Abstract
Objectives:
Human gingival fibroblasts (hGFs) play a major role in the maintenance and repair of gingival connective tissue. The mitogen insulin with IGFs etc. synergizes in facilitating wound repair. Although curcumin (CUR) and insulin regulate apoptosis, their impact as a combination on hGF in wound repair remains unknown. Our study consists of: 1) analysis of insulin-mediated mitogenesis on CUR-treated hGF cells, and 2) development of an in vitro model of wound healing.
Materials and Methods:
Apoptotic rate in CUR-treated hGF cells with and without insulin was observed by AnnexinV/PI staining, nuclear morphological analysis, FACS and DNA fragmentation studies. Using hGF confluent cultures, wounds were mechanically created in vitro and incubated with the ligands for 48 h in 0.2% fetal bovine serum DMEM.
Results:
CUR alone showed dose-dependent (1–50 μM) effects on hGF. Insulin (1 μg/ml) supplementation substantially enhanced cell survival through up-regulation of mitogenesis/anti-apoptotic elements.
Conclusions:
The in vitro model for gingival wound healing establishes that insulin significantly enhanced wound filling faster than CUR-treated hGF cells over 48 h. This reinforces the pivotal role of insulin in supporting CUR-mediated wound repair. The findings have significant bearing in metabolic dysfunctions, e.g. diabetes, atherosclerosis, etc., especially under Indian situations.
KEY WORDS: Curcumin, fibroblast, gingival healing, insulin, regeneration
Introduction
Curcumin (CUR) is an indispensable part of Indian civilization and so is insulin interspersed with our lives where little is known about their combined role in wound repair.[1–5] Healthy fibroblasts[6] and extracellular matrix (ECM)[7,8] maintain the structural integrity of soft connective tissues, critical for wound repair. The utility of fibroblasts as “architects and caretakers of connective tissues” regulating autocrine/paracrine/juxtacrine-mediated epithelial cell differentiation is unclear.[9] In periodontitis, loss of spatial juxtaposition between specialized epithelium, gingiva and gingival ligament connective tissues occurs.[6,10–12] CUR is known to enhance wound contraction and shorten the healing time in rodents.[13] It induces apoptosis in cell lines where reactive oxygen species (ROS) is a common denominator.[14–16] hGF displays heterogeneous responses to growth factors[3–6,17] whereas insulin enhances mitogenesis in soft-tissue regeneration.[3,6,18–21]
The present study tests the hypothesis that insulin reverses CUR-mediated apoptosis in hGF thereby facilitating wound repair in vitro. This mirrors the processes under intact in vivo situations where CUR and insulin remain to be an integral part of the Indian scenario.
Materials and Methods
Curcumin, bovine insulin, Dulbecco's Modified Eagle's Medium (DMEM), N-[2-Hydroxyethyl] piperazine-N′-2-ethanesulfonic acid (HEPES), penicillin, streptomycin, gentamicin sulfate, Dulbecco's Phosphate Buffered Saline (PBS), propidium iodide (PI), Annexin V-FITC Apoptosis Detection Kit and ribonuclease-A were purchased from Sigma Chemical Co., St. Louis, MO, USA. Fetal calf serum (FCS) was procured from GIBCO BRL Laboratories, New York, USA. All other chemicals used were of analytical grade.
Culture of Human Gingival Fibroblasts
Human gingival fibroblast (hGF) cell line routinely maintained in our laboratory was used in the present study whose isolation and development has been described by us elsewhere. For the experiments, the cells were propagated in DMEM supplemented with 0.2% FBS in a humidified CO2 incubator at 37°C.[14,15] The study was approved by the Institutional Ethics Committee.
Treatment
Curcumin (1 mM) was dissolved in absolute alcohol and diluted with DMEM to obtain 1 μM, 10 μM, 25 μM and 50 μM concentrations. Bovine insulin (1 mg/ml) was solubilized in HCl and diluted to 1 μg/ml with DMEM.[22]
Annexin V-FITC and PI Staining Analysis
For evaluating apoptosis, 0.2 × 106 hGF cells were plated onto 35 mm culture dishes containing cover slips and treated with ligands as reported previously.[23] Following staining according to manufacturer's protocol, the cells were analyzed on Nikon Eclipse Ti E200 fluorescence microscope and photographed (excitation 490 nM and emission 525 nM).
