Abstract
Aim:
Shorea robusta (Sal), an important traditional Indian medicinal plant used in various ailments and rituals and the indigenous use of the resin of this plant as a medicament for treatment of various inflammatory conditions is well documented in literature. In the present study, ethanolic extract of S. robusta resin (SRE) was evaluated for its analgesic activity by making use of different central and peripheral pain models.
Materials and Methods:
The analgesic activity of SRE was assessed by employing different pain models such as, i) hot plate and tail flick tests for central analgesia, ii) acetic acid- induced writhing (peripheral analgesic model), iii) formalin-induced hind paw licking (both central and peripheral model), iv) carrageenan-induced hyperalgesia (peripheral analgesic model) and v) post-surgical pain (peripheral analgesic model).
Results:
The extract produced significant central and peripheral analgesic effects, as is evident from increase in reaction time in hot plate and tail flick tests, inhibition in writhing counts in acetic acid-induced writhing test, inhibition of licking time in formalin-induced hind paw licking, increased pain threshold in paw withdrawal latency in carrageenan-induced hyperalgesia and increased paw withdrawal threshold in post-surgical pain.
Conclusion:
The results of the present study demonstrate marked antinociceptive effects of SRE.
KEY WORDS: Carrageenan, hot plate, post-surgical pain, resin, Shorea robusta, tail flick
Introduction
Shorea robusta (Sal) belongs to the family Dipterocarpaceae (two-winged fruit), which is most commonly found in Indonesia, but can also be seen in Malaysia, the Philippines and certain parts of Northern India. The various parts of the plant are traditionally used in India for the treatment of diverse ailments. The leaves are used to treat wounds, ulcers, itching, leprosy, gonorrhea, cough, earache and headache. The oleoresin exuded from the cut bark has astringent and detergent properties. In Unani medicine, the resin is used for treating menorrhagia, enlargement of the spleen and for relieving eye irritations. In Ayurveda, the leaves are used as anthelmintic and alexiteric. The powdered stem, bark or bark paste is applied to stop bleeding and promote healing of cuts among the tribal inhabitants of southern Bihar and the Kondhs of south-western Odisha, India.[1] The resin obtained from the plant is considered as an astringent and a detergent and is used with honey or sugar in dysentery and bleeding piles and also for fumigating the rooms of ill people. It is also given in gonorrhea and for weak digestion. Its bark decoction is used as drops for ear problems and the fruits for diarrhea.[2] S. robusta leaf extract was found to possess significant analgesic activity[3] and its resin along with some other constituents has also shown potential in wound healing.[4]
In view of the importance of medicinal plants as a potential source of cheaper, safer and effective remedies for treating diseases in animals, and the traditional use of S. robusta resin (SRE) in various disorders, the present study was undertaken with special emphasis on the study of the analgesic effects of its alcoholic extract.
Materials and Methods
Plant Material
Pure S. robusta resin was obtained from Odisha, India where the plant grows naturally.
Preparation of Extract
Total 250 g of resin powder was extracted with 1 L of 70% ethanol in a soxhlet apparatus at 60-75°C. The extract was concentrated by evaporation. The yield was about 16.80%. The solidSRE was dissolved by using 1 % v/v Tween-80 as a vehicle for oral administration.
Phytochemical study for qualitative analysis of active principles
Qualitative analysis of SRE for the presence of various medicinally important active phytochemicals such as alkaloids, anthraquinones, flavonoids, saponins, tannins, sterols, reducing sugars, glycosides, resins and triterpenes was carried out as per the methods described earlier.[5,6]
Experimental Animal
Healthy Wistar albino rats (150-250 g)/Swiss mice (18- 20 g) of approximately the same age were used for the study. They were grouped into 5 groups of 6 animals each into a clean polypropylene cage and were maintained on a balanced ration obtained from the Feed Technology Unit of the Institute. Fresh drinking water was offered to the animals daily ad libitum. The experiments were carried out in accordance with the guidelines of Animal Ethics Committee, IVRI, Izatnagar.
Group I: Control group treated with 1% Tween-80 p.o.
Group II: Test group treated with SRE 30 mg/kg p.o.
Group III: Test group treated with SRE 100 mg/kg p.o.
Group IV: Test group treated with SRE 300 mg/kg p.o.
Group V: Test group treated with etoricoxib 10 mg/kg p.o. in peripheral analgesic model, or morphine 5 mg/kg i.p. in central analgesic model.
