Abstract
Background:
Shivering is a common problem during neuraxial anaesthesia. Neuraxial anaesthesia impairs thermoregulatory control and up to a 56.7% incidence of shivering has been reported.
Aim:
To evaluate the effectiveness of prophylactic use of intravenous ketamine, clonidine and tramadol in control of shivering and to note any side-effects of the drugs used.
Setting and Design:
Randomised double-blind study.
Methods:
This study was conducted in 200 ASA grade I and II patients. Neuraxial block was performed with 2.8 mL (14 mg) of 0.5% bupivacaine heavy in all patients. The patients were randomly allocated into four groups of 50 each to receive saline as placebo (group P), ketamine 0.5 mg/kg (group K), Clonidine 75 mcg (group C) and Tramadol 0.5 mg/kg (group T). Temperature and hemodynamic parameters were recorded at every 5-min interval. Shivering was graded from 0 to 4 grades and, if grade 3 shivering occurred, the study drug was considered as ineffective and intravenous pethidine 25 mg was given as rescue drug.
Statistical Analysis:
Data among groups was compared using one-way ANOVA. The incidence of shivering and side-effects were compared using the chi-square test.
Results:
The incidence of grade 3 shivering showed a statistically significant difference (P=0.001) in group P (27/50) as compared with the other groups (group K=5/50, group C=2/50, group T=4/50). No drug showed any statistically significant advantage over the other. No major hemodynamic changes were seen with prophylactic use of test drugs; however, sedation score was significantly higher in group K (P<0.05) as compared with the other groups.
Conclusion:
The prophylactic use of ketamine, clonidine and tramadol were effective in preventing shivering during neuraxial anaesthesia without causing any major untoward side-effects.
Keywords: Clonidine, ketamine, neuraxial blockade, shivering, tramadol
INTRODUCTION
Shivering is distressing for the patients undergoing surgery under both regional and after general anaesthesia. The main causes for shivering intra/post-operatively are temperature loss, decreased sympathetic tone and systemic release of pyrogens.[1] Shivering increases expenditure of cardiac and systemic energy,[2] resulting in increased oxygen consumption and carbon dioxide production, lactic acidosis and raises the intraocular and intracranial pressure. It also interferes with haemodynamic monitoring intraoperatively.
Regional anaesthesia produces vasodilatation, which facilitates core to peripheral redistribution of heat. It also increases sweating threshold and decreases vasoconstriction and shivering threshold.[3] Intra- and post-operative management of shivering are usually done by external heating (forced air warming, warming blankets, warmed fluids) or pharmacological interventions. Various drugs from different groups like opioids, 5-hydroxytryptamine receptor (5-HT3) antagonists, N-methyl D-aspartate (NMDA) receptor antagonists, cholinomimetics and biogenic amines have been used in the literature.[4–7]
Shivering under neuraxial anaesthesia is a common problem faced by anaesthetists; therefore, a randomized double-blind study was conducted using commonly available drugs like ketamine, clonidine and tramadol to assess their efficacy when used prophylactically to control shivering under neuraxial blockade.
METHODS
After institutional approval and informed consent, 200 patients of ASA grade 1 and 2, belonging to either sex, aged between 18 and 65 years, undergoing lower abdominal or lower limb surgery were included in the study. Exclusion criteria were any patients suffering from neuromuscular disease, hyperthyroidism, history of cardiopulmonary disease, psychological disease, refusal to participate or temperature >38°C or <36.5°C. Following a detailed pre-anaesthetic checkup along with relevant investigations, patients were brought to the operation theatre (OT) and relevant monitoring attached. All the patients were pre-loaded with Ringer lactate 10 mL/kg before giving neuraxial blockade. The study drug was coded and presented to the anaesthetist not involved in the management of the patient and administered by intravenous (i.v) route just before giving the block. The study drug and saline were pre-heated to 37°C before administering them to the patient. The patients were randomized into four groups of 50 patients each as follows:
Group P: Patients received 10 mL normal saline
Group K: Patients received ketamine 0.5 mg/kg i.v. diluted to 10 mL in saline
Group C: Patients received clonidine 75 mcg i.v. diluted to 10 mL in saline
Group T: Patients received tramadol 0.5 mg/kg i.v. diluted to 10 mL in saline.
