Abstract
Objectives:
Present study was carried out to evaluate acute and subacute toxicity and efficacy of Seabuckthorn (Hippophae rhamnoides) based herbal antioxidant supplement (HAOS).
Materials and Methods:
In vivo toxicity studies were performed in male balb ‘C’ mice by oral administration. Acute toxicity study was done at doses ranging from 2000 to 10 000 mg/ kg while in subacute studies, HAOS was given at doses of 2000, 4000, and 8000 mg/kg body weight. Animals were observed for any toxic sign and symptoms periodically. At completion of study animals were sacrificed; their hematological, biochemical parameters were analyzed and histopathology of vital organs was done. In vivo efficacy studies in human volunteers were done and the levels of vitamin A and Vitamin C in blood samples were analyzed in comparison to a similar commercially available formulation.
Results:
No mortality and any clinical signs of toxicity were found in HAOS administered group of animals. There were no significant alterations in hematological and biochemical parameters. Histopathological analysis of vital organs showed normal architecture in all the HAOS administered groups. Human studies showed an increase of 32% and 172% in Vitamin A and Vitamin C levels respectively in term of bioavailability.
Conclusion:
The data obtained indicate no toxicity of this antioxidant supplement up to the highest dose studied. Efficacy in terms of increased bioavailability of vitamin A and C in human volunteers indicates the clinical usefulness of the supplement.
KEY WORDS: Acute and subacute toxicity, herbal antioxidant supplement, seabuckthorn, histopathology and bioavailability
Introduction
Herb based medicines or food supplements are popular, effective and generally safe remedies against variety of diseases and are being used widely the world over. This assumes significance since a World Health Organization survey has found that around 70%–80% of the world's population rely on non-conventional medicine, mainly of herbal source, in the primary healthcare sector.[1] However, in many countries including India, many a times the nutritional/beneficial claims made for a herbal based supplement or drug are not backed by sufficient preclinical and animal toxicity data, which is otherwise a prerequisite for any allopathic formulation. A scientifically carried out screening is therefore important in order to ascertain safety and efficacy of traditional and herbal products and also to establish the active components in them.[2] Toxicity, safety, and efficacy data for any herbal preparation in suitable animal models as per regulatory norms can greatly help in predicting toxicity and providing guidelines for selecting a safe dose in humans. We have formulated a herbal antioxidant supplement (HAOS) using seabuckthorn pulp and extract of indigenous medicinal plants of high altitudes that tone up the gastric and intestinal functions, including the exocrine and gastrointestinal motility functions. Nutraceutical profiling is an important but very uncommon practice during the development of any herbal formulation. As per regulatory norms, the developed formulation has been evaluated for its nutraceutical value and stability by a certified outside agency, viz., Food Research and Action Center (FRAC), Delhi, India and has been found to be rich in antioxidant contents [Table 1].
Table 1.
Nutraceutical profile of herbal antioxidant supplement
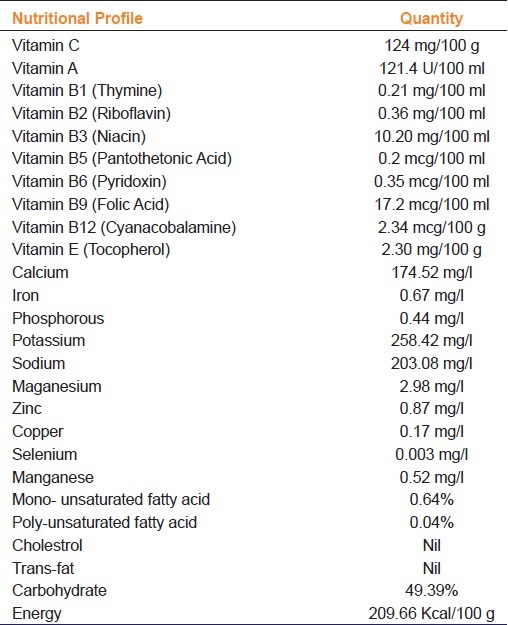
At high altitude (13 000 feet and above), the local inhabitants, treckkers, soldiers, and other population suffer from chronically upset gastrointestinal system, indigestion, loss of appetite, and probably malabsorption leading to weight loss and reduced efficiency which is further accentuated by harsh and extreme weather conditions with low atmospheric pressure and oxygen concentration.[3] We believe that at such altitudes this formulation may prove to be a supplementary food of therapeutic value for the geriatric population, children suffering from malnutrition, and other susceptible population. Vitamin A, vitamin C, vitamin E, and carotenoids are major dietary antioxidants which significantly neutralize the adverse effects of free radicals in the body.[4] The daily requirement of Vitamin C at high altitude is more than at sea level.[5] The developed supplement formulation contains sufficient quantities of vitamins A, E, K, β-carotene, phytosterols and fatty acids [omega-3 (linolenic acid) and omega-6 (linoleic acid), oleic acid and lower saturated fatty acid], to neutralize the toxic effects of free radicals.[6,7] As a natural immune enhancer, Vitamin A maintains the stability of the immune system and keeps the supervisory role of the system normal. β- Carotene, which is the principal source of vitamin A acts as an antioxidant which plays an important role in decreasing the incidence of cancer,[8,9] cardiovascular diseases,[10,11] aging and acts as an immunological regulator.[12]
The present study was carried out to assess the safety and tolerability for long term use of the developed product. Acute and subacute toxicity studies were carried out in suitable animal models. Human volunteer studies were done to estimate the change in bioavailability of vitamin A and vitamin C in comparison to a similar commercially available product.
