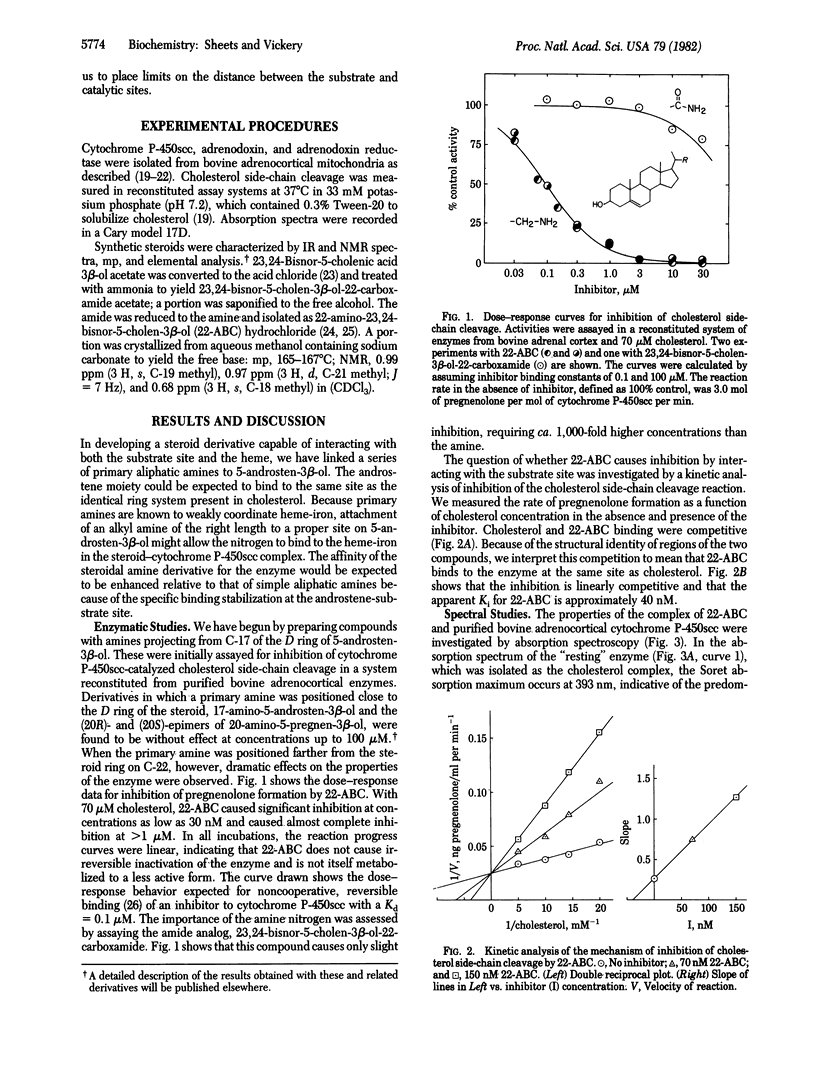
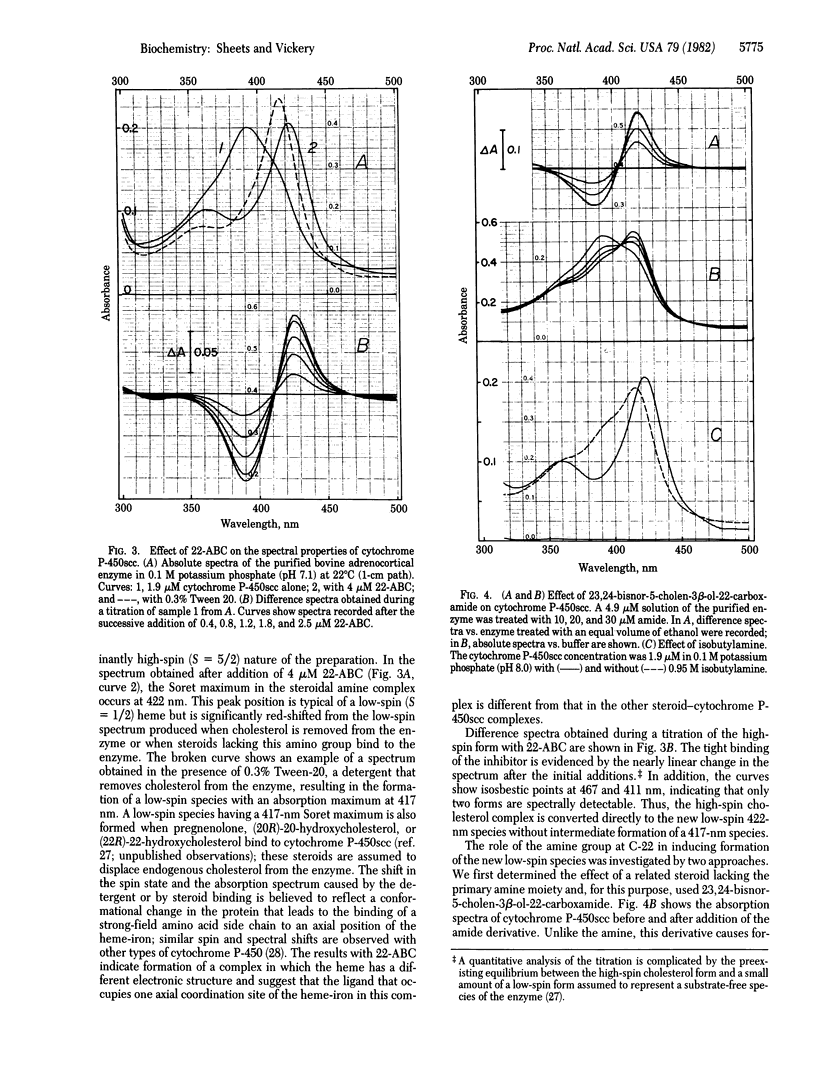
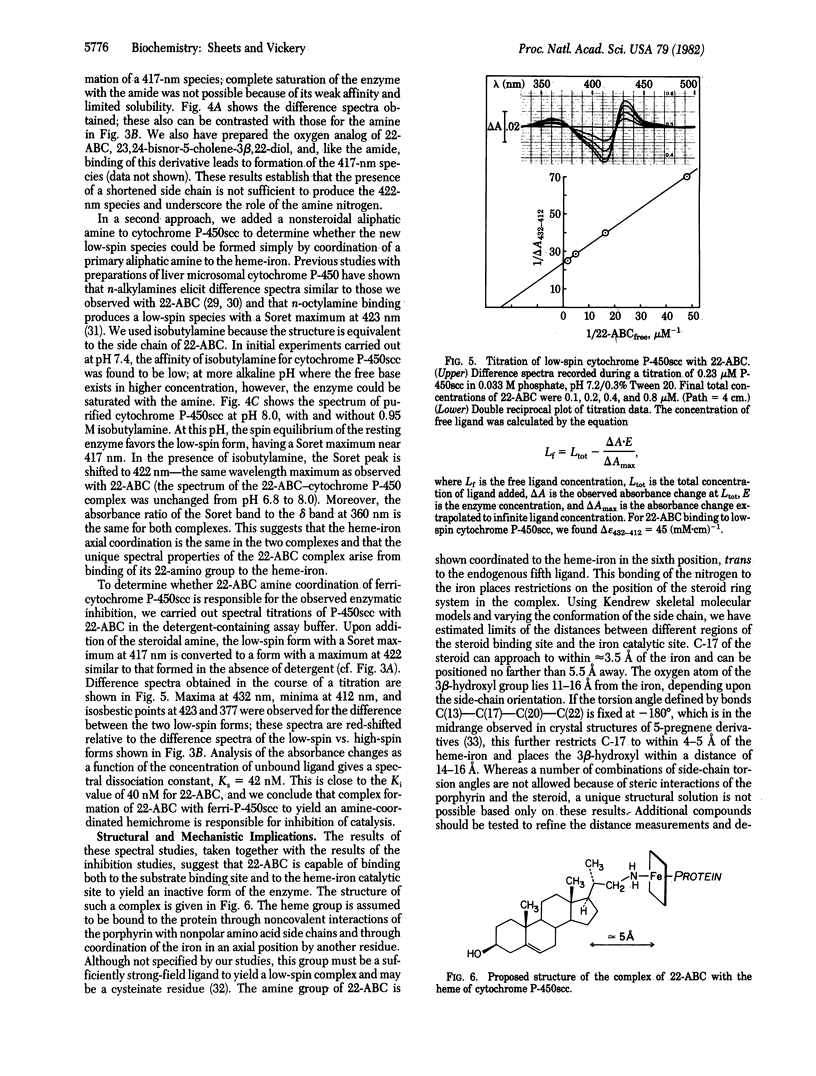
Abstract
As an approach to "mapping" the active site of the cytochrome P-450 that catalyzes cholesterol side-chain cleavage, designated cytochrome P-450scc, we have synthesized steroid derivatives with the potential to interact with both the substrate binding site and the heme-iron catalytic site of the enzyme. The effects of these substrate analogs were studied with cytochrome P-450scc purified from bovine adrenal cortex. One derivative, 22-amino-23,24-bisnor-5-cholen-3 beta-ol, was found to be a potent inhibitor of pregnenolone formation in a reconstituted enzyme system, and a kinetic analysis of the inhibition showed that binding of the derivative is competitive with respect to cholesterol. The spectral properties of a stable complex formed between the steroidal amine and the purified cytochrome suggest that the 22-amine group coordinates directly to the heme-iron. A model for the structure of this inhibitor-enzyme complex is proposed in which the 5-androstene ring system of the steroid occupies the substrate binding site, and the amine group of the side chain occupies an axial coordination position of the Fe(III) center. This places limits on the distance between these two domains in the enzyme and offers support for proposed mechanisms of cytochrome P-450-catalyzed oxygen-insertion reactions in which an iron-bound oxidant directly attacks the substrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein S., Middleditch B. S., Gut M. Mass spectrometric study of the enzymatic conversion of cholesterol to (22R)-22-hydroxycholesterol, (20R,22R)-20,22-dihydroxycholesterol, and pregnenolone, and of (22R)-22-hydroxycholesterol to the lgycol and pregnenolone in bovine adrenocortical preparations. Mode of oxygen incorporation. J Biol Chem. 1975 Dec 10;250(23):9028–9037. [PubMed] [Google Scholar]
