Abstract
Trimethoprim-sulfamethoxazole (TMP/SMX) is a widely prescribed antimicrobial for the management of several uncomplicated infections. It is commonly used for the treatment and prophylaxis of Pneumocystis jirovecii pneumonia (PCP) in the HIV-infected population. The adverse reaction to TMP/SMX is more frequent and severe in HIV-infected patients as compared to the general population. Here, we report a case of Stevens-Johnson syndrome (SJS) secondary to TMP/SMX. The patient had a generalized cutaneous reaction with involvement of the eyes, oral cavity, and genitals. He had elevated hepatic alanine aminotransferase and aspartate aminotransferase enzyme. TMP/SMX therapy was stopped and supportive treatment was started. His condition improved after eight days of stopping TMP/SMX therapy.
KEY WORDS: Desensitization, HIV infection, glutathione enzyme, P. jirovecii pneumonia, Steven Johnson syndrome, trimethoprim-sulfamethoxazole
Introduction
Steven Johnson syndrome (SJS) is characterized by sudden onset of erosion of mucous membranes (predominantly oral mucosa, lips, and conjunctivae) with widespread blistering of the skin involving up to 10% of the body surface area.[1] It is caused by several factors such as infections, drugs, and malignancies. Trimethoprim-sulfamethoxazole (TMP/SMX) is a widely prescribed antimicrobial for the management of several uncomplicated infections. In immunocompetent persons, the adverse reactions to TMP/SMX occur in approximately 1-3% of persons. In contrast, in HIV-infected population, a much higher incidence of adverse reactions has been reported with frequencies ranging from 40-80%.[2] We report a HIV-infected man who developed SJS while receiving TMP/SMX therapy.
Case Report
A 33-year-old male presented with complaints of intermittent low grade fever for two months, cough with expectoration for one and half month, loss of weight, and appetite for one month. He also had exertional dyspnea for the last one month. On examination, the patient was afebrile with a pulse rate of 92/min, respiratory rate of 30/min and blood pressure of 120/70 mmHg. Mild pallor was present. There was no cyanosis, icterus, clubbing, edema or significant lymphadenopathy. Respiratory system examination revealed bilateral coarse crepitation's. Rest of the systemic examination was normal. Investigations revealed: Hemoglobin 9.2 gm/dL, total leucocyte count 7500/cumm with an absolute lymphocyte count of 1500/cumm, platelet count of 2.1 lac/cumm and erythrocyte sedimentation rate of 76 mm at the end of 1 hr. Sputum for acid fast bacilli was negative. Chest X-ray showed bilateral basal reticulo-nodular opacities. HIV-ELISA was positive. In view of the clinical presentation and investigations, a diagnosis of Pneumocystis jirovecii pneumonia (PCP) was considered and the patient was started on TMP/SMX doubles strength two tablets thrice daily. After ten days of starting TMX/SMX therapy, the patient developed high grade fever, redness and pricking sensation in eyes, and burning sensation in throat. Within the next 48 hours he developed blepharitis, conjunctival congestion, erythema of the eyelids, and oral mucosal erosions [Figure 1]. Painful, erythematous, maculopapular, and vesicular lesions appeared all over the body including genitals [Figure 2]. There were multiple ulcerations in the buccal mucosa, floor of the mouth, and ventral surface of the tongue. Nikolsky's sign was positive. The patient was hemodynamically stable with a pulse rate of 102 per/min and a blood pressure of 110/80 mmHg. The alanine aminotransferase and aspartate aminotransferase levels were 250U/L (10-40U/L) and 155U/L (10-35U/L), respectively. TMP/SMX therapy was stopped immediately. The patient was treated aggressively with intravenous dexamethasone 6 hourly, intravenous fluids, prophylactic antibiotics, anti-allergic drugs and local treatment of lesions. After eight days the eruptions began to clear. Re-epithelialization of the previously sloughed areas occurred and the general condition of the patient remained satisfactory. A diagnosis of TMP/SMX induced SJS was made. This can be considered as a ‘probable’ adverse drug reaction as per causality assessment with Naranjo's Scale.[3] He was discharged after a month by which time the skin had healed with post-inflammatory hypopigmentation. The ocular and mucosal lesions cleared completely. His repeat liver function tests on discharge were normal. Desensitization to TMP/SMX was not attempted and the patient was discharged on dapsone prophylaxis for PCP.