Nuclear Morphological Studies
The cells were cultured and treated in situ on coverglass as for Annexin/PI analysis, washed with chilled PBS and fixed with chilled 70% ethanol. Thereafter, 40 μg/ml PI in PBS was added for 1 h at room temperature. The coverslips were then mounted in glycerine and photographed with Nikon Eclipse Ti E200 fluorescence microscope (excitation 536 nM and emission 617 nM).
Analysis of Cell Cycle
hGF cells (0.2 × 106) were plated on a 6-welled plate and treated as above. Subsequently, the cells were collected by trypsinization and permeabilized with chilled 70% ethanol for 1 h at 4°C.[23] After washing with chilled PBS, the cells were resuspended in 500 μl of PBS containing PI (40 μg/ml) and RNase (100 μg/ml). Flow cytometry was performed on a Beckton–Dickinson Fluorescence-Activated Cell Sorter (FACS) employing the CellQuest Software.
DNA Fragmentation Analysis
For qualitative DNA Fragmentation Studies, DNA was extracted from hGF cells exposed to 50 μM CUR with and without insulin (1 μg/ml).[24]
Wounding Assay
0.2 × 106 hGF cells in a 6-welled plate in DMEM medium supplemented with penicillin (100 μg/ml), streptomycin (200 μg/ml), gentamicin (50 μg/ml) and 0.2% FBS were cultured at 37°C. Following monolayer formation, one wound/well was created with a sterile 200 μl pipette tip. The monolayer with the wound was washed with growth medium, pre-incubated for 24 h and then exposed to 1, 10, 25 or 50 μM CUR with and without insulin (1 μg/ml). Post-48 h, the wound repair was observed using Nikon Eclipse Ti E200 fluorescence microscope and photographed. Additionally, relative wound closure was calculated using the formula (X0 – Xyy)/(C0 – Cyy), where X0is the scratch width at time 0; Xyy is the width after time yy exposure; C0 is the width of the scratch at time 0; Cyy scratch with after yy time exposure to the control.
Statistical Analysis
The results were expressed as mean ± SE from one of the three similar experiments each performed in triplicate. Student's t-test was used to determine the level of significance.
Results
Annexin/propidium iodide
Induction of early apoptosis by CUR and its reversal by insulin in hGF cells was evaluated by Annexin V-FITC/Propidium Iodide (PI) staining [Figure 1]. In control untreated cells, 1 μM CUR with and without insulin display absence of green fluorescence indicating healthy, viable cells in the absence of apoptosis. Beginning with sparse green fluorescence at 10 μM, fair amount of staining was subsequently observed. At 25 μM CUR, the onset of apoptosis through visualization of red spots in addition to intense green color was noticed. The phenomena intensified with 50 μM CUR. Noticeably with insulin, the green fluorescence declined at both 25 and 50 μM CUR with near complete dissipation of red spots.
Figure 1.
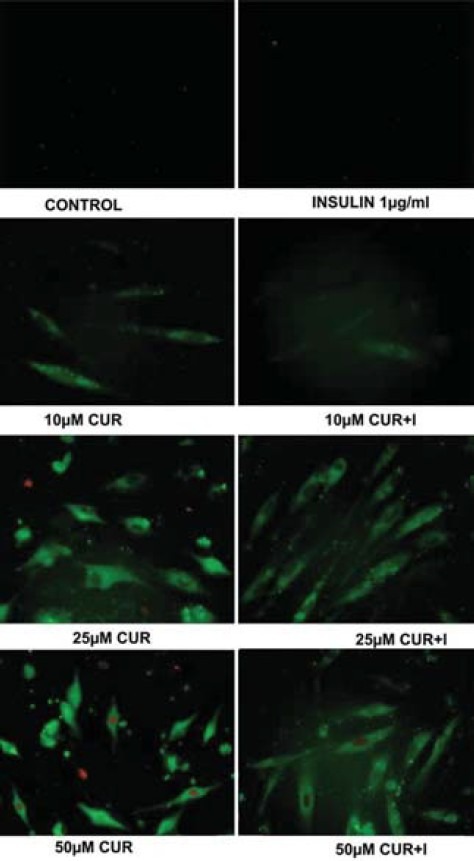
Detection of apoptosis in hGF cells by annexin V-FITC/PI staining of curcumin (CUR) and curcumin + insulin (I) treated cells (Nikon Eclipse Ti E200 Fluorescence Microscope, 400×)
Nuclear Morphological Studies
Propidium iodide staining reveals nuclear morphology of hGF cells exposed to CUR with and without insulin. Control- and insulin-treated hGF cells depict elliptical, normal nuclei with uniform red fluorescence with minimal debris. No discernible change in the nuclear morphology was observed at 1/10 μM CUR in the presence and absence of insulin as compared to control. CUR initiated chromatin condensation at 25 25 μM, illustrated by shrinkage of nucleus with convoluted, deformed, deranged morphology. At 50 μM CUR, the number of deformed nuclei increased significantly. The frequency of disfigured nuclei decreased when the cells were treated with 25/50 μM CUR plus insulin [Figure 2].