Experimental Procedure
Models for assessing analgesic activity
Hot plate test
In this experiment, the central analgesic activity of SRE was assessed in a male albino mice, as per the method described by Eddy and Leimbach.[7] Overnight fasted male albino mice were placed individually on a thermostatically controlled heated metal plate (Ugo Basile, Italy) within a restraining perspex cylinder and the reaction time of each mouse was recorded. The temperature of the hot plate was maintained at 55 ± 0.5°C. The reaction time was considered as the time elapsed between placing of the mouse on the hot plate and appearance of signs of acute discomfort, characterized by flicking or licking of the hind paw, forepaw or jumping in an attempt to escape from the pain. The mice showing initial reaction time of 10 sec or less were selected for this study and were divided into 5 groups (6 in each group). After regrouping, animals in Groups II to IV were orally administered with the test substance, SRE at the dose rate of 30, 100 and 300 mg/kg b.wt., respectively. Group V received the standard drug morphine at the rate of 5 mg/kg i.p. and the control group (Group I) received a comparable volume of vehicle. Thereafter, the reaction time of each mouse was recorded at 1, 3 and 5 h after the drug administration with a cut-off time of 30 sec. The increase in reaction time in drug-treated groups was compared with that of the control group.
Tail flick method
The central analgesic activity of SRE was studied in tail withdrawal assay, as described by D’Amour and Smith.[8] Radiant heat was applied to the base of the tail using a tail flick unit (Ugo-Basile, Italy) and the latency time for removal of the tail from the stimulus was recorded. The intensity of the heat stimulus was set to elicit a tail flick within 10-12 sec. A cut-off time of 20 sec was used to prevent tissue damage. Mice weighing 18- 25 g were randomly divided into 5 groups of 6 animals each. After recording the baseline latency (0 h), Groups II to IV were administered SRE at the rate of 30, 100 and 300 mg/kg. Group I (control group) received comparable volume of vehicle. Group V received the standard drug morphine hydrochloride (5 mg/kg b.wt. i.p.). The tail withdrawal latencies were measured at 0, 1, 3 and 5 h after the drug administration.
Formalin-induced Hind Paw Licking
The formalin-induced hind paw licking assay was performed as described by Corea and Calixto.[9] Adult albino mice were randomly divided into 5 groups of 6 animals each. Groups II to IV were orally administered SRE in doses of 30, 100 and 300 mg/kg, respectively. Group I served as a control receiving a proportionate amount of vehicle, while Group V received the reference drug etoricoxib (10 mg/kg, orally).
Briefly, 1 h after the drug administration, animals were injected with formalin (2.5%, 20 μL) sub-plantarly in 1 hind paw and the duration of paw licking as index of nociception was monitored at 0-5 min (early phase) and 15-30 min (late phase).
Acetic acid-induced Writhing in Mice
This experiment was carried out in female mice (18-25 g) using 6 animals in each group, according to the method described by Witkin et al.[10] They were divided into 5 groups, where Groups II to IV received SRE at the dose rate of 30, 100 and 300 mg/kg b.wt., respectively (orally) while Group V was administered with the standard drug etoricoxib (10 mg/kg, orally) 1 h prior to the injection of acetic acid. The control group received the vehicle. The effect of the extract and etoricoxib on acetic acid-induced writhing was observed in comparison to control. Writhing in animals was produced by i.p. administration of 300 mg/kg acetic acid (3%) solution. Each mouse was then put into a big glass cylinder and the total number of writhing episodes for a period of 20 min after the injection of acetic acid was counted. The percent inhibition of writhing count of the treated group was calculated from the mean writhing count of the control group.
Carrageenan-induced Hyperalgesia
The peripheral analgesic activity of SRE was studied as per the method described by Chan et al.[11] Adult male Wistar rats weighing 180-200 g were divided into 5 groups of 6 animals each. A 150 μL of 3% solution of carrageenan was administered through an intraplantar injection. Vehicle, SRE or etoricoxib (10 mg/kg dose) was administered orally 2 h following the carrageenan injection. The paw withdrawal latency response of the rat to increase pressure on the carrageenan-injected paw using analgesiometer (Ugo-Basile, Italy) was recorded at 0, 1, 3 and 5 h time point and compared with the vehicle-treated group.
Post-surgical Pain
Effect of SRE on post-surgical pain was assessed by the method described by Brennan et al.[12] Fresh female Wistar rats weighing 180-200 g were divided into 6 groups of 6 animals each. Incision pain was induced in the rats as described by Brennan et al.[12] with minor modifications. Briefly, rats were anaesthetized with ether and the plantar surface of the left hand paw prepared in a sterile manner. A 1 cm longitudinal incision was made with a number 10 scalpel, through skin and fascia of the plantar aspect of the paw, starting 0.5 cm from the proximal edge of the heel and extending toward the toes. The plantaris muscle was elevated and incised longitudinally. Following hemostasis with gentle pressure, the skin was opposed with 2 single interrupted sutures using 5-0 nylon. The wound site was covered with povidone-iodine and antibiotic powder.