The i.v. fluids used were pre-heated to 37°C before using them for the patients. The temperature of the OT was maintained at 24±1°C for all the patients. Neuraxial anaesthesia was instituted at either L3-4 or L4-5 interspaces using 2.8 mL (14 mg) of hyperbaric bupivacaine 0.5% (with 8.5% dextrose) using a 25 gauge quincke's spinal needle. During the intraoperative period, after noting the baseline parameters, pulse rate, non-invasive blood pressure (NIBP), oxygen saturation, temperature (core and surface) and level of sensory block were assessed at 5-min intervals. Sensory block was assessed every 5 min till there was no change in the level of anaesthesia, and every 15 min thereafter. The core temperature was measured by a nasopharyngeal thermometer and surface temperature by an axillary thermometer.
Shivering was graded using a scale validated by Tsai and Chu[8] : grade 0=no shivering, 1=piloerection but no visible shivering, 2=muscular activity in only one group, 3=muscular activity in more than one muscle group but not generalized and 4=shivering involving the whole body. During surgery, the shivering scale was recorded at 5-min intervals up to 90 min of surgery. The prophylaxis was regarded as ineffective if the patients exhibited grade 3 shivering any time during the study and then i.v. pethidine 25 mg was administered as a rescue drug.
Side-effects such as hypotension, nausea, vomiting, hallucinations and sedation were also recorded. Hypotension was defined as a decrease in mean blood pressure (MBP) of more than 20% from the baseline. Hypotension was treated with i.v. incremental bolus dose of mephentermine 3 mg and a further i.v. infusion of Ringer lactate. If patients developed nausea and vomiting, i.v. metoclopramide 10 mg was administered. Hallucination as a side-effect was defined as a false sensory experience where the patients reported that they saw, heard, smelled, tasted or felt something that was non-existent. The attending anaesthetist also assessed the degree of sedation on a 5-point scale.[9] 1=fully awake and oriented, 2=drowsy, 3=eyes closed but arousable to command, 4=eyes closed but arousable to mild physical stimulation and 5=eyes closed but unarousable to mild physical stimulation.
Statistical analysis
All parameters were analyzed using SPSS 11.0 and STAT 9.0 software. The data among groups were compared using one-way ANOVA. The within-group data were analyzed using repeated-measure analysis of variance followed by Bonferroni's post hoc testing. The incidence of shivering and side-effects were compared using the chi-square test. The data was expressed as mean±SD. A value of P<0.05 was considered as statistically significant. Further, with the power of 80% and at the 5% significance level, it can be concluded that the sample size of 50 is sufficient enough to evaluate the effectiveness of prophylactic use of intravenous ketamine, clonidine and tramadol in controlling shivering and for analyzing any side-effects of the drugs used.
RESULTS
The demographic profile and the median level of sensory blockade in all the four groups were comparable [Table 1]. When the hemodynamic parameters (heart rate and mean blood pressure) were compared, the patients in the ketamine group had a higher heart rate and mean blood pressure during the first 30 min of observation as compared with the other three groups; however, thereafter, the two were comparable in all the four groups [Figures 1 and 2]. Hypotension occurred in 20% (10/50), 12% (6/50), 16% (8/50) and 12% (6/50) patients in the placebo, ketamine, clonidine and tramadol groups, respectively, which was statistically insignificant (P=0.631). Bradycardia occurred in two (4%), one (2%), three (6%) and two (4%) patients in the placebo, ketamine, clonidine and tramadol groups, respectively (P=0.791). There was a greater fall in core body temperatures in the control group as compared with the study drug groups [Figure 3].
Table 1.
Patient characteristics and median level of sensory blockade
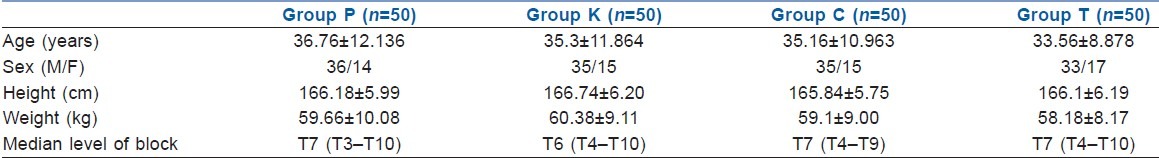
Figure 1.
