Abstract
Objectives:
Setarud (IMOD™) is a herbal medicine with beneficial effect for patients suffering Human immunodeficiency virus (HIV) infection and has been approved for IV (intra venues) injection. The beneficial effect of IMOD administration for acquired immune deficiency syndrome (AIDS) patient has been proved in previous clinical trials. Here the in vitro inhibitory effect of IMOD against HIV-1, Herpes simplex virus (HSV) and murine leukemia viruses (MLV) was evaluated.
Materials and Methods:
HIV single cycle replication and HSV plaque reduction assays were used to evaluate the anti-viral effect. The level of HIV replication was monitored by p24 capture Enzyme-linked immunosorbent assay (ELISA). The single round infection [with green fluorescent protein (GFP) reporter MLV and HIV], virucidal and time-of-additions (HSV) assays were utilized to determine the mode of anti-viral activity. The toxicity of IMOD for cells was monitored by XTT (sodium 3_-[1 (phenylaminocarbonyl)- 3,4-tetrazolium]-bis (4-methoxy-6-nitro)benzene sulfonic acid) cell proliferation assay kit.
Results:
IMOD inhibited 50% of HIV-1 and HSV replication (IC50) at 6.5 × 10-4 and 4.3 × 10-3V/V concentrations, respectively. The IC50 value against HIV-1 and MLV infection were 6 × 10-4V/V and 4.9 × 10-4V/V. Virucidal assay showed that IMOD reduces the potency of HIV and HSV particles to 41 and 54% of control, respectively. Time-of-addition study revealed that IMOD inhibits the replication of HSV at a stage after penetration of virions to the target cells.
Conclusions:
Data from this study indicate that IMOD has significant anti-viral activity against HIV, HSV and MLV. Setarud could be subjected to further investigation after isolation of the constituents and determination of the toxic components.
KEY WORDS: Anti-viral activity, human immunodeficiency virus, setarud (IMOD™)
Introduction
Human immunodeficiency virus (HIV) is a retrovirus, and the infectious cause of acquired immunodeficiency syndrome (AIDS).[1] AIDS is characterized by the depletion in the count of CD4-positive T lymphocytes. Despite the considerable success of anti-retroviral therapy (HAART), AIDS remains one of the most important world health problems.[2,3] Emergence of resistance strains and toxicity of current available drugs make it impetus to find new anti-HIV therapy strategies.[4,5] Setarud (IMOD™) is a natural medicine and includes herbaceous components manufactured by Parsroos Company in Iran (www.Parsroos.com). IMOD has been patented in Europe (WO/2007/087825) for its immunomodulatory capacities and also for its benefits for increasing CD4 count in HIV positive patients.[6] Setarud is an herbal compund comprising of ethanolic Rosa canina (fruit), Urtica Dioica and Tanacetum Vulgare (leaf and stem) extracts. This medicine further comprises of Selenium and Urea.[6] IMOD has been introduced to the market as the 4 ml ampoules containing 120 mg of Setarud. Previous studies show that IMOD administration can increase the CD4 cell count in HIV infected patients.[7] Intravenous (IV) administration of IMOD (125 mg) for 12 weeks increases the cluster of differentiation 4 (CD4) cells count 2 or 3 fold, and this induction is stable even after 6 months. It was also shown that patient with CD4 count less than 400 could benefit more from IMOD compared with controls.[7] The clinical investigations showed that IMOD is safe for administration and does not have any serious adverse effects in patients even after months of the drug administration.[7,8] The in vitro investigations showed no mutagenic or genotoxic effects for this agent.[9] IMOD is also known as an immunomodulatory, and its effect in sepsis and inflammatory bowel disease has been investigated.[10,11] In addition to the immunomodulatory potential, hypercholesterolemia and hepatic-protection were also reported for IMOD.[12,13]
IMOD is currently being introduced as a commercial medicine for treatment of AIDS in Iran. There is no data available about the anti-viral activity of IMOD. Here, the in vitro anti-HIV-1 (NL4-3), Herpes simplex virus type [HSV (KOS)] and murine leukemia virus (MLV) potential of IMOD were investigated versus its cellular toxicity. Also the specific action of this agent against HIV-1 virions is evaluated by using a retrovirus control (MLV). The mechanism of anti-viral activity of IMOD was investigated by performing virucidal and time -f-addition study in the HIV and HSV models.
