to the left using a wedge.
Pulse rate, blood pressure, respiratory rate and SPO2 were monitored at 0, 5, 10, 15 and 30 min and thereafter every 30 min until the women delivered. Motor block was assessed by modified bromage scale and the level of sensory block was recorded. Pain relief was assessed using visual analogue scores (VAS)[6] (0 = no pain, 10 = worst possible pain experienced). When the parturient experienced pain equivalent to a VAS score of 5, epidural top-up was administered. The time interval between initiation of intrathecal analgesia and patient developing pain equivalent to VAS score 5 was defined as the duration of intrathecal analgesia, and was noted. Parturients of group S received 10 mL of 0.125% bupivacaine with 0.30 mcg/mL of sufentanil and group F received 10 mL of 0.125% bupivacaine with fentanyl 2.5 mcg/mL through an epidural catheter. Time of onset of epidural analgesia, total number of top-ups and duration between successive top-ups were recorded. Foetal heart rate (FHR) was continuously monitored using a cardiotocograph. Progress of labour was recorded in a partogram. The mode of delivery in the form of normal vaginal delivery, instrumental delivery or caesarean section was observed. Side-effects such as pruritis (rated as none, minimal, moderate and severe),[7] hypotension (fall of >20% from baseline systolic reading), motor blockade of limbs, shivering, sedation (categorized as none, mild, moderate and severe for awake, drowsy, sleepy and unarousable)[7] and bradycardia (heart rate<60) were noted.
Neonatal outcome in the form of APGAR scores at 1 min and 5 min and need for intensive care were noted. All the data were recorded by a resident blinded to the drug administered during conduct of labour analgesia throughout the study period. The parturient was monitored for 2 h following delivery and the epidural catheter was removed. They were questioned after 24 h about their views on the procedure and the satisfaction. Enquiry about the symptoms related to post-dural puncture headache (PDPH) was made.
Statistical analysis
Data were analyzed using software version SPSS 12.0. Demographic data were analysed using analysis of variance. Unpaired t-test and chi-square tests were used where appropriate. Sample size of 60 with 30 parturients in each group was determined with power of study of 80%. Data were expressed as mean ± SD. Standard tests of significance were applied to determine the P value. P<0.05 was considered significant.
RESULTS
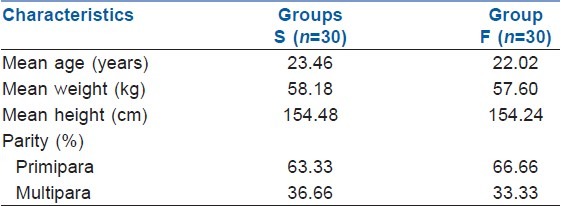
There were no significant differences between the groups with respect to maternal demographic characteristics, parity, cervical dilation at initiation of labour analgesia, duration of labour and delivery characteristics [Table 1].
Table 1.
Maternal demographic characteristics
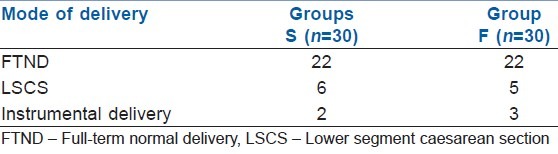
The mode of delivery data suggests no significant difference between the groups regarding incidence of caesarean, instrumental or vaginal deliveries [Table 2].
Table 2.
Mode of delivery
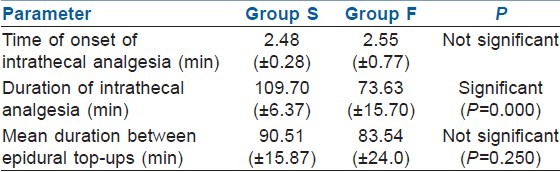
The mean duration of intrathecal analgesia was 109.7 (±6.3) min for group S and 73.63 (±15.76) min for group F. The mean time of onset of epidural analgesia was 2.48 (±0.28) min and 2.55 (±0.77) min for group S and group F, respectively. The mean duration between epidural top-ups was 90.51 (±15.8) min for group S and 83.54 (±24.64) min for group F [Table 3]. The number of additional bolus supplementary analgesic top-ups were comparatively less in the sufentanil group. Eight women among group S and nine among group F experienced transient initial motor blockade (bromage score 1). None of the women had any motor blockade at the end of 1 h. The highest level of sensory block attained was T6 in 63.33%, T8 in 36.66% among group S and 66.66% and 33.33% among group F, respectively.
