Abstract
Background
Although angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) are equally important in the treatment of hypertension, there is less evidence whether they have equal cardiovascular and cerebrovascular protective effects, especially in elder hypertensive patients. This study aims to clarify this unresolved issue.
Methods
This cross-sectional study included clinical data on 933 aged male patients with hypertension who received either an ARB or ACEI for more than two months between January 2007 and May 2011. The primary outcome was the composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. The secondary endpoints were unstable angina, new atrial fibrillation, and transient ischemic attack.
Results
The median follow-up time was 24 months. Age, drug types, cerebral infarction history, renal dysfunction history were the independent predictors of the primary endpoint. The risk of an occurrence of a primary endpoint event was higher in the ARB group than the ACEI group [P = 0.037, hazard ratios (HR): 2.124, 95% confidence interval (95% CI): 1.048–4.306]. The Kaplan-Meier method also suggests that the rate of primary endpoint occurrence was higher in the ARB group than the ACEI group (P = 0.04). In regard to the secondary endpoints, there were no significant differences between the two treatment arms (P = 0.137, HR: 1.454, 95% CI: 0.888–2.380). Patient age and coronary heart disease history were independent predictors of the secondary endpoint.
Conclusion
ACEI were more effective than ARB in reducing cardiovascular and cerebrovascular morbidity and mortality in aged patients with hypertension.
Keywords: Angiotensin receptor blocker, Angiotensin converting enzyme inhibitor, Renin-angiotensin-aldosterone system, Angiotensin type 2 receptor
1. Introduction
Activation of the renin-angiotensin-aldosterone system (RAAS) plays an important role in both the pathophy-siology of hypertension and atherosclerotic vascular disease. Angiotensin receptor blockers (ARB) attenuate RAAS activation by competitively inhibiting the binding of angiotensin II (Ang II) to Ang II type I receptor (AT1R). Thus far, reports suggest that ARB also have many clinical benefits, including effective blood pressure lowering,[1]–[3] improvements in left ventricular remodeling of infarcted hearts,[4],[5] inhibition of diabetic renal disease,[6],[7] and reduction in stroke rates.[8]
The American College of Cardiology/American Heart Association (ACC/AHA) Guidelines recommend the use of angiotensin converting enzyme inhibitors (ACEI) for the treatment of heart failure, left ventricular dysfunction, myocardial infarction, diabetic nephropathy, left ventricular hypertrophy, carotid atherosclerosis, proteinuria or microalbum-inuria, atrial fibrillation, and metabolic syndrome. In the 2007 European Society of Cardiology/European Society of Hypertension (ESC/ESH) Guidelines, the recommended use of ARB beyond the treatment of hypertension expanded to include heart failure and post-myocardial infarction. In the clinic, ARB are typically used as an alternative to ACEI, primarily in elderly patients, as they are intolerant of its side effects, e.g., coughing. Although both classes of drugs are equally important in antihypertensive treatment, there is less evidence on whether they have equal cardiovascular and cerebrovascular protective effects. In this study, we compared the effects of ARB and ACEI on cardiovascular and cerebrovascular morbidity and mortality in aged patients with hypertension and assessed other corresponding prognostic factors.
2. Methods
2.1. Patients
Male patients aged 65 years or older with established hypertension were enrolled between January 2007 and May 2011 at the Chinese PLA General Hospital. The diagnostic criteria used for hypertension were according to the definition used by the World Health Organization and World Hypertension League (i.e., systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg at any three clinic visits). All of the enrolled patients have been taking either an ARB or ACEI for more than two months. The exclusion criteria were as follows: a history of using both ARB and ACEI, secondary hypertension, acute myocardial infarction, percutaneous transluminal coronary angioplasty or coronary artery bypass graft within the past three months, clinically relevant valvular disease, a cerebrovascular incident in the past three months, severe hepatic disease, severe chronic renal failure, hyperthyroidism, primary cardiomyopathy, pulmonary heart disease, malignancy, rheumatism, connective tissue disease, or multiple organ failure. A total of 933 cases were selected, including 348 cases in the ACEI group and 585 cases in the ARB group.