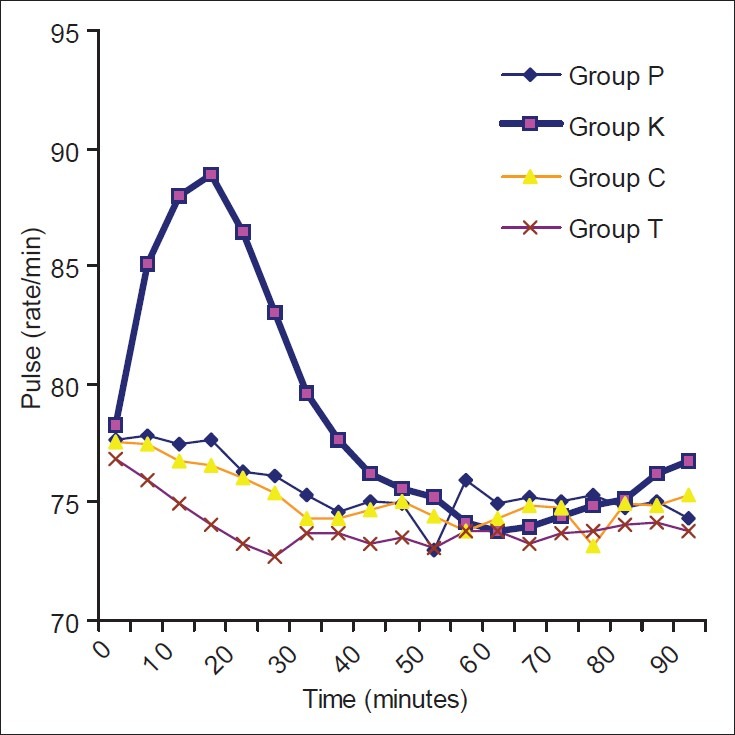
Trends in pulse rate in the four groups
Figure 2.
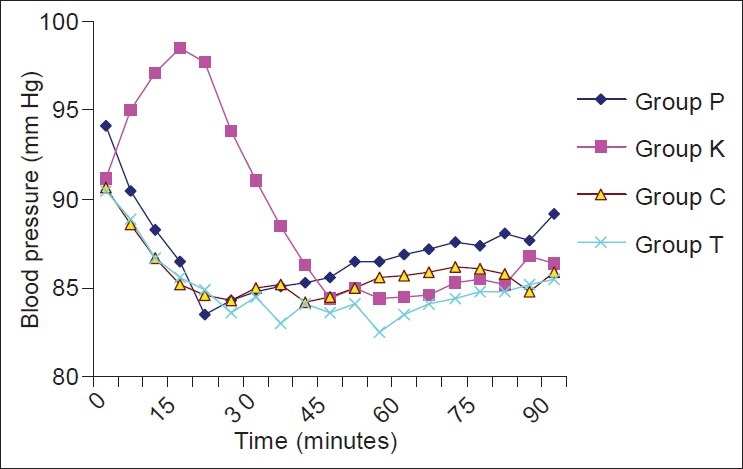
Trends in mean blood pressure in the four groups
Figure 3.