Materials and Methods
Test substance
The herbal antioxidant supplement (HAOS) was provided by Defence Institute for High Altitude Research (DIHAR), Leh, India. All other chemicals and solvents used in the study were of analytical grade.
Animals
The experiments were conducted on male balb ‘C’ mice weighing 26 ± 2 g obtained from experimental animal facility of Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi. All animal experiments were approved by the Institutional Animal Ethical Committee and confirmed to general national guidelines on the care and use of laboratory animals. The animals were kept for one week acclimatization and maintained at controlled temperature and hygiene conditions. They were provided food and drinking water ad libitum and housed on 12 hours day and night cycle.
Human subjects
Healthy human volunteers (n = 6) of age group 19-27 years were randomly selected for the study. The study was approved by Institutional Human Ethical Committee duly constituted for the purpose. The subjects were briefed about the procedures involved in the study and informed consent was taken from each of the volunteer.
Treatment Regimen
Acute toxicity study
Male Balb ‘c’ mice weighing 26 ± 2 g were selected and divided into six groups having six animals in each group. The animals were fasted overnight and HAOS was administered orally at the dose levels 0, 2000, 4000, 6000, 8000, and 10000 mg/kg body weight respectively in six different groups. Animals were observed individually after dosing for a total of 14 days[13] for any clinical sign of toxicity and mortality.
Cage side observations
Observations included changes in skin, fur, eyes, mucous membranes, respiratory, circulatory, autonomic, central nervous systems, somatomotor activity and behavior pattern. Attention was also directed toward observations of tremors, convulsions, salivation, diarrhoea, lethargy, and sleep.
Body weight, food and water intake
Any change in body weight, food and water intake was recorded. The body weight changes were recorded weekly while food and water intake were observed daily.
Pathology
Overnight fasted surviving animals were weighed and humanely killed on 14th day using anesthetic chloroform. All test animals were subjected to gross necropsy.
Subacute toxicity study
Male Balb ‘c’ mice weighing 26 ± 2 g were taken and randomly divided into four groups of six animals each. Animals in Group A received distilled water only and served as controls, while those in Group B, C and D received 2000, 4000, and 8000 mg/kg per oral dose of HAOS, respectively, once daily up to one month (OECD 407 Guidelines). Toxic manifestations and mortality were monitored daily, while body weight changes were observed weekly. At the end of the study animals were sacrificed and their blood samples were collected and clinically evaluated for hematological and biochemical parameters.
Body weight, food intake and water consumption
Body weights were measured before the treatment, every week thereafter, and on the day of sacrifice. Food and water intake of the animals was observed periodically.
Organ weights
During necropsy, vital organs (lungs, heart, spleen, liver, kidneys) were carefully examined macroscopically and weighed in relation to total body weight.
Hematology
The animals were fasted overnight prior to necropsy and blood collection. Blood collection was done by cardiac puncture, in EDTA containing vials for immediate analysis of hematological parameters. Hematological parameters were determined by standard clinical procedures using an automatic hematological analyzer (Roche Integra, 400 Plus, Diagnostic Systems).