Three tests were used to assess the pain behavior:
Radiant heat stimulation
The latency to radiant heat in seconds was measured by radiant heat apparatus (Ugo-Basile, Italy), immediately prior to the surgery, 24 h post-surgery, 1, 3, and 5th h after drug administration. The paw was placed on the heat radiator and the withdrawal of paw was measured in seconds. The change in paw withdrawal latency (PWL) of the test group was compared with that of the untreated control group. Results are expressed as mean time (in s (mean) ± SE).
Mechanical stimulation
The pressure was recorded as pain threshold by Randall-Selitto assay,[13] using Randall-Selitto algesiometer (Ugo-Basile, Varese, Italy) immediately prior to the surgery, 24 h post-surgery (pre-drug), 1, 3 and 5th h after drug administration. The change in pain threshold in test group was compared with that of the untreated control groups. Results are expressed as pressure in g ± SE. Recordings for both hind paws were done.
Cold stimulation
Ice cool (4 ± 1°C) water was taken in a beaker. The right paw of the surgically incised rats (test group) and the untreated rats (control group) was submerged gently in the water and the withdrawal time (in seconds) was measured just prior to the surgery, 24 h post-surgery, 1, 3 and 5th h after drug administration. The change in withdrawal latency of the treated groups and the untreated groups was compared. Results are expressed as mean time in s ± SE.
The mechanical hyperalgesia was assessed by measuring the paw withdrawal threshold (both ipsilateral and contralateral) on exposure to mechanical stimulation (Randall and Selitto). The cold allodynia in both ipsilateral and contralateral paw was determined by measuring the paw withdrawal latency (PWL) when dipped in cold water maintained at 4°C. The tail withdrawal latency was measured using tail flick assay.
Statistical analysis
All the data were analyzed using Graphpad Instat Software using one-way ANOVA with Bonferroni's multiple comparison tests and two-way ANOVA with Bonferroni's multiple comparison test.
Results
Phytochemical analysis
The phytochemical analysis of the extract showed the presence of triterpenoids, sterols and resin.
Analgesic activity
Central analgesic activity
Hot Plate method
The reaction time following the oral administration of different doses of SRE is presented in Figure 1. SRE (300 mg/ kg) produced a significant (P < 0.001) increase in the mean reaction time throughout the observation period, i.e., at 1, 3 and 5 h, compared to the control and 30 mg/kg dose of SRE. The reference drug morphine (5 mg/kg, i.p.) also caused significant (P < 0.001) increase in the mean reaction time throughout the observation period, as compared to the control group.
Figure 1.
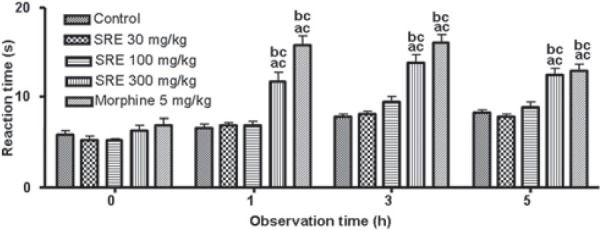
Effect of SRE on hot plate test in mice (mean ± SE, n= 6); acP < 0.001 (compared with control); bcP < 0.001 (compared with SRE 30)
Tail flick latency
The results of orally administered SRE on tail flick latencies in mice are summarized in Figure 2. SRE (300 mg/kg), at 3 and 5 h after its administration, significantly (P < 0.001 and P < 0.05, respectively) increased the tail flick latency, when compared with the control group. SRE did not show any significant and dose dependent increase in the reaction time when compared with SRE 30 mg/kg. The reference drug morphine (5 mg/kg i.p.) significantly (P < 0.001) increased the tail flick latency at 1, 3 and 5 h, as compared to the control group.
Figure 2.
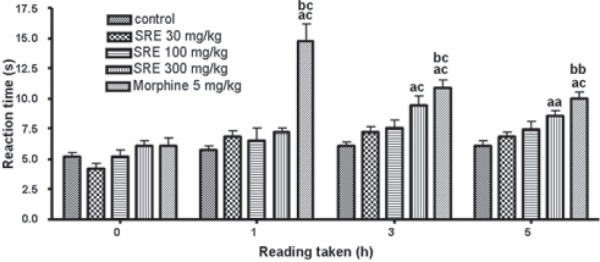
Effect of SRE on radiant heat latency in mice (mean ± SE, n= 6); aaP < 0.05 and acP < 0.001 (compared with control); bbP < 0.01 and bcP < 0.001 (compared with SRE 30)
Peripheral analgesic activity
Formalin-induced hind paw licking
The results of orally administered SRE on the formalin-induced hind paw licking are presented in Figure 3. In this model, SRE at 100 and 300 mg/kg, showed a significant (P < 0.001) reduction in the licking time suggesting analgesic activity in the early phase (0-5 min). SRE (300 mg/kg) also showed a significant (P < 0.001) decrease in the duration of paw licking when compared with SRE 30 mg/kg Reference compound, etoricoxib at 10 mg/kg also significantly (P < 0.001) reduced the paw licking duration in the early phase (0-5 min).