- Byon C. Y., Gut M. Steric considerations regarding the biodegradation of cholesterol to pregnenolone.-exclusion of (22S)-22-hydroxycholesterol and 22-ketocholesterol as intermediates. Biochem Biophys Res Commun. 1980 May 30;94(2):549–552. doi: 10.1016/0006-291x(80)91266-8. [DOI] [PubMed] [Google Scholar]
- Duax W. L., Griffin J. F., Rohrer D. C., Weeks C. M. Conformational analysis of sterols: comparison of X-ray crystallographic observations with data from other sources. Lipids. 1980 Sep;15(9):783–792. doi: 10.1007/BF02534032. [DOI] [PubMed] [Google Scholar]
- Duval J. F., Vickery L. E. 3-Methoxybenzidine: a potent inhibitor of cholesterol side chain cleavage cytochrome P-450. Steroids. 1980 Oct;36(4):473–481. doi: 10.1016/0039-128x(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Duval J. F., Vickery L. E. Inhibition of bovine adrenocortical cytochrome P-450scc by 3,3'-dimethoxybenzidine. Steroids. 1981 Jan;37(1):91–101. doi: 10.1016/0039-128x(81)90011-8. [DOI] [PubMed] [Google Scholar]
- Groves J. T., McClusky G. A. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. Evidence for a carbon radical intermediate. Biochem Biophys Res Commun. 1978 Mar 15;81(1):154–160. doi: 10.1016/0006-291x(78)91643-1. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Sligar S. G. Oxygen reduction by the P450 monoxygenase systems. Adv Enzymol Relat Areas Mol Biol. 1978;47:1–44. doi: 10.1002/9780470122921.ch1. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Lewes J. L., Lipson E. D. The role of mitochondrial cytochrome P-450 from bovine adrenal cortex in side chain cleavage of 20S,22R-dihydroxycholesterol. J Biol Chem. 1975 Mar 25;250(6):2283–2286. [PubMed] [Google Scholar]