Serum biochemistry
Blood samples for biochemical investigations were collected in plain tubes and centrifuged at 4000 rpm at 4°C for 10 min to obtain the serum. Analysis of biochemical parameters, namely, Aspartate aminotransferase (AST), alanine aminotransferase (ALT) alkaline phosphatase (ALP), cholesterol (CHL), triglycerides (TG) and glucose (GLU) was done with a reagent kit of Randox laboratory Ltd, UK using an automated analyzer (DREL 3000 HACH).
Histopathology
The animals were sacrificed by chloroform anesthesia and after the last dosing. Stomach, intestine, liver, kidney and spleen of the animals were surgically removed and fixed with 10% buffered formalin. Standard histopathological procedures were followed to observe any microscopic changes in the organs.
In vivo efficacy studies in human volunteers
After overnight fasting, the volunteers were fed 20 gram of HAOS as a bolus followed by 100 ml of drinking water. Their blood samples were collected at 0, 1, 2, 4, 6, and 24 h. The samples were appropriately processed and sent for the estimation of Vitamin A and Vitamin C levels to a certified laboratory (Religare SRL Diagnostics, Mumbai, India). The estimation was done using HPLC method.
Statistical Analysis
Numerical data are presented as mean ± S.D. Differences between groups were analyzed using analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test. Minimum criterion for statistical significance was set at P < 0.05 for all comparisons.
Results
Acute toxicity study
No mortality and morbidity or any signs of behavioral changes or toxicity were observed throughout the 14-day period after single oral administration of HAOS up to the dose levels of 10,000 mg/kg body weight. Morphological characteristics (fur, skin, eyes, and nose) appeared normal. No tremors, convulsion, salivation, diarrhea, lethargy or unusual behaviors such as self mutilation, walking backward and so forth were observed; gait and posture, reactivity to handling or sensory stimuli, grip strength were all normal. There was no significant change in body weights between control and treatment groups.
Subacute toxicity study
The animals were healthy with no differences being noted with respect to the control group. No significant changes were observed in weight of treated groups as compared to control [Figure 1] and no mortality was observed during the experimental procedures. The weight of vital organs (liver, kidney, heart, spleen and stomach) was not significantly affected in experimental animals as compared to control group (Data not shown).
Figure 1.
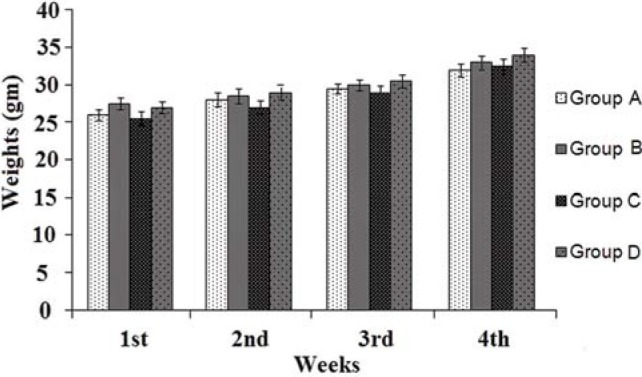
Comparative body weight changes in animals during sub acute toxicity experimentation
Hematology
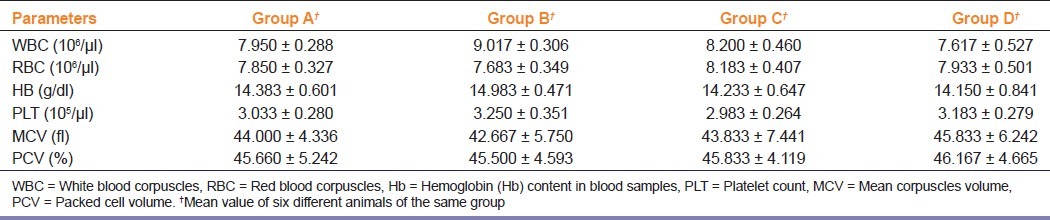
The effect of repeated dose of orally administered HAOS on hematological parameters is presented in Table 2. Hematological analysis showed no significant change in treatment groups as compared to the control group.
Table 2.