Figure 3.
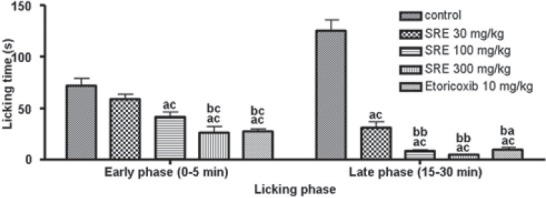
Effect of SRE on formalin-induced hind paw licking in mice (mean ± SE, n= 6); acP < 0.001 (compared with control); baP < 0.01 and bcP < 0.001 (compared with SRE 30)
In the inflammatory phase (15-30 min), SRE at 30, 100 and 300 mg/kg significantly (P < 0.001) reduced the paw licking time, as compared to the control group. SRE (100 and 300 mg/kg) showed a significant (P < 0.01) decrease in the duration of paw licking when compared with SRE 30 mg/kg. The reference drug etoricoxib also reduced the paw licking duration significantly (P < 0.001).
Acetic acid-induced writhing
The data on the effect of SRE on the number of writhing movements induced by i.p. acetic acid in mice are presented in Figure 4. SRE at 30, 100 and 300 mg/kg, significantly (P < 0.001) inhibited the writhing counts. SRE (100 and 300 mg/kg) showed a significant (P < 0.05) decrease in the inhibition of the writhing movements when compared with SRE 30 mg/kg. The reference drug etoricoxib (10 mg/kg) also significantly (P < 0.001) inhibited the writhing counts, compared to the control.
Figure 4.
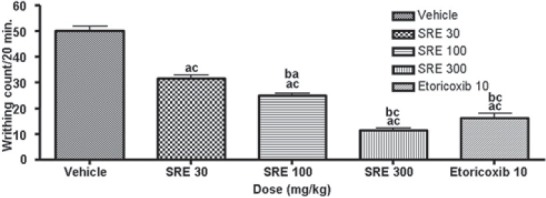
Effect of SRE on acetic acid-induced writhing in mice (mean ± SE, n= 6); acP < 0.001 (compared with control); baP < 0.05 and bcP < 0.001 (compared with SRE 30)
Carrageenan-induced hind paw mechanical hyperalgesia
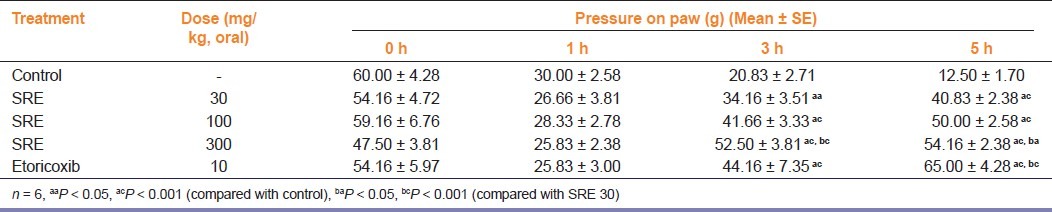
The results of orally administered SRE on carrageenan-induced hyperalgesia, as determined by the mechanical pressure, are summarized in Table 1. SRE in all the doses employed in this study significantly (P < 0.001) increased the paw withdrawal latency at 3 and 5 h of drug administration. SRE (100 and 300 mg/kg) showed significant increase in the paw withdrawal latency as compared to SRE 30 mg/kg. Reference drug etoricoxib (10 mg/kg) also significantly increased paw withdrawal latency as compared to control group at similar observation period.
Table 1.