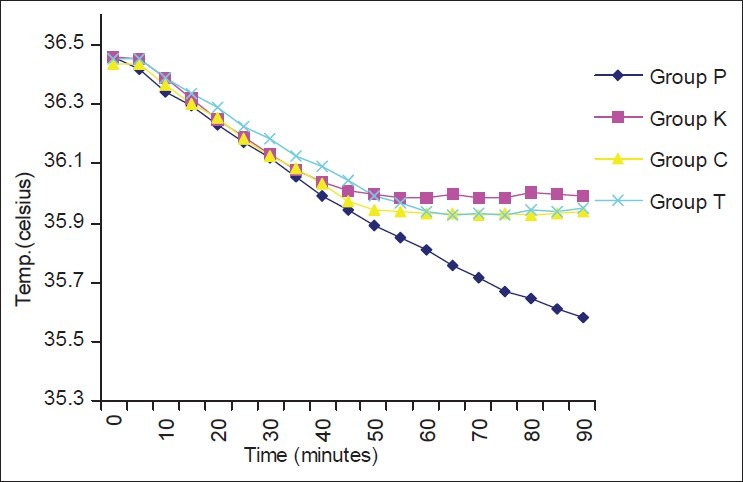
Change in core temperature with time in the four groups
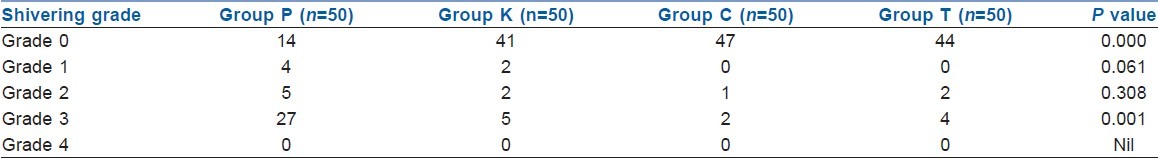
Twenty-eight percent (14/50) people did not shiver at all in the control group, while 82% patients (41/50) in the ketamine group, 94% patients (47/50) in the clonidine group and 88% patients (44/50) in the tramadol group did not shiver at all [Table 2], which was statistically significant (P=0.000). In the control group, 27 patients (54%) exhibited grade 3 shivering; however, only five patients (10%) in the ketamine group, two patients (4%) in the clonidine group and four patients (8%) in the tramadol group exhibited grade 3 shivering [Table 2], which was statistically significant (P=0.001). However, the drugs used for the study were equally efficacious in controlling shivering when used prophylactically, and no drug showed any significant advantage over the other.
Table 2.
Incidence of different grades of shivering in the four groups
No significant untoward side-effects were seen in all the four groups. Vomiting was only observed in the tramadol group in only 2% (1/50) patients, which was not statistically significant (P=0.389). Nausea was observed in one of 50 patients (2%) in the placebo group and two of 50 patients (4%) in the tramadol group; however, there was no nausea experienced in the patients in the ketamine and clonidine groups (P=0.293). Hallucination was not observed in any of the groups. Sedation score (grades 3 and 4) was significantly higher in the ketamine group (P<0.05) as compared with the other groups [Table 3].
Table 3.
Sedation score in the four groups

DISCUSSION
Our study has shown that prophylactic use of ketamine, clonidine and tramadol were effective in preventing shivering during neuraxial anaesthesia without causing any untoward side-effects. Ketamine is a competitive receptor antagonist of NMDA, which has a role in thermoregulation at various levels. It increases blood pressure, heart rate and cardiac output because of direct sympathetic stimulation and inhibition of norepinephrine uptake into the post-ganglionic sympathetic nerve endings, and may decrease core to peripheral redistribution of heat.[10] Ketamine may cause side-effects such as hallucination. Tramadol is a centrally acting analgesic that has weak opioid agonist properties. It also inhibits serotonin and norepinephrine uptake in the spinal cord and is effective in the treatment of post-operative shivering after regional and general anaesthesia.[11] Tramadol may cause nausea and vomiting. Tramadol has a low risk of respiratory depression, tolerance and dependence. Clonidine is a centrally acting α2, agonist and the anatomic target of its anti-shivering effect can be found at three levels. It decreases the thermoregulatory threshold for vasoconstriction and shivering as the hypothalamus contains high-density α2 receptors. It also reduces spontaneous firing in locus coeruleus – a pre-shivering centre in the pons. The α2 receptors also get activated to release dynorphine (opioid agonist), nor epinephrine and acetylcholine. The depressor effects of these neurotransmitters at the dorsal horn modulate cutaneous thermal inputs in addition to noxious and mechanoreceptive transmission.[12] Clonidine can cause hypotension and bradycardia as a side-effect.
In our study, the incidence of shivering was 54% (in the placebo group), which is comparable with the incidence reported by Sagir et al.,[13] Bilotta et al.[14] and Dhorigol et al.[15] However, Sia[16] reported a lower incidence of shivering (40%), but conducted the study under extradural blockade with 15–20 mL of 2% mepivacaine, with a median level of sensory blockade of T9 as compared with T7 in control group in our study. Moreover, the spinal anaesthesia causes a two- to three-level higher level of autonomic blockade than the sensory level achieved, as compared with extradural, in which autonomic blockade is the same or one level higher than the sensory level achieved.