Effect of herbal antioxidant supplement on hematological parameters
Serum biochemistry
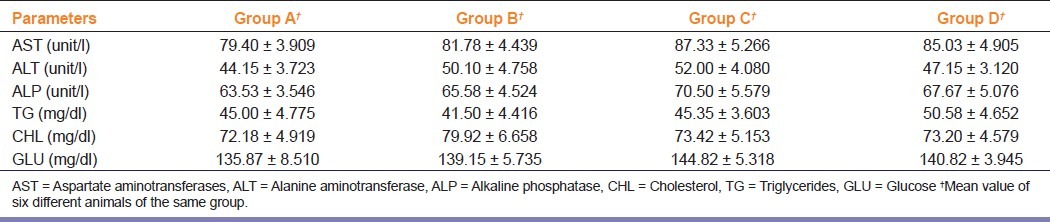
The effect of repeated dose of orally administered HAOS on serum toxicity markers (AST, ALT, ALP, CHL, TG, and GLU) is presented in Table 3. Results show that there was no significant change in values of treated animals as compared to controls.
Table 3.
Effect of herbal antioxidant supplement on serum toxicity marker enzymes
Histopathology
No abnormalities were detected in pathological examinations of the tissues during microscopic examination of internal organs in comparative histology of tissues of control and test animals. HAOS did not affect the histology of vital organs, viz., lungs, heart, spleen, and liver [Figure 2].
Figure 2.
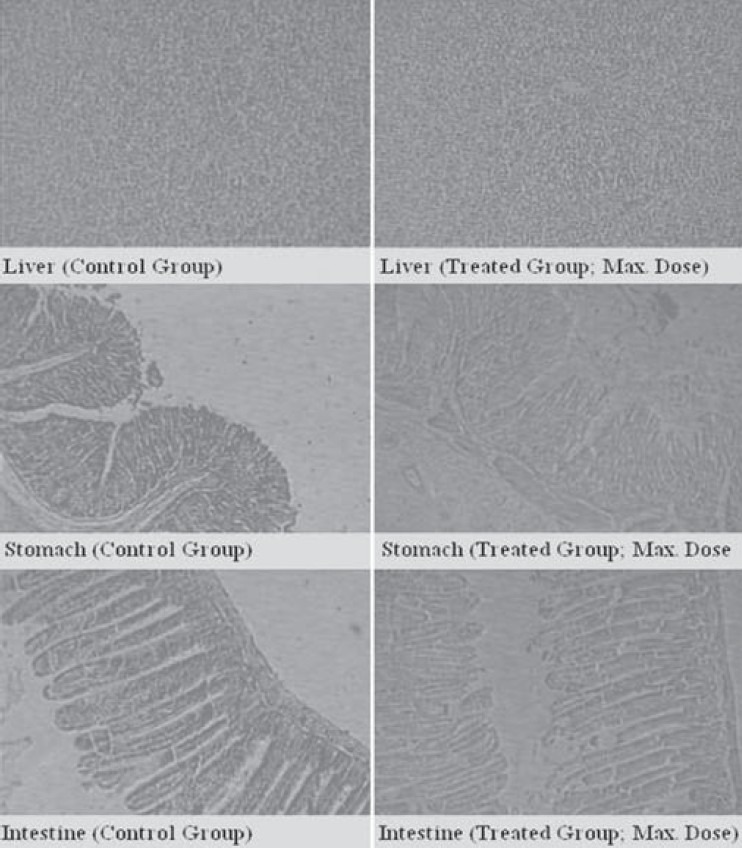
Comparative histomicrographs of vital organs in control and treatment (maximum dose) group
In vivo human study
The average per cent increase in blood vitamin A concentration after a single dose oral administration of HAOS over 6 h was found to be 32.24% of the baseline level in comparison to the control group who had consumed similar quantity of a commercially available antioxidant supplement (CAAS). Blood vitamin C concentration was increased by 172% of the baseline value over 5 h.
Discussion
In acute toxicity study, there was no mortality up to a maximum dose of 10 000 mg/kg body weight of HAOS after per oral administration. The changes in body weight have been used as an indicator of adverse effect of HAOS.[14] Since no remarkable changes were observed in animal behavior, body weight and organ weight at all dose levels in treated mice as compared to control group, it can be inferred that HAOS is nontoxic at the doses administered. Data analysis of animals’ blood parameters can be translated for risk evaluation in humans, since changes in hematological system have a higher predictive value for human toxicity. Sub-acute toxicity studies also showed no significant changes in the hematological parameters between control and treated groups. There was a transient increase in hemoglobin and WBC counts. An increase in hemoglobin level might be due to the increased absorption of iron in presence of vitamin C and the observed increase in WBC levels may indicate the impact of HAOS on the immune system of treated group. The results indicate that HAOS is neither toxic to the circulating red cells, nor does it interfere with their production.
