Sir,
Helminth infections affect large human populations in the tropical region and lead to undernourishment, anemia and pneumonia like conditions.[1] Despite advances in mode of transmission and treatment of parasites, there are no efficient products to control the helminth infection and haphazard use of some drugs has resulted in causing gastrointestinal resistance.[2,3] Thus there is a need to search and develop new chemical drugs for helminth control. From eternal era, mushrooms are an integral part of human diet. A variety of mushrooms are known to have a therapeutic potential. Pleurotus species is one of the widely consumed mushrooms, and is known for its immunomodulatory and anti-inflammatory potential. There is no scientific information about the anthelmentic potential of mushroom. Therefore, the aim of the current study was to evaluate the anthelmentic potential of the Oyster Mushroom Pleurotus florida.
The mushroom basidiocarp was obtained from Mycology Research Laboratory, Rani Durgavati University, Jabalpur (M.P.). The voucher specimen was deposited in Mycology Research Laboratory, Rani Durgavati University, and Jabalpur (M.P.) (HDBJ #43). Mushrooms were dried in shade, coarsely powered and used for preparation of extracts. The powder was extracted with ethanol: water (1:1) by stirring for 48 hours and filtered through Whatman No. 4 filter paper. The residue was then extracted with two additional 200 ml portions of ethanol: water (1:1) as described above. The combined extracts was then evaporated at 40°C to dryness, and stored at 4°C for further use. Preliminary myochemical screening was done by standard methods.[4] IR study of extract was done.
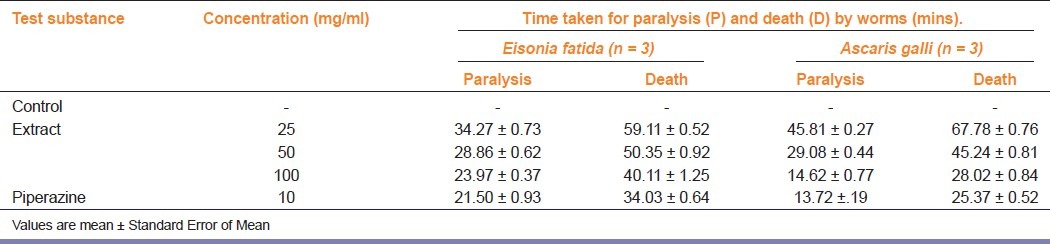
The roundworms (Ascaridia galli) were obtained from the intestines of freshly slaughtered fowls. Earthworms (Eisonia fatida African type) (0.8-2 g) were collected from Madhya Pradesh Veterinary University, Jabalpur (M.P.), India. They were used immediately after collection. Proper authentication was done by the eminent of the Veterinary University [Figure 1]. Piperazine citrate (10 mg/ml) was used as a reference standard. The anthelmintic assay was carried as per the method of Ajaiyeoba et al.[5] with necessary modifications. Formulations (4 ml) containing different concentrations of crude extracts (10, 50, and 100 mg/ml in distilled water) were prepared, and three worms (same type) were placed in it. Time for paralysis was noted as when no movement of any sort could be observed, except when the worms were shaken vigorously. Time for death of worms were recorded after ascertaining that worms neither moved when shaken vigorously or when dipped in warm water (50°C).
Figure 1.
IR Spectra of hydroethanolic extract of P. florida
Condensed tannins[6] and phenolics[7] are reported to show anthelmintic activity. Total soluble phenolic compounds in the extract were measured according to the method of Singleton and Rossi.[8] Condensed tannin content in mushroom crude extracts was determined using a colorimetric method.[9] The total phenolic content was 717.2 ± 3.03 mg GAE/100g whereas condensed tannin content was 175.2 ± 2.58 mg CE/100g. The preliminary myochemical screening showed the presence of glycosides, amino acids, carbohydrates, flavonoids and tannins. After a concise stimulant effect on motility, earthworms lost their movements, became paralyzed and finally died on exposure to the extract. Formulation containing 25, 50,100 mg/ ml of hydroethanolic extract of Pleurotus florida caused dose dependent paralysis, causing loss of motility to loss of response to external stimuli, which ultimately progressed to death [Table 1]. Helminth infections of the gastrointestinal tract of humans adversely affect the health standards of huge inhabitants all around the globe. In the investigation of compounds with anthelmintic activity, a number of substances have been screened using different species of worms, for example, earthworms, Ascaris, Nippostrongylus and Heterakis. Among these species, earthworms and round worms have been used widely for the early assessment of anthelmintic compounds in vitro because they show resemblance to intestinal “worms” in their response to anthelmintics and are easily obtainable. It has been demonstrated that all anthelmintics are lethal to earthworms, and a substance toxic to earthworms is commendable for investigation as an anthelmintic.[10]
Table 1.
Anthelmintic activity of Pleurotus florida extract as seen in the study
Pleurotus spp. are promising as medicinal mushrooms, and exhibit hematological, antiviral, antitumor, antibiotic, antibacterial, hypocholesterolic and immunomodulation activities. Pleurotus florida contains a large number of myochemicals like terpenoids, tannins, steroidal glycosides and carbohydrates. Chemically, tannins are polyphenolic compounds. Some synthetic phenolic anthelmintics e.g. niclosamide, oxyclozanide, bithionol etc., are reported to interfere with energy generation in helminth parasites by uncoupling oxidative phosphorylation. Another possible anthelmintic effect of tannins is that they can bind to free proteins in the gastrointestinal tract of host animal or glycoprotein on the cuticle of the parasite, and may cause death. It can be concluded that hydroethanolic extract of P. florida showed significant anthelmintic activity when compared with the standard anthelmintic drug. Further studies are required to evaluate the active components in vivo which may be responsible for the anthelmintic activity.
References
- 1.Prevention and control of intestinal parasitic infections. Technical Report Series. Geneva: World Health Organization; 1987. Report of WHO Expert Committee; p. 749. [PubMed] [Google Scholar]
- 2.Bundy DA. Immunoepidemiology of intestinal helminthic infection I: The global burden of intestinal nematode disease. Trans R Soc Trop Med Hyg. 1994;8:259–61. doi: 10.1016/0035-9203(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 3.Coles GC. Anthelmintic resistance and the control of worms. J Med Microbiol. 1999;48:323–5. doi: 10.1099/00222615-48-4-323. [DOI] [PubMed] [Google Scholar]
- 4.Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. New Delhi: Springer Private Limited Press; 1998. [Google Scholar]
- 5.Ajaiyeoba EO, Onocha PA, Olarenwaju OT. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm Biol. 2001;39:217–20. [Google Scholar]
- 6.Zafar I, Kamran AM, Muhammad NK. Anthelmintic effects of condensed tannins. Int J Agric Biol. 2002;4:438–40. [Google Scholar]
- 7.Aremu AO, Ndhlala AR, Fawole OA, Light ME, Finnie JF, Van Staden J. In vitro pharmacological evaluation and phenolic content of ten South African medicinal plants used as anthelmintics. S Afr J Bot. 2010;76:558–66. [Google Scholar]
- 8.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 9.Xu BJ, Chang SK. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:S159–66. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 10.Mali RG. In vitro Anthelmintic activity of stem bark of Mimusops elengi linn. Pharmacogn Mag. 2007;3:73–6. [Google Scholar]


