Abstract
Objective:
Role of nitric oxide (NO) in reversing morphine anti-nociception has been shown. However, the interaction between NO and naloxone-induced pain in the hippocampus is unknown. The present study aimed to investigate the involvement of molecule NO in naloxone-induced pain and its possible interaction with naloxone into cortical area 1 (CA1) of hippocampus.
Materials and Methods:
Male Wistar rats (250–350 g), provided by Pasteur Institute of Iran, were housed two per cage with food and water ad libitum. The animals’ skulls were cannulated bilaterally at coordinates adjusted for CA1 of hippocampus (AP: -3.8; L: ±1.8– 2.2: V: 3) by using stereotaxic apparatus. Each experimental group included 6–8 rats. To induce inflammation pain, the rats received subcutaneous (s.c.) injections of formalin (50 μL at 2.5%) once prior to testing. To evaluate the nociceptive effect of naloxone, the main narcotic antagonist of morphine (0.1–0.4 mg/kg) was injected intraperitoneally (i.p.) 10 min before injection of formalin. Injections of L-arginine, a precursor of NO, and NG-Nitro-L-arginine Methyl Ester (L-NAME), an inhibitor of NO synthase (NOS), intra-CA1, were conducted orderly prior to the administration of naloxone. The pain induction was analyzed by analysis of variance (ANOVA).
Results:
Naloxone at the lower doses caused a significant (P<0.01) pain in the naloxone-treated animals. However, pre-administration (1–2 min) of L-arginine (0.04, 0.08, 0.15, 0.3, 1.0, and 3.0 μg/rat, intra-CA1) reversed the response to naloxone. But, the response to L-arginine was blocked by pre-microinjection (1–2 min) of L-NAME (0.15, 0.3, 1.0, and 3.0 μg/rat), whilst, L-arginine or L-NAME alone did not induce pain behavior.
Conclusion:
NO in the rat hippocampal CA1 area is involved in naloxone-induced nociception.
KEY WORDS: Formalin test, naloxone, nitric oxide, pain
Introduction
Hippocampus involves a variety of biological functions including declarative learning and memory, anxiety,[1] sensorimotor integration,[2] arousal,[3] and nociception.[4] It is also implicated in affective-motivational response to noxious–aversive events.[5] The endogenous opioids are released by noxious stimulation, thereby limiting the intensity of the pain sensation.[6] Numerous studies have shown the effects of an opioid receptor antagonist, naloxone, in modulating of pain.[7] Naloxone, a phenanthrene compound, which is structurally related to morphine,[8] has been widely used to antagonize the effects of opioid drugs. Most of the biological effects of naloxone are classified as opioid or non-opioid depending on reversal by naloxone.[9] Naloxone exerts the opposite effects on nociception.[10] The effects appear to be dose dependent and biphasic because high doses of naloxone, both in humans and animals, induce pain lowering the thermal and mechanical nociceptive threshold whereas low doses exert analgesia and increase the nociceptive threshold.[11] Naloxone increases pain at high doses in the presence of opioid drugs or activation of endogenous opioid system.[12]
Nitric oxide (NO) is a molecule implicated in the actions of opioids.[4] It has been indicated that opioidergic stimulation of the arginine/NO pathway causes central, spinal, or peripheral analgesia.[4] The central analgesic effect of arginine seems to be as associated with NO stimulation.[13] Thus, NO is involved in nociceptive transmission; however, the results are not clear. NG-Nitro-L-arginine Methyl Ester (L-NAME) is a specific NO synthase (NOS) inhibitor and has been shown to cause antinociception by spinal, supraspinal, local (intraplantar), or systemic administration.[14] This criterion supports our hypothesis that naloxone induces pain sensation and the NO pathway changes nociception.
The present study aimed to investigate the involvement of the molecule NO in naloxone-induced pain and its possible interaction with naloxone into cortical area 1 (CA1) of hippocampus. In this study, nociception was evaluated using inflammatory pain model promoted by a peripheral injection of formalin.
Materials and Methods
Animals
Male Wistar rats (Pasteur Institute of Iran, Tehran, Iran), weighing between 250 and 350 g, were used in the study. Rats were housed two per cage in a controlled colony room (temperature 22 ± 2°C). They were maintained under a 12:12 h light/dark cycle with water and food provided ad libitum. A group of 6–8 rats were included in each experiment. The experiments were designed as the solely naloxone or L-arginine/L-NAME plus naloxone treatments. All experiments were approved by the local committee of ethics at Shahed University.