Figure 2.
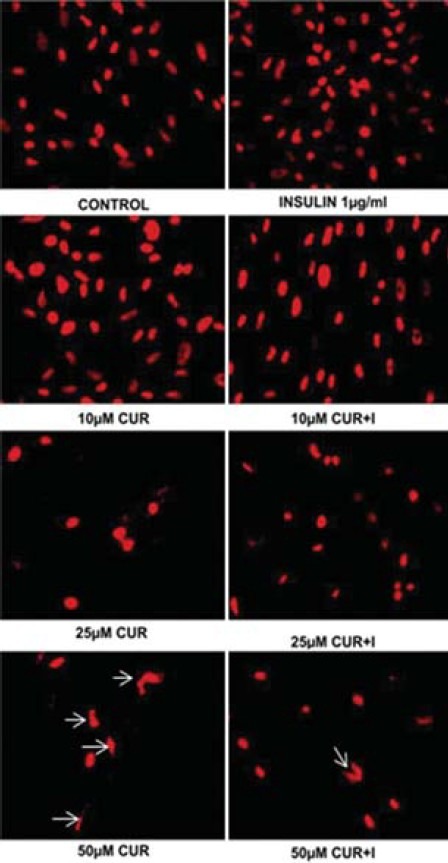
Alterations in nuclear morphology of curcumin (CUR) and curcumin + insulin (I) treated cells by propidium iodide (PI) staining (Nikon Eclipse Ti E200 Fluorescence Microscope, 400×)
Apoptosis
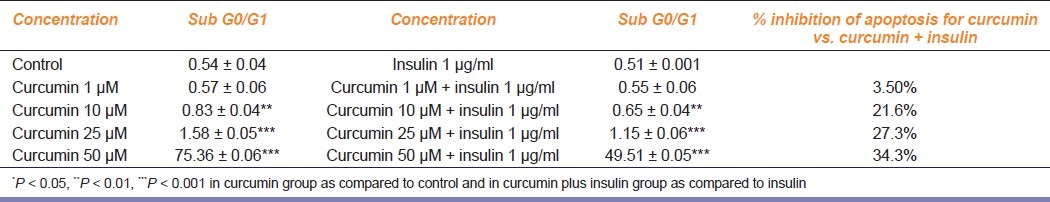
The apoptosis (Sub-G0/G1) induced by CUR with and without insulin is summarized in Table 1. Percent apoptotic cells increased significantly in dose-dependent fashion on intra-group comparison. Insulin addition showed significant decrease in percent apoptotic cells compared to corresponding CUR treatment, except at 1 μM at which the decline was insignificant [Table 1].
Table 1.
Dose-dependent inter-group (curcumin versus curcumin plus insulin) comparison of percent apoptotic cells (SubG0/G1) (data shown are the mean ± S.E. of one of the three similar experiments each performed in triplicate)
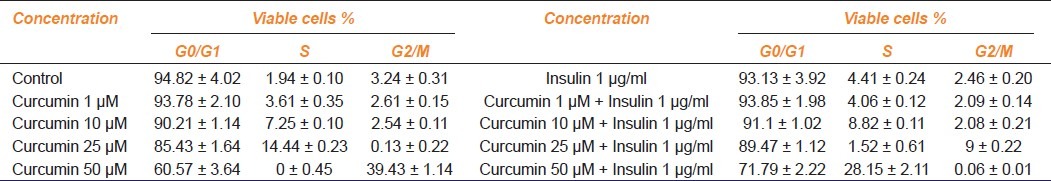
Cell Cycle Analysis
Flow cytometry was performed to reveal the stages of cell cycle [Table 2]. The cells in the control sample resolved classically into maximal G0/G1 or resting phase, minimal S or synthetic phase and residual G2/M or mitotic phase. Unaltered G0/G1, G2/M phases and elevated S phase were observed with 1/10 μM CUR. At 25 μM CUR, the S phase enhanced further with concomitant reduction in G0/G1 and G2/M phase. Noticeably with 50 μM CUR, S phase completely diminished arresting the cells in G2/M phase. The significant S phase increment in presence of insulin over control is suggestive of the mitogenic influence of the hormone. Subsequently, the same in presence of 1 μM CUR plateaus off to increase significantly in the next group i.e. 10 μM CUR. With 25/50 μM CUR plus insulin, S and G2/M phase blocks were undone to register concomitant rise in G2/M and S phases, respectively.