Materials and Methods
Reagents
Commercial intravenous (IV) ampoules of IMOD were kindly obtained from the owner company of IMOD - Parsroos. All ampoules were stored in a cold and dark place and checked for the expiration date, and expired samples were not used. In each experiment, IMOD was used freshly after vial breakage. A non-nucleoside inhibitor of HIV reverse transcriptase enzyme (Nevirapine) and Acyclovir (anti-HSV drug) were extracted from commercial tablets and used as positive controls.
Plasmids
Single cycle replicable (SCR) and GFP reporter retroviral systems (HIV-1 and MLV) were used in this study.[14,15] GFP reporter HIV-1 virions were produced by using plasmids coding viral poly-proteins (pSPAX.2), viral envelope glycoprotein (p7-HX) and packaging reporter RNA (pWPXL). Vesicular stomatitis virus surface glycoprotein (VSVG) pseudotyped single cycle replicable (SCR) HIV-1 was used in this study. SCR HIV virus was produced by using pSPAX.2, pMD2G (coding VSVG) and pmzNL4-3 plasmids.[15] pmzNL4-3 includes HIV-1 (NL4-3) provirus with a large deletion mutation in pol sequence.[16] pCL-ECO, pMD2G and pBABE-GFP plasmids were used for production of MLV virions with GFP reporter provirus.
Cell Culture and Virus Production
Human Embryonic Kidney 293 cells (HEK293T), vero and MT-2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) and Roswell Park Memorial Institute 1640 (RPMI1640) mediums (Chemicon, USA) supplemented with 10% heat inactivated fetal bovine serum (Gibco, USA) and appropriate concentrations of L-glutamine and sodium pyruvate (Sigma, United States of America). HIV and MLV virus stocks were prepared by transfection of the (HEK293T) cells. Polyfect transfection reagent (Qiagen, USA) was used according to the user manual. Virus containing supernatants were harvested at 24, 48 and 72 hours post transfection, then pooled and stored at 4-8°C. Pooled Supernatant was clarified by using 10 minute centrifuging at 104 g and filtering thought 0.22 μm filters. Viruses were stored at -70°C and determined for infectious titer.[17,18] HSV (KOS) virions were expanded in 6 - wells plates by infecting the 80 percent confluent vero cells with 1 ml of virus supernatant for each well. The supernatants were harvested and pooled every 24 hours until day 4. To clarify the virus containing supernatant, it was filtered through 0.22 μm filters and then stored at -70°C until experiments.
Human Immunodeficiency Virus Replication Assay
HIV-1 single cycle replication assay was performed using VSVG SCR HIV-1 (NL4-3) virions and HEK293T cells.[15] Cells were seeded in each well of 24-wells plate (70 × 103/well) and infected with 700ng P24 of single cycle replicable (SCR) virions in the presence of different IMOD concentrations. After 16 hours, cells were washed two times with pre-warmed DMEM (to remove the unbound virions) and then the fresh medium containing IMOD was added to the wells. Cells supernatants were analyzed for P24 load, 72 hours after infection. The level of p24 was evaluated by quantitative P24 capture ELISA (Zeptometrix, USA). Nevirapine (extracted from commercial tablets) and phosphate buffered saline (PBS) were used as the positive and negative controls, respectively.