Effect of SRE on carrageenan-induced hyperalgesia in ratsn
Post-surgical pain
Effects on post-surgical pain by radiant heat stimulation in rats
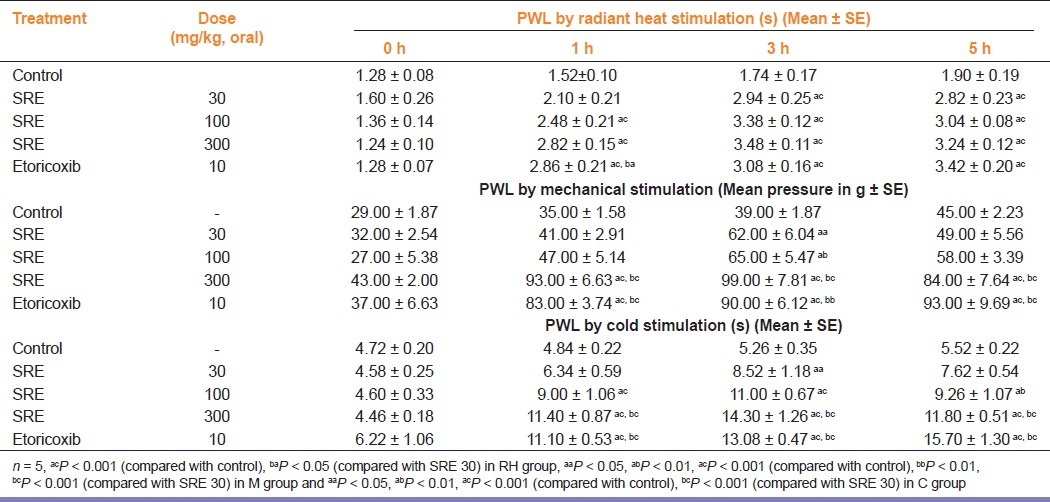
The results of orally administered SRE on paw withdrawal latencies on radiant heat stimulation are summarized in Table 2. SRE 100 and 300 mg/kg increased the paw withdrawal latency significantly (P < 0.001) when compared with the control group at 1, 3 and 5 h of observation, whereas SRE 30 mg/kg significantly (P < 0.001) increased the paw withdrawal latency at 3 and 5 h interval only. The reference drug etoricoxib (10 mg/ kg) significantly (P < 0.001) increased the tail flick latency at 1, 3 and 5 h of observation.
Table 2.
Effect of SRE on post-surgical pain in ratsn by radiant heat (RH group), mechanical (M group) and cold (C group) stimulation
Effect on post-surgical pain by mechanical stimulation in rats
The results of orally administered SRE on mechanical stimulation in post-surgical pain are summarized in Table 2. SRE in 30 and 100 mg/kg doses significantly (P < 0.05 and P < 0.01, respectively) increased the paw withdrawal latency at 3 h interval, whereas at 300 mg/kg dose, it significantly (P < 0.001) increased the paw withdrawal latency at 1, 3 and 5 h interval, as compared with the control group. SRE (300 mg/kg) showed a significant (P < 0.01) increase in the paw withdrawal latency when compared with SRE 30 mg/kg. Reference drug etoricoxib (10 mg/kg) also increased the paw withdrawal latency significantly (P < 0.001) at 1, 3 and 5 h of observation.
Effect of post-surgical pain by cold stimulation
The results of orally administered SRE on paw withdrawal latencies to cold stimulation are summarized in Table 2. SRE (30 mg/kg) increased the paw withdrawal time significantly (P < 0.05) only at 3 h of its administration, while at 100 and 300 mg/ kg doses, it increased the paw withdrawal time significantly at 1, and 3 and 5 h of observation. SRE (300 mg/kg) showed a significant (P < 0.001) increase in the paw withdrawal time when compared with SRE 30 mg/kg. Similarly, reference drug etoricoxib (10 mg/kg) increased the paw withdrawal time significantly at 1, 3 and 5 h of observation.
Discussion
The present study was carried out to evaluate the possibility of SRE in alleviating pain. The antinociceptive activity of the SRE was investigated using experimental models that employed chemical- or thermal-induced nociception, which at the same time were used to determine the effectiveness of the extract on inflammatory-mediated nociception (abdominal writhing test), non-inflammatory-mediated nociception (hot plate test and tail flick test) or both types of nociception (the formalin-induced paw licking test) and provides some evidence on the mechanism implicated in this effect.
SRE was evaluated for both central (opioid) and peripheral nonsteroidal anti-inflammatory drug (NSAID) analgesic properties. In the present study, some common nociceptive methods were employed to study the effect of the extract.[14] To assess the central mechanism of the compound in producing analgesia, hot plate and tail-flick tests in mice were employed. These methods differ from each other in their tendency to respond to nociceptive stimuli conducted through neuronal pathways. Tail flick mediates spinal reflex to a painful stimuli, whereas hot plate test involves higher brain functions and is considered to be a supraspinally organized response.[15] To assess the peripheral analgesic activity, acetic acid-induced writhing and formalin-induced hind paw licking tests were employed.
