Sir,
The interference of xenobiotics with endocrine regulatory mechanisms has been well established. These xenobiotics include natural or synthetic chemicals that interfere with the synthesis, circulating levels or peripheral action of hormones. Thyroid disruptors (TDs) or thyroid disrupting chemicals (TDCs) are a subfamily of endocrine disruptors which interfere with thyroid function by affecting the hypothalamo-pitutary-thyroid axis or directly via thyroid hormone receptors.[1] The alteration in hormonal milieu may cause developmental defects, tumors, hypo or hyper-functions of hormones. Thyroid disruptors are widely prevalent in the ecosystem as pollutants or products of day to day use. Despite their ubiquitous distribution and disastrous effects on human endocrine health, endocrine disruptors form a poorly explored class of environmental toxins with respect to public awareness and scientific studies.
Possible implications of TDs on the host depend upon the phase of development of the host and role of thyroid hormones during that particular phase. Thus, exposure to TDCs in adulthood causes an array of ill-effects different from their in-utero actions. Thyroid hormones play a very important role in development of nervous and auditory systems. Thus exposure to TDCs during pregnancy and early years of life can lead to neurodevelopmental problems leading to low IQ scores, cognitive and behavioral defects and deafness in such children.[2] In adults, thyroid hormones are responsible for maintenance of cellular metabolism and cardiovascular functions. Exposure to TDCs in adults has been linked to thyroid autoimmune disorders, thyroid neoplasms, subclinical and clinical hypothyroidism and increased cardiovascular risk due to altered lipid metabolism [Table 1].[2] In addition to developmental phase, other factors like state of iodine repletion can modify effects of exposure to thyroid disruptors. Thyroid homeostasis is least affected on exposure to benzophenones and other goitrogenic thyroid peroxidase inhibitors during iodine repletion; however, cause hypothyroidism due to significant inhibition of thyroid peroxidase in presence of iodine deficiency.[3,4] Moreover, exposure to multiple thyroid disruptors may be more harmful. TDs are widely distributed and there are higher chances of people coming into contact with multiple TDs simultaneously. The combined effects of multiple exposures often have synergistic action on thyroid axis, however, surprisingly, antagonism has also been reported.[5]
Table 1.
Thyroid disrupting chemicals, their source, mechanisms of thyroid disruption and implications on the host
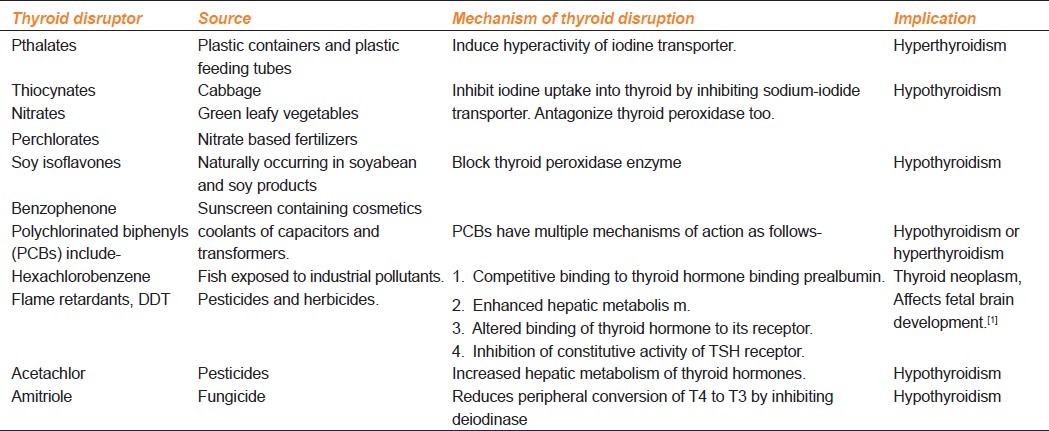
Most of the thyroid disruptors and their mechanisms have been well studied in vitro on special endocrine cell lines or recombinant enzymes and in animal experiments. These led to controversies regarding possible clinical impact of these endocrine disruptors on human endocrine systems. However, data from epidemiological studies in humans exposed either accidentally or occupationally to TDs provides conclusive evidence of alteration of hormonal milieu by these agents.[2]
In conclusion, benefits of studying the implications of TDs with respect to human ecosystem helps in correlation of risk of thyroid disorders and its clinical impact. Additionally, dissemination of such information helps in public education to minimize exposure to TDs by adopting measures like avoiding plastic materials for food and water storage and consumption, and optimizing diet by balancing the risk of thyroid disruption induced by soy and fish with their nutritional benefits.
References
- 1.Lyn Patrik ND. Thyroid disruption: Mechanisms and clinical implications in human health. Altern Med Rev. 2009;14:326–46. [PubMed] [Google Scholar]
- 2.Korrick SA, Sagiv SK. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr. 2008;20:198–204. doi: 10.1097/MOP.0b013e3282f6a4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmutzler C, Bacinski A, Gotthardt I, Huhne K, Ambrugger P, Klammer H, et al. The ultraviolet filter benzophenone 2 interferes with the thyroid hormone axis in rats and is a potent in vitro inhibitor of human recombinant thyroid peroxidase. Endocrinology. 2007;148:2835–44. doi: 10.1210/en.2006-1280. [DOI] [PubMed] [Google Scholar]
- 4.Chandra AK, Mukhopadhyay S, Lahari D, Tripathy S. Goitrogenic content of Indian cyanogenic plant foods & their in vitro anti-thyroidal activity. Indian J Med Res. 2004;119:180–5. [PubMed] [Google Scholar]
- 5.Crofton KM. Thyroid disrupting chemicals: Mechanisms and mixtures. Int J Androl. 2008;31:209–23. doi: 10.1111/j.1365-2605.2007.00857.x. [DOI] [PubMed] [Google Scholar]