Single Round Infection Assays
GFP reporter HIV-1 and MLV virions were used to perform single round infection assays. MT-2 cells were placed into each well of 96-wells plate at the confluence of 2 × 104 cells per well. Cells were infected with appropriate infectious titer of virus containing supernatant in the presence of different concentrations of IMOD. Nevirapine and PBS were used as positive and negative controls. Cells were incubated for 16 hours to let the virions enter into the cells. After that, fresh medium (200 μl) and IMOD were added to the wells. Cells were harvested 72 hours post infection and transferred into the flowcytometry tubes. The GFP positive cells populations were analyzed by using flowcytometry method.[14]
Herpes simplex virus plaque reduction assay
The inhibitory activity against HSV replication was studied using plaque reduction assay. Vero cells were seeded in each well of 6-wells plates (3.9 × 105 cells/well) containing 5% Fetal bovine serum (FBS) supplemented DMEM and cultured for 24 hours to form a monolayer with at least 96% confluence. Cell monolayer was infected with 60 Plaque forming units (PFU) HSV virions for 1 hour and then washed with DMEM and covered with 2% methylcellulose supplemented fresh medium. Plates were maintained for 72 hours in a carbon dioxide (CO2) incubator. After that, the overlay medium was removed and cell monolayer was washed twice, fixed with methanol and stained with 0.5% crystal violet. Number of formed plaques was counted by direct vision.
Cytotoxicity Assay
The cellular toxicity of IMOD for target cells (HEK and MT- 2) was investigated using the XTT cell proliferation assay kit (Roche, Germany) according to the manufacturer protocol.[19] HEK (3 × 104/well) and MT-2 (5 × 104/well) cells were cultured in 96- well plates containing 250 μl of complete medium supplemented with different IMOD concentrations. After 72 hours of incubation, cells medium was replaced with fresh phenol-red free medium. XTT solution was added into the wells and plates were incubated for additional 2 hs. The optic density (OD) of 96-wells plate was measured using an ELISA reader instrument at the test and reference wavelength of 450 nm and 630 nm.
Virucidal Assay
The virucidal effect of IMOD was investigated against HIV and HSV virions. HIV (600 ng P24) and HSV (60PFU) virions was incubated with 10-2V/V concentration of IMOD in a final volume of 5 μl. Virions were incubated for 0.5 and 5hs (37°C) with IMOD and then the replication of virions was measured as described above.
Time-of-addition study
The inhibitory effect of IMOD on different intervals of HSV virions replication was monitored by time-of-addition study. IMOD (final concentration of 10-2V/V) was added into the cells environment at different stages of HSV replication include before (-2 and -6 hours), during (0 hour) and after infection (2 and 6 hours). The plaque reduction assay was performed as described above. Cellular monolayers were washed to remove IMOD in the wells which it had been added before (-2 and -6 hours) and during (0 hour) infection.
Statistical analysis
All experiences were performed in triplicate and two independent experiments. Results from experiments are expressed as the mean ± Standard Deviation (SD). The 50 percent of values (IC50 and CC50) were calculated by Microsoft Excel. Student's unpaired t-test was used to calculate P values for means between test and control samples. A P-value of less than 0.05 was considered to be statistically significant.
Results
The anti- human immunodeficiency virus - 1 and Herpes simplex virus potential of IMOD
The inhibitory activity of IMOD for HIV-1 and HSV replication was evaluated as well as its cytotoxicity [Figure 1]. The inhibitory activity of IMOD for the replication of HIV-1 virions was detectable at 10-5V/V. IMOD completely inhibits HIV-1 at the 10-2V/V concentration. Results from HIV-1 replication assay showed that IMOD inhibits 50% of HIV-1 virions replication at the concentration (IC50) of 6.5 × 10-4V/V. IMOD was also potent for inhibition of HSV replication with IC50 of 4.3 × 10-3. The cytotoxicity was assessed by measuring the proliferation of HEK293T cells cultured in the presence of IMOD. Low IMOD concentration (10-4 and 10-5V/V) slightly raised proliferation however its higher concentration was toxic for both HEK and MT-2 cells. Results showed that proliferation of the target cells was decreased to 50% of negative control in the presence of 5.4 × 10-3V/V concentration (CC50 of IMOD).
Figure 1.