Opiates (morphine and its derivatives) exert their analgesic activity by interacting with various receptors both at spinal and supraspinal sites. Morphine produces spinal analgesia by inhibiting the release of substance-P from the primary pain afferents in the spinal cord, and also by antagonizing the action of substance-P post-synaptically on the dorsal-horn interneurons and on the output neurons of the spinothalamic tract that convey nociceptive information to the higher centers of the brain.[16] Opiates at supraspinal sites like medulla, mid-brain, third ventricle, limbic and cortical areas, etc. induces analgesia as they alter the processing and interpretation of the pain impulses as well as the inhibitory impulses to descending pathways to the spinal cord. It is now evident that μ, κ3, d and d2 are the opioid receptor sub-types primarily responsible for the supraspinally mediated analgesic action of opiates and spinal analgesia appears to be mediated through μ2, d2 and κ1 receptors.[17] Morphine in a dose of 5 mg/kg b.wt. showed a significant analgesic effect in both hot plate and tail flick nociceptive tests. The analgesic action presented by SRE involves supraspinal as well as spinal components as demonstrated by the utilization of the hot and tail flick tests, respectively. The results suggest that SRE has a central analgesic effect, as evidenced by the prolonged delay in response when mice were subjected to a nociceptive stimulus in the tail flick test and also by the increase in the reaction time of the mice in the hot plate test.
The peripheral analgesic effect was tested by acetic acid-induced writing in mice. This is a visceral pain model used for screening the effectiveness of the analgesic agents. It utilizes noxious chemical irritation of peritoneum and measures the pain response. The acetic acid-induced writhing is a standard test to analyze the pain sensitivity to opiates as well as to non-opiate analgesics.[18] The associated nociceptive response is believed to involve the release of endogenous substances like, bradykinin and prostanoids among others that stimulate the nociceptive endings.[19] The peripheral analgesic effect tested by this model produced a significant decrease in writhing counts at 30, 100 and 300 mg/kg b.wt. Reference drug etoricoxib also offered relief from visceral pain in this model. Thus, the results of this writhing test alone did not ascertain whether the antinociceptive effects are central or peripheral.
The formalin-induced paw licking test was carried out to further strengthen the evidence of the antinociceptive activity of the extract seen in both the abdominal writhing and the hot plate and tail flick tests. The formalin test is considered as a valid and reliable model of persistent nociception[20] and involves 2 distinct phases, a neurogenic pain that corresponds to the early phase, followed by an inflammatory pain that is accompanied by the release of inflammatory mediators designated as the late phase.[21] The first phase of pain (lasting the first 5 min) corresponding to the acute neurogenic pain, is attributed to direct activation of nociceptors and primary afferent fibers by formalin causing the release of bradykinin and tachykinins[11,22] and the activation of the transient receptor potential vanilloid 1 (TRPV1) channel.[23] This phase is inhibited by opioid analgesics.[23] The second phase (lasting from 15 to 30 min after injection of formalin) is due to an inflammatory reaction caused by tissue injury leading to the release of histamine, serotonin, prostaglandin and excitatory amino acids.[24] This late phase is inhibited by NSAIDs and opioid analgesics.[25] SRE at 100 and 300 mg/kg significantly decreased the paw licking time in both the phases of formalin test. As has been reported for morphine,[26] a central action can be suggested for SRE. The analgesic effect of SRE in hot plate and tail flick tests in which SRE significantly increased the reaction time further confirms its central action. Based on our observations, SRE possesses antinociceptive activity against chemically and thermally induced nociception, and against both inflammation- and non-inflammation-mediated nociception.
The drugs used to treat chronic inflammatory conditions are not as effective and display pharmacological effect acting only on the inhibition of production of prostanoids but not interfering with other important elements of the cascade of inflammation. Thus, the pharmacological validation of natural products with established use in folk medicine in these types of conditions is very important. The pharmacologic effect of SRE and standard drug etoricoxib was evaluated in a model of mechanical hypernociception induced by important flogistic agents such as carrageenan. Our study demonstrated the effect of SRE in the mechanical hypernociception induced by intradermal injection of carrageenan in rats. Carrageenan is an inflammatory agent that is largely used as a pharmacological tool for investigating inflammatory hyperalgesia in rats and mice.[27] When injected intradermally on the plantar surface of animal's hind paw, it induces an inflammatory process associated with hyperalgesia.[28] Tissue injury originating after the injection of carrageenan involves the release of different chemical mediators such as PGE2,[29] mast cells products histamine and serotonin,[30] neuropeptides[31] and cytokines (TNFα and IL-1β),[23] among others.[32] This effect was similar when compared with animals treated with etoricoxib. This result suggests that SRE may be interfering with different pathways involved in the inflammatory pain signaling.