Transaminases (AST and ALT) and ALPs are generally used as indices for liver and kidney damage respectively.[15,16] No significant change was found in serum levels of AST, ALT, and ALP enzymes post-HAOS administration. HAOS therefore did not provoke any detrimental effect on liver and kidney. The nontoxicity of HAOS on specific organs was further confirmed by histopathological assessment. Histopathological examination of selected vital organs (heart, lung, liver, kidney, spleen and stomach) from both treated and control animals showed normal architecture, suggesting no microscopic changes and morphological disturbances were caused due to oral administration of HAOS at all dose levels.
In vivo efficacy study data in term of bioavailability of vitamin A and C after a single oral dose in human volunteers indicates that HAOS is significantly richer in terms of antioxidant property. Since the antioxidant levels play a vital role in body's normal physiological systems,[17,18] the increased bioavailability of these vitamins (antioxidants) suggests that the developed HAOS may be more efficacious and exhibit better nutritive value than the similar commercially available antioxidant supplement.
The results strongly suggest that the herbal antioxidant supplement is safe and well tolerated at the tested oral doses since no deleterious changes were observed in animal macro-parameters, behavior and habits, hematology, serum biochemistry and histopathological parameters. Further, the developed herbal antioxidant supplement was found to be nontoxic when oral acute and repeated dose toxicity studies were performed. Bioavailability studies in humans along with animal toxicity profile of the developed HAOS have shown very encouraging results, suggesting a long term, therapeutic/nutritive potential of the supplement.
Acknowledgments
This task project was sponsored by Defence Institute of High Altitude Research (DRDO), Leh, India (Sanction No.: DIHAR/ASSIGN/09/2009).
Footnotes
Source of Support: Defence Institute of High Altitude Research (DRDO), Leh, India.
Conflict of Interest: Nil.
References
- 1.Güvenç A, Kendir G, Eken A, Aydin A. Evaluation of inorganic compounds of erica L.species (Ericaceae) native to Turkey. FABAD J Pharm Sci. 2007;32:121–5. [Google Scholar]
- 2.Chakravarty B. Herbal medicines. Safety and efficacy guidelines. Regul Aff J. 1993;4:699–701. [Google Scholar]
- 3.Vats P, Singh SN, Shyam R, Singh VK, Singh SB, Banerjee PK, et al. Leptin may not be responsible for high altitude anorexia. High Alt Med Biol. 2004;5:90–2. doi: 10.1089/152702904322963753. [DOI] [PubMed] [Google Scholar]
- 4.Monsen ER. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100:637–40. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 5.Weisburger JH. Vitamin C and disease prevention. J Am Coll Nutr. 1995;14:109–11. doi: 10.1080/07315724.1995.10718480. [DOI] [PubMed] [Google Scholar]
- 6.Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92:119–24. [Google Scholar]
- 7.Upadhyay NK, Kumar R, Siddiqui MS, Gupta A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189. (Published online 2011 March 20. doi: 10.1093/ecam/nep189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290:201–8. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 9.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S–69S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 10.Bast A, Haenen G. The toxicity of antioxidants and their metabolites. Environ Toxicol Pharmacol. 2002;11:251–8. doi: 10.1016/s1382-6689(01)00118-1. [DOI] [PubMed] [Google Scholar]
- 11.Santos G, Padrón ÁS, De Souza R, Marques L. Effect of beta-carotene supplementation on the blood pressure of rats. Rev Nutr. 2007;20:39–45. [Google Scholar]
- 12.Burri BJ. Beta-carotene and human health: A review of current research. Nutr Res. 1997;17:547–80. [Google Scholar]
- 13.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Sith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and animals. Regul Toxicol Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 14.Teo S, Stirling D, Thomas S, Hobermann A, Kiorpes A, Khetani V. A 90 day oral gavage toxicity study of d-Methyl penidate and DL methyl penidate in sprague-dawley rats. Toxicology. 2002;179:183–96. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 15.Hayes A.W, editor. Principles and Methods of Toxicology. 5th edition. New York, NY: Informa Healthcare; 2007. [Google Scholar]
- 16.Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of swiss albino mice. Sci Pharm. 2002;70:135–46. [Google Scholar]
- 17.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nut. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 18.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]