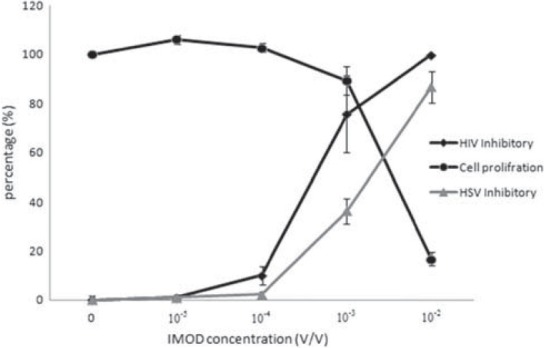
The anti-viral potential of IMOD. Significant reduction in Human immunodeficiency virus and Herpes simplex virus (HSV) replication was observed from 10-4V/V IMOD concentration. It able to completely block virions at 10-2V/V. toxicity of Setarud (IMOD) for target cells is also shown in this figure. IMOD reduced 50 percent of cell proliferation at 5.4 × 10-3V/V concentration
The inhibitory effect of IMOD for inhibition of human immunodeficiency virus and murine leukemia virus infection
To identify the specific inhibitory activity of IMOD for HIV, the anti-viral activity of this reagent for a retrovirus control (MLV) was examined. MT-2 cells were infected with GFP reporter HIV-1 and MLV virions in the presence of different IMOD concentrations. The result from counting the GFP positive cells by flowcytometer is shown in Figure 2. The reduction in number of GFP positive cells was seen from 10-5V/V IMOD concentration in both HIV and MLV infected cells. The infection of MT-2 cells with HIV-1 and MLV (retrovirus control) virions was inhibited by IMOD with IC50 of 6 × 10-4V/V and 4.9 × 10-4, respectively. Almost all of both MLV and HIV-1 virions infection was blocked by IMOD at 10-2V/V concentration.
Figure 2.
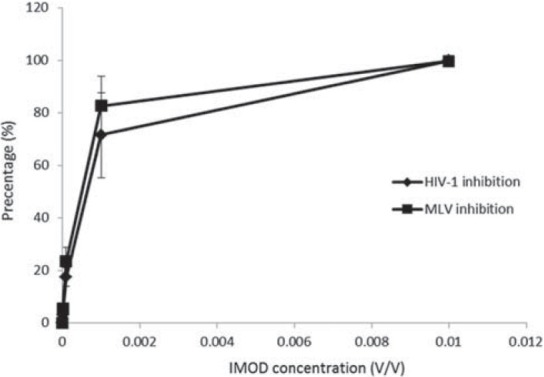
Anti-viral activity of Setarud (IMOD) against retrovirus control (Murine leukemia virus – MLV) over Human immunodeficiency virus - 1 (HIV-1). Significant inhibition of MLV and HIV-1 virions infection can be seen from 10-4V/V concentration of IMOD. Almost all of both retroviruses infection was blocked at 10-2V/V concentration. As it can be seen in this figure, there is no difference between inhibition capacity of IMOD for HIV-1 and MLV virions
The mode of anti-viral effect
Data from this study showed that IMOD significantly inhibited HIV, MLV and HSV with similar dose response pattern. The mode of action of this agent against HSV and HIV was studied using virucidal and time-of-addition studies. HIV and HSV virions were incubated for 0.5 and 5 hs with 10-2V/V of IMOD and then the replication of virions were investigated in replication and plaque formation assays. The HIV replication was 56 and 41% of the negative control after 0.5 and 5 hs incubation with IMOD, respectively [Figure 3]. After 0.5 and 5 hours incubation of HSV virions with this agent the replication capacity was decreased to 77 and 54%, respectively. Time-of-addition study was performed to study the effect of IMOD on different stages of HSV virions replication. IMOD (10-2V/V) inhibited the 92 and 97 percent of HSV replication when was added into the cells environment 2 and 6 hs after infection [Figure 4]. Presence of IMOD before or during infection did not inhibit HSV virions replication.
Figure 3.