Postoperative pain models are utilized for screening the pathophysiology of hyperalgesia and were performed in this study in rats via incision. Pain response in rats was evaluated by mechanical, cold and heat stimuli. Decrease in weight bearing measured by Randall-Selitto analgesiometer (paw pressure), decrease in PWL measured by cold hyperalgesia and radiant heat stimuli assay were assessed. The time course of hypersensitivity is in accordance with previous reports using alternative behavioral measurements.[12] We found that mechanical, cold and radiant heat hyperalgesia were maximal on day 1 post-incision and resolved over similar time courses.
Investigation on the potency and efficacy of the test compound on mechanical, cold and radiant heat hyperalgesia was undertaken on day 1 post-incision. This time point allowed the assessment of the action of analgesics on an established pain state and removed the possibilities of development of behavioral hypersensitivity during surgery or in the immediate postsurgical period. We have demonstrated that mechanical hyperalgesia, cold hyperalgesia and radiant heat hyperalgesia can be reversed by SRE and reference drug (etoricoxib). The effects are dose-dependent with maximum effect elicited by the highest dose.
Past studies have demonstrated[33] on the action of cyclooxygenase (COX) inhibitors on plantar incision-induced pain. Three of these demonstrated that post-surgical intrathecal administration of COX-2 inhibitors has no effect on incision-induced pain behavior. In contrast to these, Yamamoto et al.[34] showed that oral administration of indomethacin or a selective COX-2 inhibitor (JTE522) 5 min following plantar incision can reduce tactile allodynia, as assessed with von Frey filaments. They further reported that there is no information on the efficacy of celecoxib, naproxen or etoricoxib in the rat model; however, flunixin, a potent NSAID, when given subcutaneously in the immediate postsurgical period and daily thereafter, produced an anti-allodynic effect.[34]
In our experiments, SRE and etoricoxib effectively reversed incision-induced decrease in PWL suggesting their usefulness in post-surgical pain. These results are in agreement with those of Yamamoto et al.[34] and Stewart and Martin[29] in demonstrating that systemically administered NSAIDs and selective COX-2 inhibitors are effective in a rat model of incisional pain.
The present study demonstrated the antinociceptive activity of SRE at both peripheral and central levels. Although further studies are needed in order to know the mechanism behind the observed antinociceptive action, in view of the need for new, safe and effective therapies, and taking into account the adverse effects associated with the drugs currently used, SRE represents an important and promising source of herbal medicine for the treatment of pathologies for which no efficacious treatment exists, such as chronic pain. Finally, the antinociceptive action demonstrated in the present study supports, at least in part, the ethno-medical uses of this plant.
Acknowledgments
The authors extend their sincere thanks to the Director and Joint Director (Academic) of Indian Veterinary Research Institute for funding this project.
Footnotes
Source of Support: Indian Veterinary Research Institute.
Conflict of Interest: No.
References
- 1.Ganesan S, Venkateshan G, Bhanumathy N. Medicinal plants used by ethnic group Thottianaickans of Semmalai hills, Tiruchirrapalli district, Tamil Nadu. Indian J Tradit Knowl. 2006;5:245–52. [Google Scholar]
- 2.Chandel KP, Shukla G, Sharma N. Biodiversity in Medicinal and Aromatic Plants in India: Conservation and Utilization. New Delhi: National Bureau of Plant Genetic Resources; 1996. [Google Scholar]
- 3.Jyothi G, Carey WM, RaviKumar B, KrishnaMohan G. Antinociceptive and antiinflammatory activity of methanolic extract of leaves of Shorea robusta. Pharmacol online. 2008;1:9–19. [Google Scholar]
- 4.Datta HS, Mitra SK, Patwardhan B. Wound healing activity of topical application forms based on ayurveda. Evid Based Complement Alternat Med. 2011;2011:134378. doi: 10.1093/ecam/nep015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paech K, Tracey MV. Modern methods of plant analysis. Berlin: Springer-Verlag; 1956. [Google Scholar]
- 6.Harborne JB. Photochemical Methods: A Guide to Modern Techniques of Plant Analysis. London: Chapman A & Hall; 1973. [Google Scholar]
- 7.Eddy NB, Leimbach D. Systemic analgesics II. Dithienylbutenyl and dithienylbutenyl. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 8.D’Amour GE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–8. [Google Scholar]