Experimental Procedure
Rats were anesthetized by intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (20 mg/kg) provided by Veterinary Organization of Iran. Each anesthetized animal was then placed in a stereotaxic apparatus, with the incisor bar set at approximately 3.0 mm below horizontal zero to achieve a flat skull position. An incision was made to expose the rat skull. Two holes were drilled in the skull at stereotaxic coordinates: AP: -3.8 mm posterior to bregma and L: 1.8 ± 2.2 mm. Two guide cannulae (21-gauge) were inserted into the holes. For animals receiving bilateral injections into the cortical area 1 (CA1) of the hippocampus, the guide cannulae were lowered 2.5 mm below bregma through the holes. The guide cannulae were anchored with a jeweler's screw, and the incision was closed with dental cement. After surgery, dummy inner cannulae that extended 0.5 mm beyond the guide cannulae were inserted into the guide cannulae and left in the place until injections were made. A total of six rats were excluded because of incorrect cannulae placements. All animals were allowed to recover for one week before behavioral testing.
Formalin test
Seven days after surgery, each rat was placed in a transparent acrylic cage and allowed to move freely for 15–20 min to habituate. A mirror was placed under the cage to allow an unobstructed view of the animal's paws to the behavioral observer. Each rat was restrained and received 50 μL subcutaneous injection of 2.5% buffered formalin into the right hind paw and was placed in the observation box for the next 60 min. The initial 5 min shows nociceptive behavior (early or acute phase). The behavior reappears in next 10–15 min (pause) and then slowly diminishes over the subsequent 40–60 min (late or chronic phase). The observations were continuously rated by a 4-point scale as follows:
Score of 0 denotes normal use of the injected paw (i.e., the plantar surface of the paw comes into full contact with the floor of the observation box and the animal's weight is evenly distributed between hind paws). A score of 1 indicates careful use of the injured paw, with some part of the paw in contact with the floor; the animal limps when walking. A score of 2 indicates elevation of the paw. A score of 3 denotes vigorous shaking or licking of the injured paw (distinct from normal grooming behavior). The nociceptive effect of naloxone (0.1–0.4 mg/kg, i.p.) was tested 10 min after administration of the drug by the above-mentioned test using a single formalin injection.[4] In the cortical area 1, the NO agent, L-arginine, was first injected followed by naloxone administration. L-NAME (0.15–3 μg/rat), the NOS inhibitor, was pre-injected to L-arginine (0.04–3 μg/rat) intra-CA1. Vehicles were administered intra-CA1 or intraperitoneal as 1 μL/rat or 1 mL/kg respectively.
Intra-hippocampal injection
The animals were gently restrained by hand; the dummy cannulae were removed from the guide cannulae, and the drugs were directly injected into the CA1 region of the hippocampus through guide cannulae by using injection needles (27-gauge) connected by polyethylene tubing (0.3 mm internal diameter) to a 5.0-μL glass Hamilton syringe. The injection needles projected a further 0.5 mm ventral to the tip of the guides. The injection volume was 1 μL for all groups (0.5 μL/side) and injected over a 30-s period. The injection cannulae were left in the guide cannulae for an additional 60 s to facilitate the diffusion of the drugs.
Cannula position verification
After testing of behaviors, animals were anesthetized by overdose of chloroform. The animals were decapitated using a guillotine to verify their cannulae position; an ink (0.5 μL of 1% aquatic methylene blue solution) was injected into the guide cannulae, using the same injection set-up. To aid in verification, the rats’ brains were removed and fixed in a 10% formalin solution for 48 h before sectioning. Sections were taken through the brain areas of cannulae placement for verification.[4]
Drugs
The drugs, Naloxone hydrochloride (Tolid Daru co., Tehran), L-arginine (Sigma Chemical Co., USA), NG-nitro-L-arginine methyl ester (L-NAME; Research Biochemical Inc., USA), were prepared freshly in sterile 0.9% NaCl solution. Naloxone was injected i.p. and NO agents, L-arginine or L-NAME, were bilaterally injected into the hippocampal CA1 area (in a volume of 1 μL/rat). Vehicle injections were of the appropriate volume of 0.9% physiological saline.