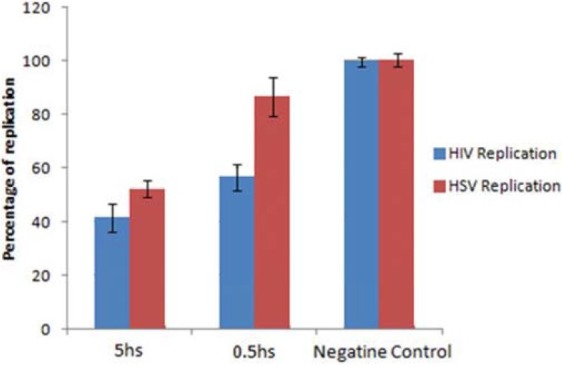
The virucidal effect of Setarud (IMOD) for Human immunodeficiency virus (HIV) and Herpes simplex virus (HSV) virions. The virions were incubated with 10-2V/V of IMOD for 0.5 and 5 hours and then the viral replication was investigated by HIV replication and HSV plaque reduction assays. The amount of HSV and HIV virions replication after exposure to the IMOD is shown here
Figure 4.
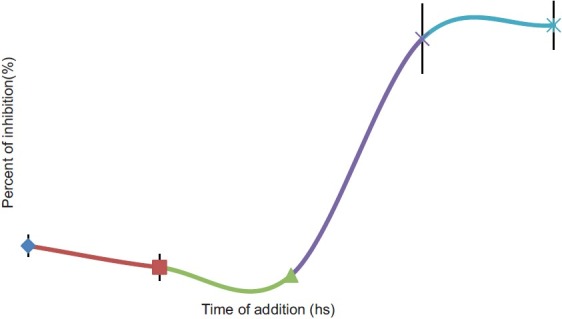
The inhibitory effect of Setarud (IMOD) on different stages of Herpes simplex virus (HSV) infection. IMOD was added to the cell enviroment in different intervals of HSV replication including before (-6 and 02 hours), during (0 hour) and after (+2 and +6) infection. Results showed that IMOD is not effective for inhibition of virions if is added to cells enviroment before viral penetrion. It significantly inhibits viral replication if is added after infection
Discussion
The adverse-effects of currently available drugs and continuous emergence of resistance against them has encouraged further studies for new and more effective anti-HIV agents. Medicinal plants are diverse and chemically complex. Herbal compounds provide widespread pharmacological and biological properties.[20] Previous studies show the anti-HIV activities of extracts from some medicinal plants.[21] Many anti-HIV-1 active compounds have been determined in botanicals and some of them discovered to block HIV at various stages of viral life cycle. IMOD is a commercial herbal anti-AIDS agent in Iran and currently is using for treatment of HIV-1 infected patients however the direct effect of this reagent on HIV-1 virions has not been investigated yet. The immunomodulatory activity of IMOD has been shown by Mahmoodpoor, et al.[11] It is suggested that the anti-AIDS potential of this drug is linked to its interaction with immune system components,[7] although according to data from this study IMOD would have anti-viral property. Reduction in HIV-1 virions replication was detectable in the presence of as low as 10-5V/V IMOD concentration. This finding implies that IMOD would have direct effect on HIV virions rather than just immunomodulatory activity. To determine the exact anti-viral properties of IMOD the inhibitory activity against HIV-1, HSV and MLV viruses was examined as well as its cellular toxicity in vitro condition.
Results of clinical trials showed that patients, with CD4 count less than 400 cells per ml or stage III, treatment with IMOD for at least 3 months significantly raised the CD4 cells in comparison with participants who did not receive treatment. The increase of the number of CD4 cells was higher than control after stopping the treatment (for six months).[7] Evidences about the in vitro anti-viral activity of two major constituents of the IMOD (T. Vulgare and Urtica dioica) exists.[22,23] It has been shown that the ethyl acetate extract of aerial parts of T. Vulgare and isolated compound parthenolide have anti-HSV-1 activity.[22] Urtica dioica is a plant usually used as folk medicine in Italy for treating numerous diseases first of all Herpes zoster infection. The anti-viral activity of this medicinal plant was previously reported.[23] Current study showed that Setarud inhibits the 50 percent of SCR HIV-1 (NL4-3) virions replication (IC50) at the 65 × 10-5V/V concentration. IMOD is highly potent for inhibiting HIV-1 virions even in very low concentration, therefore it would help AIDS patients via this mode of action.