- Hrycay E. G., Gustafsson J. A., Ingelman-Sundberg M., Ernster L. Sodium periodate, sodium chloride, organic hydroperoxides, and H2O2 as hydroxylating agents in steroid hydroxylation reactions catalyzed by partially purified cytochrome P-450. Biochem Biophys Res Commun. 1975 Sep 2;66(1):209–216. doi: 10.1016/s0006-291x(75)80315-9. [DOI] [PubMed] [Google Scholar]
- Hume R., Boyd G. S. Cholesterol metabolism and steroid-hormone production. Biochem Soc Trans. 1978;6(5):893–898. doi: 10.1042/bst0060893. [DOI] [PubMed] [Google Scholar]
- Jefcoate C. R., Gaylor J. L., Calabrese R. L. Ligand interactions with cytochrome P-450. I. Binding of primary amines. Biochemistry. 1969 Aug;8(8):3455–3463. doi: 10.1021/bi00836a049. [DOI] [PubMed] [Google Scholar]
- Jefcoate C. R., Gaylor J. L. Ligand interactions with hemoprotein P-450. II. Influence of phenobarbital and methylcholanthrene induction processes on P-450 spectra. Biochemistry. 1969 Aug;8(8):3464–3472. doi: 10.1021/bi00836a050. [DOI] [PubMed] [Google Scholar]
- Lichtenberger F., Nastainczyk W., Ullrich V. Cytochrome P450 as an oxene transferase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):939–946. doi: 10.1016/0006-291x(76)90682-3. [DOI] [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Light D. R., White-Stevens R. W., Orme-Johnson W. H. Steroid binding properties of beef adrenal cortical cytochrome P-450 which catalyzes the conversion of cholesterol into pregnenolone. J Biol Chem. 1979 Mar 25;254(6):2103–2111. [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J., Hrycay E. G., Peterson J. A., Estabrook R. W. Possible higher valence states of cytochrome P-450 during oxidative reactions. Biochem Biophys Res Commun. 1974 Sep 23;60(2):695–702. doi: 10.1016/0006-291x(74)90296-4. [DOI] [PubMed] [Google Scholar]