Statistical analysis
All results are expressed as mean ± SEM. Data was analyzed using one-way analysis of variance (ANOVA) or two-way ANOVA followed by appropriate post-hoc analysis (Tukey-Kramer). P<0.05 was considered to be statistically significant.
Results
Naloxone dose response in the rat formalin test
Naloxone pre-testing significantly induced pain in the naloxone treated animals as compared to the controls in chronic phase (F4,25= 5.018, P<0.01). However, the effect of the narcotic drug in acute phase was not significant. The post-hoc analysis indicated that the opioid antagonist produces the pain at the lower doses. Thus, a dose of 0.1 mg/kg of the drug was used for the rest of the experiments [Figure 1].
Figure 1.
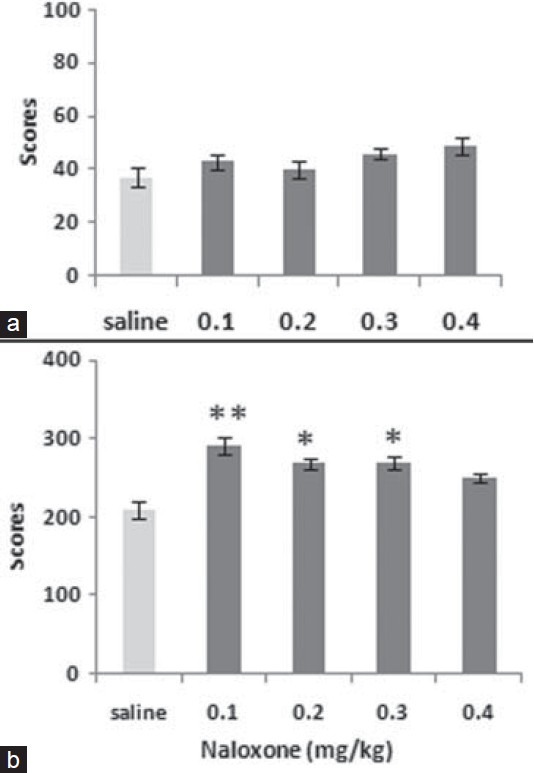
The figure shows the dose response to naloxone (0.1–0.4 mg/ kg) both in acute (a) and chronic (b) phases in Wistar rats. Values are mean ± SEM. Post hoc analysis show *P<0.05, **P<0.01 compared to control
L-Arginine effect on the response to Naloxone in the rat formalin test
Pretreatment of L-arginine intra-CA1 markedly reversed naloxone-induced pain in both phases. The calculation of the data by ANOVA indicated the significant effects in the acute phase (F6,36= 2.351, P<0.05) and chronic phase (F6,36= 3.776, P<0.01)]. Further analysis indicated that the NO precursor L-arginine significantly reversed the narcotic drug's pain induction at the lower doses. According to the findings, a dose of 0.08 μg/rat of the agent was used for the rest of the experiments [Figure 2].
Figure 2.
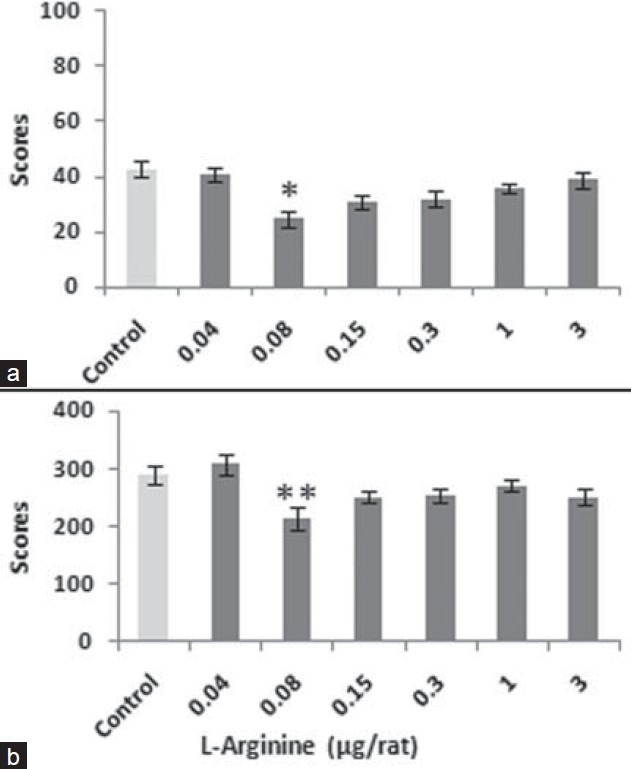
The figure shows the dose response to L-arginine (0.04–3 μg/rat) both in acute (a) and chronic (b) phases when this agent was pre-administered to naloxone (0.1 mg/kg, i.p.) before formalin test. Values are as mean ± SEM. Post hoc analysis show *P<0.05, **P<0.01 compared to control
To show interaction between NO and naloxone in pain processing, the data obtained by single L-arginine (data not shown) and those of L-arginine together with naloxone were analyzed by two-way ANOVA. According to the mentioned analysis, a significant interaction between naloxone and L-arginine (F6,72= 3.303, P<0.05) was indicated.