Figure 1.
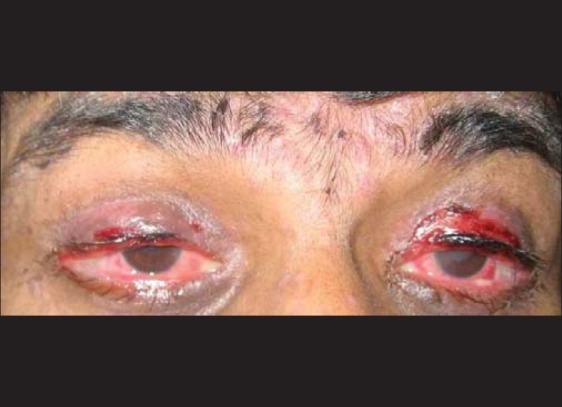
Clinical photograph showing conjunctival congestion, erythema of the eyelids, and blepharitis
Figure 2.
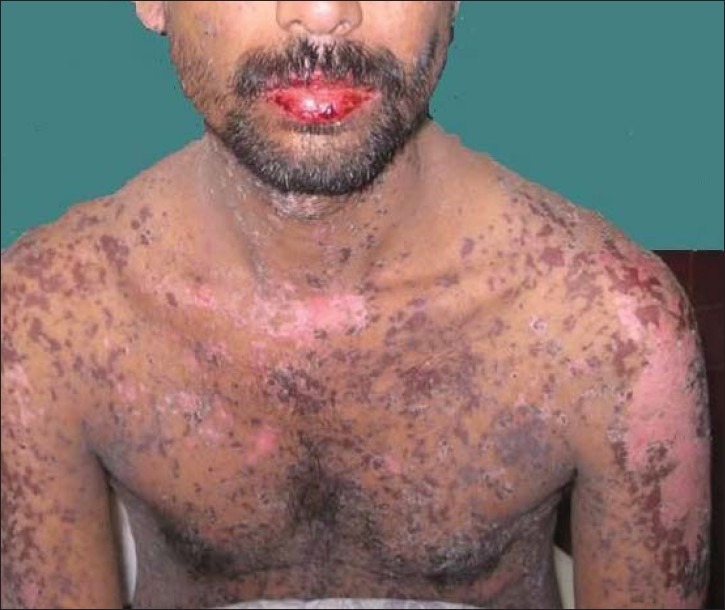
Clinical photograph showing cutaneous lesions all over the body with oral mucosal erosions
Discussion
TMP/SMX is commonly used for the treatment and prophylaxis of Pneumocystis jirovecii pneumonia (PCP) in HIV-infected patients. In addition to its efficacy in preventing PCP, TMP/SMX also protects against toxoplasmosis and several bacterial infections.[4] However, the administration of TMP/SMX is hampered by the high incidence of hypersensitivity reactions in these patients. These reactions typically occur during the second week of therapy. The most common adverse reactions include nausea, vomiting, anorexia, dermatological reactions such as pruritis, urticaria and less commonly Steven Johnson Syndrome (SJS), neutropenia, exfoliative dermatitis, erythema multiforme and toxic epidermal necrolysis.[5] A PUBMED search using the keywords ‘Trimethoprim-sulfamethoxazole’ AND ‘Steven Johnson syndrome’ AND ‘human immunodeficiency virus’ revealed less than ten cases being reported in literature till date.