IMOD contains R. Canina which showed anti-oxidant activity in former studies.[24] Furthermore the R. Canina has free radical scavenging[25] and anti-ulcerogenic[26] effects. On the other hand, the flavonoid compounds extracted from T. Vulgare leaf showed anti-inflammatory activity.[27] Another constituent of IMOD (Urtica dioica) has been used for treatment of various ailments in folk medicine.[28,29] Leaves of this plant showed anti-inflammatory[30] and cell proliferative[31] effects in previous studies. Considering the biological activity of each composition of Setarud the effect of this medicine on cellular proliferation was studied here. Presence of IMOD in target cells environment at concentration as low as 10-5V/V was inducer for target cells proliferation, while reduction in cell proliferation was observed in the presence of higher concentrations. A 50 percent decrease in cellular proliferation (CC50) was observed at 5.4 × 10-3V/V IMOD concentration. This data indicates that IMOD could have cellular toxicity in higher doses, however it is safe in low concentration.
IMOD showed robust inhibitory activity against HIV and HSV replication [Figure 1] which is in consistent with previous report about anti-viral activity of T. Vulgare and Urtica dioica.[22,23] To verify the mode of anti-viral action, the infection inhibitory of IMOD was investigated and compared for murine leukemia virus (MLV) as well of HIV-1 using GFP reporter virions. Comparison of the result obtained from these tests shows whether inhibitory effect of IMOD for HIV-1 is specific or it could inhibit a retrovirus control as well.[32] The infection process of both retroviruses (HIV-1 and MLV) was inhibited with IC50 values of 6 × 10-4V/V and 4.9 × 10-4V/V, respectively. This result indicates that anti-HIV-1 activity of IMOD is not due to specific action against viral component so it probably acts by affecting cellular pathways. On the other hand, the results from cell proliferation assay emphasis that the in vitro anti-HIV activity of IMOD mostly related to its effect on the target cells.
Further investigation of mode of anti-viral activity was performed by evaluating the direct effect of IMOD on viral particles in virucidal assays. The HIV and HSV virions were incubated with IMOD (10-2V/V) for 0.5 and 5 hs. The replication of HIV virions decreased to 41 percent of control [Figure 3] which indicates IMOD may be active against HIV particles. According to the time-of-addition study, IMOD was a strong inhibitor for the HSV virions when it was added to the cells environment after infection [Figure 4]. Presence of IMOD before infection did not inhibit viral replication. These findings reveal that IMOD inhibit viral replication at a stage after penetration of virions to the cells. Considering the similar tone of anti-viral action for HIV, MLV and HSV in addition to data from time-of-addition and virucidal s tudies, it is suggested that indicates that IMOD inhibits viral infection by affecting cellular pathways or has a direct effect on viral particles.
Conclusion
Data from this study shows that IMOD has mild to low anti-retroviral activity in vitro. IMOD has also similar anti-viral activity against a Deoxyribonucleic acid virus (HSV). IMOD inhibits the MLV and HIV virions and has significant activity for inactivating viral particles. These data emphasize that the anti-HIV-1 potential of IMOD is related to its effect on viral particles or cellular machineries. On the other hand, IMOD is a crude herbal extract and hence its fractionation could help to separate the toxic constituents, and also help to determine the active compounds and the exact mechanism of action. Further research is thus warranted in this field to establish the findings.
Acknowledgments
This study is financially supported by the Pasteur institute of Iran and performed under the supervision of the Parsroos Company. We wish to extend a special thanks to Dr. Khoramkhorshid for providing us with the IMOD vials for the research.
Footnotes
Source of Support: Pasteur institute of Iran.