- 9.Corea CR, Calixto JB. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993;110:193–8. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkin LB, Hebner CF, Gaddi F, O’Keefe E, Spitaletta P, Plumer AJ. Pharmacology of 2 amino-indane hydrochloride (SU-8629): A potent non-narcotic analgesic. J Pharmacol Exp Ther. 1961;133:400–8. [PubMed] [Google Scholar]
- 11.Chan CC, Boyce S, Brideau C, Ford-Hutchinson AW, Gordon R, Guay D, et al. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and non human primate stomach. J Pharmacol Exp Ther. 1995;274:1531–7. [PubMed] [Google Scholar]
- 12.Brennan T, Vandermeulen EP, Gebhant GE. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 13.Randall LO, Sellito J. A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacol. 1957;3:209–19. [PubMed] [Google Scholar]
- 14.Santa-Cecília FV, Vilela FC, da Rocha CQ, Dias DF, Cavalcante GP, Freitas LA, et al. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J Ethnopharmacol. 2011;133:467–73. doi: 10.1016/j.jep.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandin in nociceptive processing in the rat. Eur J Pharmacol. 1992;219:427–33. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opoids and pain regulation. Pain Headache. 1987;9:129–59. [PubMed] [Google Scholar]
- 17.Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin Neurophamacol. 1993;16:1–5. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Siegmund E, Cadnus R, Lu G. A method for evaluating both non-narcotic and narcotic analgesics. Proc Soc Exp Biol. 1957;95:729–31. doi: 10.3181/00379727-95-23345. [DOI] [PubMed] [Google Scholar]
- 19.Berkenkopf JW, Weichmann BM. Production of prostacyclin in mice following intraperitoneal injection of acetic and, phenylbenzoquinone and zymosan: its role in the writhing response. Prostaglandins. 1988;36:693–709. doi: 10.1016/0090-6980(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 20.Vasudevan M, Gunnam KK, Parle M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol. 2007;109:264–70. doi: 10.1016/j.jep.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Leal LK, Fierreira AA, Bezerra GA, Matos FJ, Viana GS. Antinociceptive, antiinflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. J Ethnopharmacol. 2000;70:151–9. doi: 10.1016/s0378-8741(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 22.Colvin AC, Wang CF, Soens MA, Mitani AA, Strichartz G, Gerner P. Prolonged cutaneous analgesia with transdermal application of amitriptyline and capsaicin. Reg Anesth Pain Med. 2011;36:236–40. doi: 10.1097/AAP.0b013e31820c2c30. [DOI] [PubMed] [Google Scholar]
- 23.Shibate M, Ohkubo T, Takashi H, Inuki R. Modified formalin test: characteristic biphasic pain response. Pain. 1984;38:347–52. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 24.Correa CR, Kyle DJ, Charkraverty S, Calixto JB. Antinociceptive profile of pseudopeptide B2 bradykinin receptor antagonist NPC 18688 in mice. Br J Pharmacol. 1996;117:552–8. doi: 10.1111/j.1476-5381.1996.tb15226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damas J, Liegeois JF. The inflammatory reaction induced by formalin in rat paw. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:220–7. doi: 10.1007/pl00005345. [DOI] [PubMed] [Google Scholar]
- 26.Xie YF, Wang J, Huo FQ, Jia H, Tang JS. Validation of a simple automated movement detection system for formalin test in rats. Acta Pharmacol Sin. 2005;26:39–45. doi: 10.1111/j.1745-7254.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Vale ML, Benevides VM, Sachs D, Brito GA, da Rocha FA, Poole S, et al. Antihyperalgesic effect of pentoxifylline on experimental inflammatory pain. Br J Pharmacol. 2004;143:833–44. doi: 10.1038/sj.bjp.0705999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitriteinvolvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–20. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 29.Oh-Ishi S. Regulation of biological functions by thekallikrein-kinin system. Yakugaku Zasshi. 1997;117:739–48. doi: 10.1248/yakushi1947.117.10-11_739. [DOI] [PubMed] [Google Scholar]
- 30.Kocher L, Anton F, Reeh PW. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain. 1987;299:363–73. doi: 10.1016/0304-3959(87)90051-0. [DOI] [PubMed] [Google Scholar]
- 31.Coderre TJ, Melzack R. Central neural mediators ofsecondary hyperalgesia following heat injury in rats: neuropeptidesand excitatory amino acids. Neurosci Lett. 1991;1319:71–4. doi: 10.1016/0304-3940(91)90339-u. [DOI] [PubMed] [Google Scholar]
- 32.Cunha TM, Verri WA, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanicalinflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto T, Sakashita Y, Nozaki-Taguchi N. Anti-allodynic effect of oral COX-2 selective inhibitor on post-operative pain in the rat. Can J Anaesth. 2000;47:354–60. doi: 10.1007/BF03020953. [DOI] [PubMed] [Google Scholar]