Table 3.
Onset and duration of analgesia
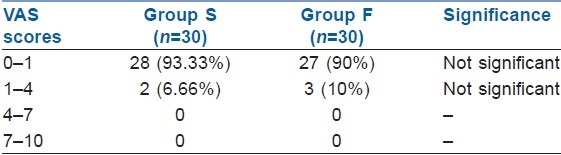
The pain scores during the first and second stages of labour were comparable without any significant difference between the groups using VAS scores (VAS: 0 = no pain, 10 = worst pain experienced); 93.3% and 90.0% women had VAS scores less than 1, indicating excellent pain relief by the CSE technique in both groups [Table 4].
Table 4.
Mean visual analogue scale scores
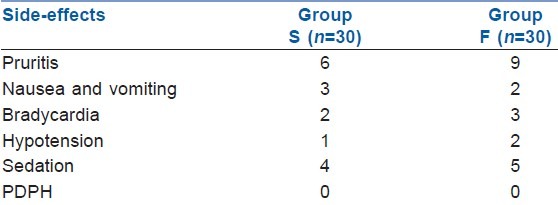
No significant alterations in intrapartum vital parameters like SPO2, mean arterial pressure, pulse rate and FHR were noted between the groups. The incidence of side-effects did not differ between the groups [Table 5]. Pruritis, shivering, nausea and vomiting, sedation, bradycardia and hypotension were reported and treated accordingly. The most common side-effect was pruritis, which was reported among 20% and 30% of the women belonging to groups S and F, respectively. Nine parturients (four in group S and five in group F; P=NS) experienced mild sedation during the study period. None of the parturients had moderate or severe sedation.
Table 5.
Side-effects
Neonatal outcome was assessed based on APGAR scores at the 1st and the 5th minutes, which did not differ between the groups. None of the neonates had scores less than 7 at 5 min. Maternal satisfaction was assessed based on subjective assessment as excellent, good, satisfactory and poor. 93.33% of the women of group S and 90% of the women of group F opined labour analgesia as excellent. None of the parturients from either group had symptoms suggestive of PDPH.
DISCUSSION
Highly lipid-soluble synthetic opioids such as sufentanil and fentanyl are being increasingly used along with very low concentrations of local anaesthetic agents such as bupivacaine (0.0625–0.125%) and ropivacaine (0.2–0.25%) to provide excellent relief of pain during labour.[4] Unlike hydrophilic opiates (morphine) or intermediate lipid-soluble opioids (meperidine), lipophillic opioids do not spread rostrally in cerebrospinal fluid to any great extent, and they tend to have fairly segmental analgesic profiles.[8]
Quality of analgesia and overall satisfaction scores were found to be comparable in both groups in our study. In a dose response study, the analgesic potency of epidural sufentanil was reported to be approximately five-times that of fentanyl when it was administered as the sole analgesic after lidocaine anaesthesia for caesarean delivery.[9] Addition of bupivacaine to intrathecal opioid prolongs duration of labour analgesia compared with either drug used alone.[10] Evidence suggests that bupivacaine potentiates the binding of morphine to opioid receptors, especially the highly dense kappa receptors, as the result of an associated conformational change in opioid receptors.[11] We observed that duration of analgesia provided by intrathecal sufentanil and bupivacaine was 109.70±6.37 min as compared with 73.63±15.76 min by group F. This difference was statistically significant. These are similar to previously reported results using sufentanil: 114±26 min[10] and 99 min.[12] This prolonged duration of analgesia with sufentanil could be attributed to the known superiority of sufentanil over fentanyl in terms of potency.[9,13] Parturients receiving sufentanil required less total dose of bupivacaine than those receiving fentanyl.[14] Similarly, the number of supplementary bupivacaine top-ups was comparatively less in the sufentanil group (group S 1.22±0.75 and group F 2.06±0.081).