Conflict of Interest: No.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–71. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Arora DR, Gautam V, Gill PS, Mishra N. Recent advances in antiretroviral therapy in HIV infection. J Indian Med Assoc. 2010;108:29–34. [PubMed] [Google Scholar]
- 3.Wilkin TJ, Shalev N, Tieu HV, Hammer SM. Advances in antiretroviral therapy. Top HIV Med. 2010;18:66–92. [PubMed] [Google Scholar]
- 4.Cohen J. HIV/AIDS. Tangled patent dispute over ‘free’ drug-resistance database. Science. 2009;323:1156–7. doi: 10.1126/science.323.5918.1156. [DOI] [PubMed] [Google Scholar]
- 5.Obiako OR, Murktar HM, Ogoina D. Antiretroviral drug resistance--implications for HIV/AIDS reduction in sub-saharan Africa and other developing countries. Niger J Med. 2010;19:302–10. [PubMed] [Google Scholar]
- 6.Novitsky YA, Madani H, Gharibdoust F, Farhadi M, Farzamfar B, Mohraz M. Inventors; Parsroos corporation. Use of a combination of ethanolic rosa sp., urtica dioica and tanacetum vulgare extracts, further comprising selenium and urea and having been exposed to a pulsed electromagnetic field, for the preparation of a medicament for immunostimulation and/or treatment of hiv infections. EU patent WO/2007/087825. 2007 Aug 9; Other Published Materials. [Google Scholar]
- 7.Mohraz M, Khairandish P, Kazerooni PA, Davarpanah MA, Shahhosseiny MH, Mahdavian B, et al. A clinical trial on the efficacy of IMOD in AIDS patients. DARU. 2009;17:277–83. [Google Scholar]
- 8.Khairandish P, Mohraz M, Farzamfar B, Abdollahi M, Shahhosseiny MH, Madani H, et al. Preclinical and phase 1 clinical safety of setarud (IMOD™), a novel immunomodulator. DARU. 2009;17:148–57. [Google Scholar]
- 9.Khorramkhorshid HR, Novitsky YA, Abdollahi M, Shahhosseiny MHS, Madani H, Rahimi R, et al. Studies on potential mutagenic and genotoxic activity of setarud. DARU. 2008;16:223–8. [Google Scholar]
- 10.Baghaei A, Esmaily H, Abdolghaffari AH, Baeeri M, Gharibdoost F, Abdollahi M. Efficacy of setarud (IMOD®), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian Journal of Biochemistry and Biophysics. 2010;47:219–26. [PubMed] [Google Scholar]
- 11.Mahmoodpoor A, Eslami K, Mojtahedzadeh M, Najafi A, Ahmadi A, Dehnadi-Moghadam A, et al. Examination of setarud (IMOD™) in the management of patients with severe sepsis. DARU. 2010;18:23–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Azonov JA, Khorramkhorshid HR, Novitsky YA, Farhadi M, Ghorbanoghli Z, Shahhosseiny MH. Protective effects of setarud (IMOD™) on development of diet-induced hypercholesterolemia in rabbits. DARU. 2008;16:218–22. [Google Scholar]
- 13.Khorramkhorshid HR, Azonov JN, Novitsky YA, Farzamfar B, Shahhosseiny MH. Hepatoprotective effects of setarud against carbon tetrachloride-induced liver injury in rats. Indian J Gastroenterol. 2008;27:110–2. [PubMed] [Google Scholar]
- 14.Speelmon EC, Livingston-Rosanoff D, Li SS, Vu Q, Bui J, Geraghty DE, et al. Genetic association of the antiviral restriction factor TRIM5alpha with human immunodeficiency virus type 1 infection. J Virol. 2006;80:2463–71. doi: 10.1128/JVI.80.5.2463-2471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabihollahi R, Sadat SM, Vahabpour R, Aghasadeghi MR, Memarnejadian A, Ghazanfari T, et al. Development of single-cycle replicable human immunodeficiency virus 1 mutants. Acta Virol. 2011;55:15–22. doi: 10.4149/av_2011_01_15. [DOI] [PubMed] [Google Scholar]
- 16.Rezaei A, Zabihollahi R, Salehi M, Moghim S, Tamizifar H, Yazdanpanahi N, et al. Designing a non-virulent HIV-1 strain: Potential implications for vaccine and experimental research. J Res Med Sci. 2007;12:227–34. [Google Scholar]