Effect of pre-injection of L-NAME on response to the administration of L-arginine prior to naloxone in the rat formalin test
Pre-injection of L-NAME, intra-CA1, prior to injection of L-arginine before of testing of naloxone-pain behavior showed a significant reversal effect in the experimental groups in a comparison to the controls (F4,14= 5.045, P<0.01). This group of control animals received saline, intra-CA1, instead of L-NAME. Further analysis indicated that the NOS inhibitor L-NAME has a significant reversal effect at the higher doses [Figure 3].
Figure 3.
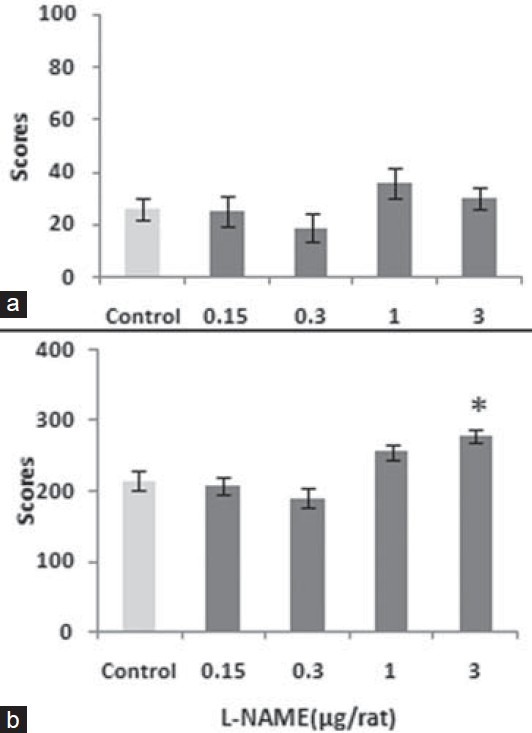
The figure shows the response to L-NAME (0.15–3 μg/rat) both in acute (a) and chronic (b) phases when pre-administration to L-arginine (0.08 μg/rat) prior to naloxone in the formalin test. Values are mean ± SEM. Post hoc analysis shows *P<0.05 compared to control
To indicate interaction between the NO and naloxone in pain processing, the data obtained by single L-NAME (data not shown) and those of L-NAME in presence of L-arginine plus naloxone were calculated by two-way ANOVA. A significant interaction was shown (F6,72= 3.155, P<0.05). However, single injection of L-NAME before testing of naloxone pain behavior showed no remarkable effect.
Discussion
The present study shows that naloxone induces pain in the rat formalin test and that the microinjection of L-arginine into rats’ cortical area 1 (CA1) prior to naloxone formalin testing blocked the naloxone-induced pain. The response to L-arginine was reversed by pre-microinjection of L-NAME. Aloisi et al.[15] has reported that subcutaneous injection of formalin into the rat hind paw induces a persistent nociceptive behavior and prolonged decrease in choline acetyl transferase activity.