Previous studies investigating the anti-shivering role of ketamine, clonidine and tramadol have shown similar results as our study. Sagir et al.[13] also found ketamine 0.5 mg/kg i.v. to be effective in controlling shivering under neuraxial blockade. Dal et al.[17] witnessed significant results with ketamine 0.5 mg/kg i.v. to prevent shivering under general anaesthesia. Gangopadhyay et al.[18] concluded that ketamine 0.5 mg/kg i.v. was effective in preventing shivering under spinal anaesthesia. Sia[16] found clonidine to be an effective drug when used prophylactically at 1 mcg/ kg i.v. to control shivering under neuraxial blockade. Tewari and others found oral clonidine 150 μg to be an efficacious drug in controlling shivering under neuraxial blockade.[19] Dhorigol and others[15] also found oral clonidine 150 μg to be effective in controlling shivering during subarachnoid block when used prophylactically. Bilotta[14] and Chan et al.[20] found tramadol to be a promising drug in doses of 0.5 mg/kg and 0.25 mg/kg i.v., respectively, in controlling shivering under neuraxial blockade. Gangopadhyay and others[18] found promising results with tramadol 1.0 mg/ kg i.v. in preventing shivering under spinal anaesthesia.
There was a greater fall in core body temperatures in the placebo group as compared with the ketamine, tramadol and clonidine groups in our study. This trend in core temperature is similar with the trends reported by Sagir[13] and Tewari et al.[19] Greater fall in core temperature in the placebo group as compared with the other groups may be because of the study drug effect.
In our study, the incidence of side-effects was not significantly different among the groups. Tramadol has the potential to cause nausea and vomiting, but the incidence of nausea and vomiting in the study groups was comparable with the placebo group. Similar results are reported in the literature.[20,21] However, Gangopadhyay et al.[18] observed a significant number of cases (20/30) of nausea and vomiting with tramadol; this high number of cases in the tramadol group could be explained by the fact that they used tramadol at 1 mg/kg i.v. as compared with 0.5 mg/kg i.v. in our study. Clonidine is known to cause hypotension and bradycardia, but the incidence of hypotension and bradycardia in the study groups was comparable with the placebo group, which was in concordance with other studies.[15,16,19] Ketamine is known to cause hallucinations, but none of the patients complained of hallucination in any of the groups. Studies done in the past support our findings.[13,17]
Ketamine causes significant grades 3 and 4 sedation as compared with placebo, clonidine and tramadol. Clonidine also has the potential to cause sedation, but, in our study, clonidine did not cause any significant sedation as compared with placebo. Ketamine causes a significant amount of sedation, which may be beneficial to us as it increases patient comfort, maintains cardiorespiratory stability, improves surgical conditions and prevents recall of unpleasant events during the surgery.[22]
CONCLUSION
We conclude that giving either ketamine 0.5 mg/ kg, clonidine 75 mcg or tramadol 0.5 mg/kg i.v. prophylactically just before neuraxial blockade significantly decreased the incidence of shivering without causing any major side-effects. Using ketamine may be more beneficial as it improves the hemodynamic profile by its sympathomimetic effects and it sedates the patient effectively, which increases patient comfort during surgery, maintains cardiorespiratory stability and prevents recall of unpleasant events during the surgery.