The increased incidence of adverse reactions to TMP/SMX in HIV-infected patients is due to the hydroxylamine derivative of sulphamethaxazole, i.e., sulphamethaxazole hydroxylamine. The deficiency of glutathione enzyme in HIV-infected patients results in a decreased capacity to scavenge these hydroxylamine derivatives.[6,7] In vitro, it has been found that as compared to CD8 cells, purified CD4 cells are more resistant to the sulphamethaxazole hydroxylamine. In HIV infection, the depletion of CD4 cells leaves a greater proportion of circulating T-cells as the more susceptible CD8 subset.[7] In addition, the immune dysfunction observed in HIV-infected patients, i.e., the polyclonal B-cell activation, polyclonal gammopathy, and decreased T-suppressor cell functions leads to an exaggerated humoral immune response to SMX or its metabolites.[2] Other factors such as viral load and concurrent medications may also increase cell sensitivity to reactive metabolites.[7] It is not clear whether the adverse reactions to TMP/SMX merely indicate or also induce progression of HIV disease. Veenstra et al. found that patients with adverse reactions to TMP/SMX have a more rapid decline in CD4 cell counts and rapid progression to AIDS and death.[4] In patients with non life-threatening adverse reactions (e.g., fever, rash), rapid or slow oral desensitization to TMP/SMX can be attempted.[8] However, in patients with severe life threatening adverse reaction to TMP/SMX, alternative drug like dapsone, pentamidine, or atovaquone should be given for PCP prophylaxis. Those who do not tolerate or have recurrent PCP infections despite alternative therapy could be subjected to a trial of desensitization to TMP-SMX under close supervision.[9]
Conclusion
This case illustrates a clinically important and life threatening adverse effect of TMP/SMX in HIV-infected patients. Due to the high incidence of such hypersensitivity reactions, physicians should monitor HIV-infected patients on trimethoprim-sulfamethoxazole therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daftarian MP, Filion LG, Cameron W, Conway B, Roy R, Tropper F, et al. Immune response to sulfamethoxazole in patients with AIDS. Clin Diagn Lab Immunol. 1995;2:199–204. doi: 10.1128/cdli.2.2.199-204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki SA. Adverse drug reaction and causality assessment scales. Lung India. 2011;28:152–3. doi: 10.4103/0970-2113.80343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veenstra J, Veugelers PJ, Keet IP, van der Ven AJ, Miedema F, Lange JM, et al. Rapid disease progression in human immunodeficiency virus type1-infected individuals with adverse reactions to trimethoprim-sulfamethoxazole prophylaxis. Clin Infect Dis. 1997;24:936–41. doi: 10.1093/clinids/24.5.936. [DOI] [PubMed] [Google Scholar]
- 5.Abusin S, Johnson S. Sulfamethoxazole/Trimethoprim induced liver failure: A case report. Cases J. 2008;1:44. doi: 10.1186/1757-1626-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora VK, Venubabu K. Cotrimoxazole induced toxic epidermal necrolysis in a suspected case of Pneumocystis carinii pneumonia with human immuno deficiency virus infection. Indian J Chest Dis Allied Sci. 1998;40:125–9. [PubMed] [Google Scholar]
- 7.Hess DA, Sisson ME, Suria H, Wijsman J, Puvanesasingham R, Madrenas J, et al. Cytotoxicity of sulfonamide reactive metabolites: Apoptosis and selective toxicity of CD8(+) cells by the hydroxylamine of sulfamethoxazole. FASEB J. 1999;13:1688–98. doi: 10.1096/fasebj.13.13.1688. [DOI] [PubMed] [Google Scholar]
- 8.Leoung GS, Stanford JF, Giordano MF, Stein A, Torres RA, Giffen CA, et al. Trimethoprim-sulfamethoxazole (TMP-SMZ) dose escalation versus direct rechallenge for Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with previous adverse reaction to TMP-SMZ. J Infect Dis. 2001;184:992–7. doi: 10.1086/323353. [DOI] [PubMed] [Google Scholar]
- 9.Douglas R, Spelman D, Czarny D, O’Hehir RE. Successful desensitization of two patients who previously developed Stevens-Johnson syndrome while receiving trimethoprim-sulfamethoxazole. Clin Infect Dis. 1997;25:1480. doi: 10.1086/516995. [DOI] [PubMed] [Google Scholar]