- 17.Svarovskaia ES, Barr R, Zhang X, Pais GC, Marchand C, Pommier Y, et al. Azido-containing diketo acid derivatives inhibit human immunodeficiency virus type 1 integrase in vivo and influence the frequency of deletions at two-long-terminal-repeat-circle junctions. J Virol. 2004;78:3210–22. doi: 10.1128/JVI.78.7.3210-3222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavrois M, Neidleman J, Yonemoto W, Fenard D, Greene WC. HIV-1 virion fusion assay: Uncoating not required and no effect of Nef on fusion. Virology. 2004;328:36–44. doi: 10.1016/j.virol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Lin PF, Blair W, Wang T, Spicer T, Guo Q, Zhou N, et al. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci U S A. 2003;100:11013–8. doi: 10.1073/pnas.1832214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patwardhan B, Gautam M. Botanical immunodrugs: Scope and opportunities. Drug Discov Today. 2005;10:495–502. doi: 10.1016/S1359-6446(04)03357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedoya LM, Sanchez-Palomino S, Abad MJ, Bermejo P, Alcami J. Anti-HIV activity of medicinal plant extracts. J Ethnopharmacol. 2001;77:113–6. doi: 10.1016/s0378-8741(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez AL, Habtemariam S, Juan-Badaturuge M, Jackson C, Parra F. In vitro anti HSV-1 and HSV-2 activity of tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action. Phytother Res. 2011;25:296–301. doi: 10.1002/ptr.3382. [DOI] [PubMed] [Google Scholar]
- 23.Uncini Manganelli RE, Zaccaro L, Tomei PE. Antiviral activity in vitro of Urtica dioica L., Parietaria diffusa M. et K. and Sambucus nigra L. J Ethnopharmacol. 2005;98:323–7. doi: 10.1016/j.jep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Kirkeskov B, Christensen R, Bugel S, Bliddal H, Danneskiold-Samsoe B, Christensen LP, et al. The effects of rose hip (Rosa canina) on plasma antioxidative activity and C-reactive protein in patients with rheumatoid arthritis and normal controls: A prospective cohort study. Phytomedicine. 2011;18:953–8. doi: 10.1016/j.phymed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Serteser A, Kargioglu M, Gok V, Bagci Y, Ozcan MM, Arslan D. Determination of antioxidant effects of some plant species wild growing in Turkey. Int J Food Sci Nutr. 2008;59:643–51. doi: 10.1080/09637480701602530. [DOI] [PubMed] [Google Scholar]
- 26.Gurbuz I, Ustun O, Yesilada E, Sezik E, Kutsal O. Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. J Ethnopharmacol. 2003;88:93–7. doi: 10.1016/s0378-8741(03)00174-0. [DOI] [PubMed] [Google Scholar]
- 27.Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from tanacetum vulgare L. Int Immunopharmacol. 2007;7:1639–50. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandis H, Karapolat S, Yildirim U, Saritas A, Gezer S, Memisogullari R. Effects of urtica dioica on hepatic ischemia-reperfusion injury in rats. Clinics (Sao Paulo) 2010;65:1357–61. doi: 10.1590/S1807-59322010001200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarhan O, Alacacioglu A, Somali I, Sipahi H, Zencir M, Oztop I, et al. Complementary-alternative medicine among cancer patients in the western region of Turkey. J BUON. 2009;14:265–9. [PubMed] [Google Scholar]
- 30.Namazi N, Esfanjani AT, Heshmati J, Bahrami A. The effect of hydro alcoholic nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: A randomized double-blind control trial. Pak J Biol Sci. 2011;14:775–9. doi: 10.3923/pjbs.2011.775.779. [DOI] [PubMed] [Google Scholar]
- 31.Harput US, Saracoglu I, Ogihara Y. Stimulation of lymphocyte proliferation and inhibition of nitric oxide production by aqueous Urtica dioica extract. Phytother Res. 2005;19:346–8. doi: 10.1002/ptr.1686. [DOI] [PubMed] [Google Scholar]
- 32.Madani N, Schon A, Princiotto AM, Lalonde JM, Courter JR, Soeta T, et al. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure. 2008;16:1689–701. doi: 10.1016/j.str.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