Addition of bupivacaine 2.5 mg and sufentanil 10 μg for labour analgesia showed that the combination, although effective, was associated with a higher incidence of hypotension and impairment of muscle power of the lower limbs, although mild in some parturients.[10] Another investigation by halving the total amount of intrathecal sufentanil and bupivacaine reduced the incidence of side-effects. However, this reduced dose regimen was associated with slower onset and shorter duration of labour analgesia.[15] As 10 mcg sufentanil is associated with severe hypotension and 2.5 mcg sufentanil produces analgesia of shorter duration and slower onset, in our study, we added 5 mcg sufentanil to bupivacaine 2.5 mg intrathecal.
CSE has an added advantage of initiating labour analgesia even at the late stages of labour. We found that analgesia through intrathecal route resulted in immediate and predictable onset of analgesia enhancing the parturient's cooperation for the insertion of epidural catheter. The duration of first and second stage of labour and the mode of delivery between the groups did not differ significantly. The incidence of caesarean delivery was 20% and 16.66% between group S and F, respectively. 6.66% of group S and 10% of group F underwent instrumental delivery. These findings do not differ grossly with the earlier reports.[16,17] Higher concentration of bupivacaine (0.25%) was used in the past, which resulted in a fairly higher incidence of motor block causing pelvic muscle relaxation, foetal malposition and maternal inability to push and a higher incidence of instrumental delivery.[4] Low concentration of local anaesthetic along with opioids has overcome this disadvantage. Incidence of motor blockade in our study groups was very minimal and was of bromage scale 1, which did not persist beyond the first hour. The incidence of cephalad extent of sensory block was identical in the two groups.
The pain relief during labour was assessed by VAS scoring and the pain scores were comparable between the groups. Maternal satisfaction was assessed based on subjective assessment on the following day of labour. It was graded as excellent in 93.33% parturients of group S and 90.0% parturients of group F, as in previous studies.[4,18,19]
Use of sufentanil and fentanil was found to be safe among neonates. The dose of opioids administered did not adversely affect the APGAR scores or cause respiratory depression among newborns in our study.
The incidence of side-effects was comparable between the groups. Pruritus was the most common side-effect between the two groups, which was not of a serious nature. The incidence of sedation and nausea was similar in the two groups, with no parturient in either group requiring any intervention.
Continuous infusion of a dilute mixture of local anaesthetics and opioids offers the advantage of stable level of analgesia and increased maternal haemodynamic stability.[4] However, automated regular bolus delivery of epidural analgesia when compared with continuous infusion is found to decrease the incidence of breakthrough pain and increased maternal satisfaction.[20] Because of logistic constraints, we used intermittent epidural dosing on demand by the parturients, which, we found, could be comparable to continuous and automated delivery system. The limitation in our study was to combine primiparous and multiparous parturients, which could have altered the results of mode of delivery.
CONCLUSION
We conclude that CSE using sufentanil and fentanyl achieved high patient satisfaction and excellent labour analgesia without serious maternal or neonatal side-effects. Sufentanil provided significantly longer duration of labour analgesia compared with fentanyl.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Wong CA. Advances in labor analgesia. Int J Womens Health. 2009;1:139–54. doi: 10.2147/ijwh.s4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frikha N, Ellachtar M, Mebazaa MS, Ben Ammar MS. Combined spinal-epidural analgesia in labour- comparison of sufentanil vs tramadol. Middle East J Anesthesiol. 2007;19:87–96. [PubMed] [Google Scholar]
- 3.Deballi P, Breen TW. Intrathecal opioids for combined spinal-epidural analgesia during labour. CNS Drugs. 2003;17:889–904. doi: 10.2165/00023210-200317120-00003. [DOI] [PubMed] [Google Scholar]