The present work showed that naloxone has pain inducing effect at the lower doses. Naloxone has biphasic effect as been reported previously. The antagonist effect at low doses enhances the morphine's analgesia potency through blocking of autoinhibition of enkephalin release.[16] In vivo studies confirm that ultra-low doses of opioid antagonists enhance opioid analgesia.[17] Furthermore, recent data lead to hypothesize that naloxone could act through the interaction with a specific and yet undetermined class of receptors, different from classical opioid receptors.[18] The effects of naloxone might be complicated by analgesic effects of an opioid compound like morphine. As previously been confirmed, the analgesic effect of ultra-low dose is obtained by blocking excitatory opioid-receptor functions in dorsal root ganglion neurons and by blocking the autoinhibition of enkephalin release.[16] L-Arginine, intra-CA1, showed an interaction with naloxone at the lower doses. The activation of NO production induces antinociception,[19] although another report has indicated that cholinergic or opioidergic stimulation of the arginine/NO pathway causes central, spinal, or peripheral analgesia.[20]
NG-nitro-L-arginine methyl ester (L-NAME) inhibited the response to L-arginine at the higher doses when it was pre-injected to L-arginine at the site of interest. L-NAME causes antinociception by spinal, supraspinal, local (intraplantar), or systemic administration.[21] In comparison, it has been indicated that the intraplantar or systemic administration of L-NAME has similar effects to other NO synthase (NOS) inhibitors in causing antinociception.[22] This contradiction may be explained by considering that the activation of the arginine/NO pathway either causes hyperalgesia or analgesia, depending on the predominant type of fibers involved in the nociceptive response or depending on the tissue level of NO.[23]
Evidence is accumulating that opioid peptides are important modulators of information processing in the hippocampus. When activated, opioid receptors play a key role in central pain modulation mechanisms, and the hippocampal formation is a structure that expresses significant densities of this kind of receptors.[24] Many physiological, pharmacological, and behavioral findings have suggested that the hippocampal formation is involved in nociception.[4] The hippocampus is assumed to play an important role in the affective and motivational components of pain perception. For example, the pyramidal cells and interneurons in the dorsal hippocampal CA1 respond to persistent noxious activation.[5] Hippocampal pathways have reduced pain behaviors; peripheral noxious stimulation alters the induction of Fos.[15] Fos and Egr1 are transcription proteins that are expressed in neurons following synaptic excitation.[15] Several opioid neuropeptides, such as enkephalin and b-endorphin, as well as morphine, promote a decrease in hippocampal ACh levels when administered intraseptally,[25] suggesting that the opioid agonists may act at the level of cholinergic cell bodies in the septal region to modulate the activity of septal cholinergic afferents terminating in the hippocampus (Ammon's horn). Our results did not show these kinds of opioidergic–cholinergic interaction in the hippocampus, but, suggest a possible nitrergic–opioidergic interaction. The present study showed that the nociception induced by naloxone is blocked by pretreatment with L-arginine microinjected into the CA1. These results can indicate the action of the opioid system inside the CA1 as NO dependent because pretreatment with L-NAME blocks the effect of L-arginine plus naloxone. On this basis, it can be concluded that the local L-arginine injection may activate nitrergic neurons, which in turn may activate pain modulators.
Acknowledgments
This study was supported in part by Deputy of Research at Shahed University (documents for the Graduate student Proposals).
Footnotes
Source of Support: This study was supported partially by Research Deputy of Shahed University.
Conflict of Interest: None declared.