Table 2.
Dose-dependent distribution of cells in G0/G1, S, G2/M phases in curcumin and curcumin plus insulin treatments (data shown are the mean ± S.E. of one of the three similar experiments each performed in triplicate)
DNA Fragmentation
1 μM, 10 μM and 25 μM CUR with and without insulin displayed no DNA fragmentation (data not shown). However, the laddering observed in presence of 50 μM CUR was restored upon insulin supplementation [Figure 3].
Figure 3.
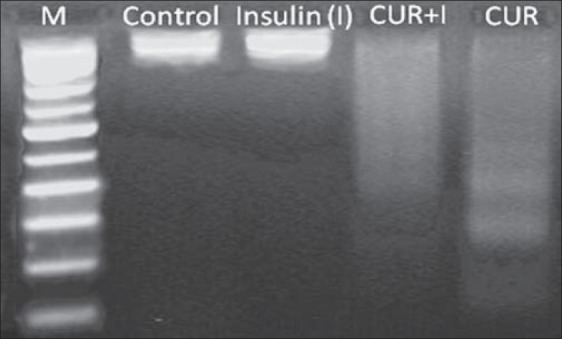
Qualitative analysis of 50 μM curcumin (CUR) with and without insulin (I), 1 μg/ml induced DNA fragmentation in hGF cells
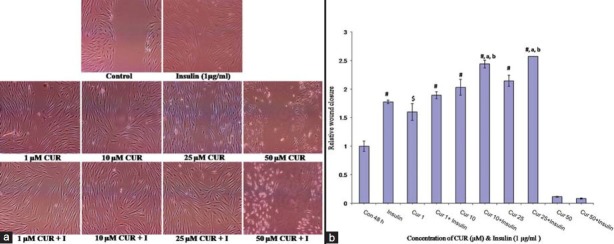
Wounding Assay
Role of different concentrations of CUR with and without insulin on proliferation/migration of fibroblasts, essential for rapid and effective repair of damaged or diseased tissue, was studied by wounding assay. In control cells, the wound gap devoid of fibroblasts was noticed post-48 h indicating poor mitotic index. Unlike control, sizable reduction in the gap width was observed with 1 μM CUR. At 10 μM CUR, the gap seemingly obliterated with fairly healthy looking cells. Subsequently, narrowing and compaction of wound at 25 μM CUR owing to visible, feeble cellular desiccation and shrinkage of cells was noticed. At 50 μM CUR, the apoptotic potential of the ligand could be visualized with occasional rounded and the majority fiber-like, shrunken cells in the wound area. From 1 to 25 μM CUR with insulin, improved closure of wound area with aggregated fibroblasts was noticed. Insulin not only failed to rescue the damage to the cells owing to 50 μM CUR but also caused marked rounding of cells with minimal, atypical, smaller fibroblasts [Figure 4a and b].
Figure 4.
Effect of cucurmin (CUR) and cucurmin + insulin (I), 1 μg/ml on wound closure in hGF monolayer (Nikon Eclipse Ti E200 Fluorescence Microscope, 400X). (a) Post-48 hrs, the wound repair was observed morphologically using Nikon Eclipse Ti E200 fluorescence microscope and photographed. (b) Relative wound closure calculated using the formula (“X0 – Xyy)/(C0 – Cyy), where X0 is the scratch width at time 0; Xyy is the width after time yy exposure; C0 is the width of the scratch at time 0; Cyy scratch with after yy time exposure to the control.
Discussion
In healthy periodontium, gingival fibroblasts are one of the most copious stromal cell types[6] regulating matrix synthesis and remodeling, injury-site-directed migration and attachment to ECM.[25] This process involves panoply of diverse elements such as age, nutrition, hormonal status etc., promoted by several growth factors.