- STONE D., HECHTER O. Studies on ACTH action in perfused bovine adrenals: the site of action of ACTH in corticosteroidogenesis. Arch Biochem Biophys. 1954 Aug;51(2):457–469. doi: 10.1016/0003-9861(54)90501-9. [DOI] [PubMed] [Google Scholar]
- Sato H., Ashida N., Suhara K., Itagaki E., Takemori S., Katagiri M. Properties of an adrenal cytochrome P-450 (P-45011beta) for the hydroxylations of corticosteroids. Arch Biochem Biophys. 1978 Sep;190(1):307–314. doi: 10.1016/0003-9861(78)90280-1. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Remmer H., Estabrook R. W. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1967 Mar;3(2):113–123. [PubMed] [Google Scholar]
- Shikita M., Hall P. F. The stoichiometry of the conversion of cholesterol and hydroxycholesterols to pregnenolone (3beta-hydroxypregn-5-en-20-one) catalysed by adrenal cytochrome P-450. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1441–1445. doi: 10.1073/pnas.71.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur J Biochem. 1967 Oct;2(3):275–285. doi: 10.1111/j.1432-1033.1967.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Suhara K., Ikeda Y., Takemori S., Katagiri M. The purification and properties of NADPH-adrenodoxin reductase from bovine adrenocortical mitochondria. FEBS Lett. 1972 Nov 15;28(1):45–47. doi: 10.1016/0014-5793(72)80673-2. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. Improved purification of bovine adrenal iron-sulfur protein. Biochim Biophys Acta. 1972 Apr 15;263(2):272–278. doi: 10.1016/0005-2795(72)90079-7. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]