References
- 1.Tai SK, Huang FD, Moochhala S, Khanna S. Hippocampal theta state in relation to formalin nociception. Pain. 2006;121:29–42. doi: 10.1016/j.pain.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–36. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Leung LS. Limbic system participates in mediating the effects of general anesthetics. Neuropsychopharmacology. 2006;31:1177–92. doi: 10.1038/sj.npp.1300909. [DOI] [PubMed] [Google Scholar]
- 4.Hashemi M, Karami M, Zarrindast MR, Sahebgharani M. Role of nitric oxide in the rat hippocampal CA1 in morphine antinociception. Brain Res. 2010;1313:79–88. doi: 10.1016/j.brainres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Khanna S, Seong Chang L, Jiang F, Chow Koh H. Nociception-driven decreased induction of Fos protein in ventral hippocampus field CA1 of the rat. Brain Res. 2004;1004:167–76. doi: 10.1016/j.brainres.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Koppert W, Filitz J, Tröster A, Ihmsen H, Angst M, Flor H, et al. Activation of naloxone-sensitive and -insensitive inhibitory systems in a human pain model. Pain. 2005;6:757–64. doi: 10.1016/j.jpain.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Tsuruoka M, Hiruma Y, Matsutani K, Matsui Y. Effects of yohimbine on naloxone-induced antinociception in a rat model of inflammatory hyperalgesia. Eur J Pharmacol. 1998;348:161–5. doi: 10.1016/s0014-2999(98)00151-4. [DOI] [PubMed] [Google Scholar]
- 8.Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gillman AG, editors. Goodman and Gillman's the Pharmacological Basis of Therapeutics. 9th ed. NewYork: McGraw Hill Press; 1996. pp. 521–55. [Google Scholar]
- 9.McNicholas LF, Martin WR. New and experimental therapeutic roles for naloxone and related opioid antagonists. Drugs. 1984;27:81–93. doi: 10.2165/00003495-198427010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Gillman MA, Lichtigfeld FJ. Naloxone analgesia: Further developments. Int J Neurosci. 1990;2:249–50. doi: 10.3109/00207459009000528. [DOI] [PubMed] [Google Scholar]
- 11.Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278:740–1. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- 12.Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–61. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Raghubir R, Srimal RC, Dhawan BN. Evidence for involvement of nitric oxide in pretectal analgesia in rat. Neuroreport. 1993;4:706–8. doi: 10.1097/00001756-199306000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Babbedge RC, Hart SL, Moore PK. Anti-nociceptive activity of nitric oxide synthase inhibitors in the mouse: Dissociation between the effect of L-NAME and L-NMMA. J Pharm Pharmacol. 1993;45:77–9. doi: 10.1111/j.2042-7158.1993.tb03686.x. [DOI] [PubMed] [Google Scholar]
- 15.Aloisi AM, Zimmermann M, Herdegen T. Sex-dependent effects of formalin and restraint on c-Fos expression in the septum and hippocampus of the rat. Neuroscience. 1997;81:951–8. doi: 10.1016/s0306-4522(97)00270-4. [DOI] [PubMed] [Google Scholar]
- 16.Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine's analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–31. doi: 10.1016/s0304-3959(99)00223-7. [DOI] [PubMed] [Google Scholar]
- 17.Shen KF, Crain SM. Ultra-low doses of naltrexone or etorphine increase morphine's antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757:176–90. doi: 10.1016/s0006-8993(97)00197-2. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzetti BB, Ferreira SH. Activation of the arginine-nitric oxide pathway in primary sensory neurons contributes to dipyrone-induced spinal and peripheral analgesia. Inflamm Res. 1996;45:308–11. doi: 10.1007/BF02280997. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata A, Umeda N, Takagi H. L-arginine exerts a dual role in nociceptive processing in the brain: Involvement of the kyotorphin-Met-enkephalin pathway and NO-cyclic GMP pathway. Br J Pharmacol. 1993;109:73–9. doi: 10.1111/j.1476-5381.1993.tb13533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Tong C, Pan HL, Cerda SE, Eisenach JC. Intravenous morphine increases release of nitric oxide from spinal cord by an alpha-adrenergic and cholinergic mechanism. J Neurophysiol. 1997;78:2072–8. doi: 10.1152/jn.1997.78.4.2072. [DOI] [PubMed] [Google Scholar]
- 21.Gao WC, Qiao JT. Nitric oxide contributes to both spinal nociceptive transmission and its descending inhibition in rats: An immunocytochemical study. Neurosci Lett. 1998;240:143–6. doi: 10.1016/s0304-3940(97)00949-x. [DOI] [PubMed] [Google Scholar]
- 22.Sakurada T, Sugiyama A, Sakurada C. Effect of spinal nitric oxide inhibition on capsaicin-induced nociceptive response. Life Sci. 1996;59:921–30. doi: 10.1016/0024-3205(96)00390-6. [DOI] [PubMed] [Google Scholar]
- 23.Granados-Soto V, Rufino MO, Gomes Lopes LD, Ferreira SH. Evidence for the involvement of the nitric oxide-cGMP pathway in the antinociception of morphine in the formalin test. Eur J Pharmacol. 1997;340:177–80. doi: 10.1016/s0014-2999(97)01399-x. [DOI] [PubMed] [Google Scholar]
- 24.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 25.Moore PK, Oluyomi AO, Babbedge RC, Wallace P, Hart SL. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991;102:198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