Studies from our laboratory indicate that synthetic molecules like Centchroman and LP 533401 in addition to nutraceuticals e.g., CUR and resveratrol have a therapeutic potential in various diseases like breast cancer and osteoporosis.[24,26,27] It has also been demonstrated that CUR (1%) as an adjunct sub-gingival irrigant results in significant reduction in redness (96%) and bleeding on probing (100%) vis-à-vis chlorhexidine and saline group during periodontitis.[20] Moreover, CUR has also been proven to possess excellent wound healing capacity.[1,2,28]
Insulin and insulin-like growth factors (IGF-I and II) activate multiple intracellular signaling pathways that are fundamental to cell growth, differentiation and survival.[29] Additionally, it has been shown that low concentrations of insulin promote cell survival and protect Chinese Hamster cells from apoptosis induced by serum starvation.[30] Therefore, we tested 1–50 μM CUR with and without insulin vis-à-vis hGF cells to mimic the situation in oral cavity, in vivo.
We investigated the role of CUR-mediated apoptosis in hGF cells and its reversal through insulin on gross cellular and nuclear morphology, annexin V/PI, flow cytometry and DNA laddering. With 1 μM CUR, no noticeable apoptotic changes were evident in hGF cells. As the apoptosis progresses, PS externalization [Figure 1], distinct changes in the nuclear morphology [Figure 2] and slight increase in Sub-G0/G1 phase was observed with 10 μM CUR further escalating at 25–50 μM [Table 1]. Supplementation of insulin promotes cell survival in 1/10 μM CUR [Table 2] and reverses the apoptotic changes induced by 25/50 μM CUR [Table 1]. This may be achieved through up-regulation of the signaling pathway(s) and associated anti-apoptotic events or both.
A novel, in vitro hGF cell based wounding assay was optimized, calibrated and validated to observe qualitative, directional cell migration and proliferation employing hGF, CUR and insulin. This affords synergism between 10–25 μM CUR and insulin to fill a wound site in hGF monolayer [Figure 4]. Similar experiments evaluating CUR, aloe vera, taurine and vitamin C have been proposed.[31] Hence, this may serve as an example for understanding in vivo gingival wound healing and its regulation with various ligands. It can also be reinforced that CUR along with insulin may enhance the wound closure with shortened healing time, beneficial for reduced risk of infection. Further, its clinical implication in management of periodontal diseases needs investigation under in vivo environment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Scharstuhl A, Mutsaers HA, Pennings SW, Szarek WA, Russel FG, Wagener FA. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: Implications for scar formation. J Cell Mol Med. 2009;13:712–25. doi: 10.1111/j.1582-4934.2008.00339.x. Epub 2008 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. Epub 2006 Jun 13. [DOI] [PubMed] [Google Scholar]
- 3.Sudhakar U, Ramakrishnan, Anand PB. Emerging role of the insulin like growth factors in pulp healing, reparative dentinogenesis and periodontal regeneration. J Conserv Dent. 2006;9:78–80. [Google Scholar]
- 4.Werner H, Katz J. The emerging role of the insulin-like growth factors in oral biology. J Dent Res. 2004;83:832–6. doi: 10.1177/154405910408301102. [DOI] [PubMed] [Google Scholar]
- 5.Han X, Amar S. IGF-1 signaling enhances cell survival in periodontal ligament fibroblasts vs. gingival fibroblasts. J Dent Res. 2003;82:454–9. doi: 10.1177/154405910308200610. [DOI] [PubMed] [Google Scholar]
- 6.Lekic PC, Pender N, McCulloch CA. Is fibroblast heterogeneity relevant to the health, diseases, and treatments of periodontal tissues? Crit Rev Oral Biol Med. 1997;8:253–68. doi: 10.1177/10454411970080030201. [DOI] [PubMed] [Google Scholar]
- 7.Thangjam GS, Agarwal P, Balapure AK, Rao SG, Kondaiah P. Regulation of extracellular matrix genes by arecoline in primary gingival fibroblasts requires epithelial factors. J Periodontal Res. 2009;44:736–43. doi: 10.1111/j.1600-0765.2008.01185.x. Epub 2009 Mar 30. [DOI] [PubMed] [Google Scholar]
- 8.Thangjam GS, Agarwal P, Khan I, Verma UP, Balapure AK, Rao SG, et al. Transglutaminase-2 regulation by arecoline in gingival fibroblasts. J Dent Res. 2009;88:170–5. doi: 10.1177/0022034508329633. [DOI] [PubMed] [Google Scholar]