ACKNOWLEDGMENTS
The authors would like to thank all the patients who participated in the study and Mr. Shukla for his help in the statistical analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92:578–96. doi: 10.1097/00000542-200002000-00042. [DOI] [PubMed] [Google Scholar]
- 2.Imrie MM, Hall GM. Body temperature and anaesthesia. Br J Anaesth. 1990;64:346–54. doi: 10.1093/bja/64.3.346. [DOI] [PubMed] [Google Scholar]
- 3.Sessler DI, Ponte J. Shivering during epidural anesthesia. Anesthesiology. 1990;72:816–21. doi: 10.1097/00000542-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Pascal A. Postanesthetic Shivering: Epidemiology, Pathophysiology and approaches to prevention and Management. Drugs. 2001;61:2193–205. doi: 10.2165/00003495-200161150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kranke P, Eberhart LH, Roewer N, Tramer MR. Single dose parenteral pharmacological interventions for the prevention of postoperative shivering: A Quantitative Systematic Review of Randomized Controlled Trials. Anesth Analg. 2004;99:718–27. doi: 10.1213/01.ANE.0000130589.00098.CD. [DOI] [PubMed] [Google Scholar]
- 6.Kim MS, Kim DW, Woo SH, Yon JH, Lee S. Effect of ramosetron on shivering during neuraxial anesthesia. Korean J Anesthesiol. 2010;58:256–9. doi: 10.4097/kjae.2010.58.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozdemir M, Usta B, Demircioglu RI, Muslu B, Sert H, Karatas OF. Magnesium sulfate infusion prevents shivering during transurethral prostatectomy with spinal anesthesia: A randomized, double blinded, controlled study. J Clin Anesth. 2010;22:184–9. doi: 10.1016/j.jclinane.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Tsai YC, Chu KS. A comparison, amitriptyline and meperidine for post epidural anaesthetic shivering. Anesth Analg. 2001;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 9.Wilson E, David A, Mackenzie N, Grant IS. Sedation during spinal anaesthesia: Comparison of propofol and midazolam. Br J Anaesth. 1990;64:48–52. doi: 10.1093/bja/64.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T, Kazama T, Sessler DI, Toriyama S, Niwa K, Shimada C, et al. Induction of anesthesia with ketamine reduces the magnitude of redistribution hypothermia. Anesth Analg. 2001;93:934–8. doi: 10.1097/00000539-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 11.De Witte J, Deloof T, De Veylder J, Housmans PR. Tramadol in the treatment of post anesthetic shivering. Acta Anaesthesiol Scand. 1997;41:506–10. doi: 10.1111/j.1399-6576.1997.tb04732.x. [DOI] [PubMed] [Google Scholar]
- 12.Alojado ME, Ohta Y, Kemmotsu O. The effect of clonidine on the activity of neurons in the rats dorsal raphe nucleus in vitro. Anesth Analg. 1994;79:257–60. doi: 10.1213/00000539-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O. Control of shivering during regional anaesthesia: Prophylactic ketamine and granisetron. Acta Anaesthesiol Scand. 2007;51:44–9. doi: 10.1111/j.1399-6576.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- 14.Bilotta F, Pietropaoli P, Sanita’ R, Liberatori G, Rosa G. Nefopam and tramadol for the prevention of shivering during neuraxial anesthesia. Reg Anesth Pain Med. 2002;27:380–4. doi: 10.1053/rapm.2002.33563. [DOI] [PubMed] [Google Scholar]
- 15.Dhorigol MG, Dhulkhed VK, Biyani A, Desai N. Randomized controlled, double-blind study to evaluate oral clonidine to prevent post-subarachnoid block shivering in patients undergoing elective urological surgery. J Anaesthesiol Clin Pharmaco. 2010;26:15–8. [Google Scholar]
- 16.Sia S. I.v. clonidine prevents post-extradural shivering. Br J Anaesth. 1998;81:145–6. doi: 10.1093/bja/81.2.145. [DOI] [PubMed] [Google Scholar]
- 17.Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth. 2005;95:189–92. doi: 10.1093/bja/aei148. [DOI] [PubMed] [Google Scholar]
- 18.Gangopadhyay S, Gupta K, Acharjee S, Nayak SK, Dawn S, Pipal G. Ketamine, tramadol and pethidine in prophylaxis of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26:59–63. [Google Scholar]
- 19.Tewari A, Katyal S, Singh A, Garg S, Kaul TK, Narula N. Prophylaxis with oral clonidine prevents perioperative shivering in patients undergoing transurethral resection of prostate under subarachnoid block. Indian J Urol. 2006;22:208–12. [Google Scholar]
- 20.Chan AM, Ng KF, Tong EW, Jan GS. Control of shivering under regional anaesthesia in obstetric patients with tramadol. Can J Anaesth. 1999;46:253–8. doi: 10.1007/BF03012605. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar S, Saxena A, Kannan TR, Punj J, Panigrahi M, Mishra S. Tramadol for postoperative shivering: A double-blind comparison with pethidine. Anaesth Intensive Care. 2001;29:149–54. doi: 10.1177/0310057X0102900209. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama T, Yokoyama T, Hanaoka K. Sedation guidelines for midazolam infusion during combined spinal and epidural anesthesia. J Clin Anesth. 2004;16:568–72. doi: 10.1016/j.jclinane.2004.02.004. [DOI] [PubMed] [Google Scholar]