- 4.Karla S, Saraswat N, Agnihotri GS. Comparision of efficacy of bupivacaine and fentanyl with bupivacaine and sufentanil for epidural labor analgesia. Saudi J Anaesth. 2010;4:178–81. doi: 10.4103/1658-354X.71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lilker S, Rofaeel A, Balki M, Carvallho JC. Comparison of fentanyl and sufentanil as adjuncts to bupivacaine for labor epidural analgesia. J Clin Anesth. 2009;21:108–12. doi: 10.1016/j.jclinane.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Thomas T, Robinson C, Champion D, McKell M, Pell M. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain. 1998;75:177–85. doi: 10.1016/s0304-3959(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 7.Connelly NR, Parker RK, Pedersen T, Manikantan T, Lucas T, Serban S, et al. Diluent volume for epidural fentanyl and its effect on analgesia in early labor. Anaesth Analg. 2003;96:1799–804. doi: 10.1213/01.ANE.0000061583.77068.0B. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet F, Blery C, Zatan M, Simonet O, Brage D, Gaudy J. Effect of epidural morphine on postoperative pulmonary dysfunction. Acta Anaaesthesiol Scand. 1984;28:147–51. doi: 10.1111/j.1399-6576.1984.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 9.Grass J, Sakima NT, Schmidt R, Michitsch R, Zuckerman RL, Harris AP. A randomized, double blind, dose response comparision of epidural fentanyl versus sufentanil analgesia after cesarean section. Anesth Analg. 1997;85:365–71. doi: 10.1097/00000539-199708000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Campbell DC, William RC, Datta S. The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anaesth Analg. 1995;81:305–9. doi: 10.1097/00000539-199508000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Tejwani GA, Rattan AK, McDonald JS. Role of spinal opioid receptors in the antinociceptive interactions between intrathecal morphine and bupivacaine. Anaesth Analg. 1992;74:726–34. doi: 10.1213/00000539-199205000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Claes B, Soetens M, Van Zundert A, Datta S. Clonidine added to bupivacaine-epinephrine-sufentanil improves epidural analgesia during childbirth. Reg Anesth Pain Med. 1998;23:540–7. doi: 10.1016/s1098-7339(98)90078-5. [DOI] [PubMed] [Google Scholar]
- 13.Herman NL, Sheu KL, Van Decar TK, Rubin JD, Gadalla F, Koff HD, et al. Determination of the analgesic dose- response relationship for epidural fentanyl and sufentanil with bupivacaine 0.125% in laboring patients. J Clin Anesth. 1998;10:670–711. doi: 10.1016/s0952-8180(98)00113-5. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Amar D, Pantuck CK. Epidural analgesia for labour and delivery: Fentanyl or sufentanil? Can J Anaesth. 1996;46:341–6. doi: 10.1007/BF03011711. [DOI] [PubMed] [Google Scholar]
- 15.Sia Alex TH, Chang JL, Chiu JW. Combination of intrathecal sufentanil 10 mcg plus bupivacaine 2.5 mg for labor analgesia: Is half dose enough? Anaesth Analg. 1999;88:362–6. doi: 10.1097/00000539-199902000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Abouleish A, Abouleish E, Camann W. Combined spinal epidural analgesia in advanced labor. Can J Anesth. 1994;41:575–8. doi: 10.1007/BF03009995. [DOI] [PubMed] [Google Scholar]
- 17.Salem IC, Fukushuma FB, Nakamura G. Side effects of subarachnoid and epidural sufentanil associated with a local anesthetic in patients undergoing labor analgesia. Rev Bras Anestesiol. 2007;57:1006–8. doi: 10.1590/s0034-70942007000200001. [DOI] [PubMed] [Google Scholar]
- 18.Kudialis SJ, Wirth RK. Comparision of sufentanil versus fentanyl with 0.125% bupivacaine for continuous labour epidural anesthesia. CRNA. 1995;6:26–30. [PubMed] [Google Scholar]
- 19.Gwen Le. Comparision of fentanyl and sufentanil in combination with bupivacaine for patient – controlled epidural analgesia during labor. J Clin Anesth. 2001;13:98–102. doi: 10.1016/s0952-8180(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 20.Lim Y, Sia AT, Ocampo C. Automated regular boluses for epidural analgesia: A comparison with continuous infusion. Int J Obstet Anesth. 2005;14:305–9. doi: 10.1016/j.ijoa.2005.05.004. [DOI] [PubMed] [Google Scholar]