- 9.Ohshima M, Yamaguchi Y, Matsumoto N, Micke P, Takenouchi Y, Nishida T, et al. TGF-β signaling in gingival fibroblast-epithelial interaction. J Dent Res. 2010;89:1315–21. doi: 10.1177/0022034510378423. Epub 2010 Aug 25. [DOI] [PubMed] [Google Scholar]
- 10.Flemmig TF. Periodontitis. Ann Periodontal. 1999;4:32–8. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 11.Socransky SS, Haffajee AD, Goodson JM, Lindhe J. New concepts of destructive periodontal disease. J Clin Periodontol. 1984;11:21–32. doi: 10.1111/j.1600-051x.1984.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 12.Page RC, Altman LC, Ebersole JL, Vandesteen GE, Dahlberg WH, Williams BL, et al. Rapidly progressive periodontitis. A distinct clinical condition. J Periodontol. 1983;54:197–209. doi: 10.1902/jop.1983.54.4.197. [DOI] [PubMed] [Google Scholar]
- 13.Bhagavathula N, Warner RL, DaSilva M, McClintock SD, Barron A, Aslam MN, et al. A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-impaired hairless rat skin. Wound Repair Regen. 2009;17:360–6. doi: 10.1111/j.1524-475X.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. Epub 2009 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atsumi T, Fujisawa S, Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin-and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005;11:236–42. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi D, Singh N, Ahmad IZ, Sharma R, Balapure AK. Antiproliferative Effects of Curcumin Plus Centchroman In MCF7 and MDA MB-231 Cells. Int J Pharm Pharm Sci. 2011;3(2):212–6. [Google Scholar]
- 17.Lackler KP, Cochran DL, Hoang AM, Takacs V, Oates TW. Development of an in vitro wound healing model for periodontal cells. J Periodontol. 2000;71:226–37. doi: 10.1902/jop.2000.71.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Shanley LJ, McCaig CD, Forrester JV, Zhao M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Investig Ophthalmol Vis Sci. 2004;45:1088–94. doi: 10.1167/iovs.03-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. Epub 2010 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynne D. Diabetes disease management in managed care organizations. Dis Manag. 2004;7:47–60. doi: 10.1089/109350704322918998. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 22.Shailubhai K, Saxena ES, Balapure AK, Vijay IK. Developmental regulation of glucosidase I, an enzyme involved in the processing of asparagine-linked glycoproteins in rat mammary gland. J Biol Chem. 1990;265:9701–6. [PubMed] [Google Scholar]
- 23.Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK. Caspase mediated enhanced apoptotic action of cyclophosphamide-and resveratrol-treated MCF-7 cells. J Pharmacol Sci. 2009;109:473–85. doi: 10.1254/jphs.08173fp. [DOI] [PubMed] [Google Scholar]
- 24.Nigam M, Ranjan V, Srivastava S, Sharma R, Balapure AK. Centchroman induces G0/G1 arrest and caspase-dependent apoptosis involving mitochondrial membrane depolarization in MCF-7 and MDA MB-231 human breast cancer cells. Life Sci. 2008;82:577–90. doi: 10.1016/j.lfs.2007.11.028. Epub 2007 Dec 15. [DOI] [PubMed] [Google Scholar]
- 25.Bartold PM, Walsh LJ, Narayanan AS. Molecular and cell biology of the gingiva. Periodontol. 2000;24:28–55. doi: 10.1034/j.1600-0757.2000.2240103.x. [DOI] [PubMed] [Google Scholar]
- 26.Nigam M, Singh N, Ranjan V, Zaidi D, Sharma R, Nigam D, et al. Centchroman mediated apoptosis involves cross-talk between extrinsic/intrinsic pathways and oxidative regulation. Life Sci. 2010;87:750–8. doi: 10.1016/j.lfs.2010.10.015. Epub 2010 Oct 27. [DOI] [PubMed] [Google Scholar]
- 27.Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–12. doi: 10.1038/nm.2098. Epub 2010 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suhag Aparna DJ, Dhan Prakash. Role of curcumin as a subgingival irrigant: A pilot study. PERIO-Periodontal Practice Today. 2007;4:115–21. [Google Scholar]
- 29.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–76. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Kwon W, Park D, Baskar PV, Kole S, Bernier M. Antiapoptotic signaling by the insulin receptor in Chinese hamster ovary cells. Biochemistry. 1998;37:15747–57. doi: 10.1021/bi9805947. [DOI] [PubMed] [Google Scholar]
- 31.Fray TR, Watson AL, Croft JM, Baker CD, Bailey J, Sirel N, et al. A combination of aloe vera, curcumin, vitamin C, and taurine increases canine fibroblast migration and decreases tritiated water diffusion across canine keratinocytes in vitro. J Nutr. 2004;134:2117S–9S. doi: 10.1093/jn/134.8.2117S. [DOI] [PubMed] [Google Scholar]