2.2. Outcome measures
The primary endpoint was the first occurrence of a cardiac event, an event defined as either sudden cardiac death, fatal myocardial infarction, or non-fatal myocardial infarction. The pre-defined secondary endpoints were the occurrence of unstable angina, new onset atrial fibrillation, or transient ischemic attack.
2.3. Data analysis
Comparisons of baseline values between the two treatment groups were performed with the use of the Chi-square test (for categorical data) or t test (for continuous data). Cox regression models were used to assess differences in clinical events between the treatment arms. Patient age, body mass index, a history of smoking, the presence of coronary heart disease, diabetes mellitus, cerebral infarction, hyperlipidemia, cardiac insufficiency, and renal inadequacy at baseline were used as a priori covariates to account for the effects of key risk predictors in the study. Treatment effects were determined with hazard ratios (HR) and their 95% confidence interval (95% CI) based on the Cox regression models. Event rates over time were presented as Kaplan-Meier curves. Only the time to the first cardiac event was considered in the primary endpoint. For secondary endpoint analyses, only the first event was counted in each category. All tests were two-sided and the significance level was set at 5%.
3. Results
3.1. Comparison of baseline characteristics
The median follow-up time of this cross-sectional study was 24 months. Patient characteristics at baseline of the two treatment groups were well balanced, with the exception of diabetes mellitus history (ARB 46.0% vs. ACEI 33.6%, P = 0.02) and renal dysfunction (ARB 14.2% vs. ACEI 10.7%, P = 0.038), (Table 1).
Table 1. Baseline characteristics of the two groups.
| Clinical characteristics | ARB group (n = 585) | ACEI group (n = 384) | P |
| Age, yrs | 78.1 ± 10.7 | 78.7 ± 11.3 | 0.418 |
| Body mass index, kg/m2 | 24.7 ± 4.2 | 24.8 ± 10.1 | 0.949 |
| Smoking | 99 (16.9%) | 76 (19.8%) | 0.065 |
| Coronary heart disease history | 370 (63.2%) | 231 (60.2%) | 0.353 |
| Diabetes mellitus history | 269 (46.0%) | 129 (33.6%) | 0.020 |
| Cerebral infarction history | 129 (22.1%) | 96 (25.0%) | 0.066 |
| Hyperlipidemia | 117 (20%) | 56 (14.6%) | 0.108 |
| Cardiac dysfunction | 45 (7.6%) | 28 (7.0%) | 0.846 |
| Renal dysfunction | 83 (14.2%) | 41 (10.7%) | 0.038 |
Data are shown as n (%) or mean ± SD. ACEI: angiotensin II converting enzyme inhibitor; ARB: angiotensin receptor blocker.
3.2. The primary endpoint
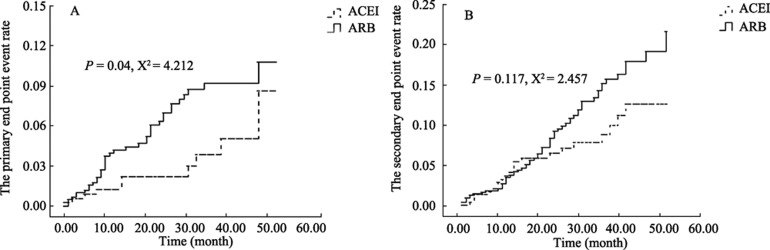
The primary endpoint (i.e., cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) was present in 38 patients (6.5%) in the ARB group and 10 patients (2.6%) in the ACEI group (HR: 2.124, 95% CI: 1.048– 4.306, P = 0.037, ARB vs. ACEI). Three patients (0.5%) on ARB therapy, but none on ACEI therapy, died from a cardiovascular event. A non-fatal myocardial infarction occurred in 16 patients on ARB therapy (2.7%) and seven patients on ACEI (1.8%) therapy. A non-fatal stroke occurred in 19 patients (3.3%) on ARB therapy and three patients on ACEI (0.7%) therapy. The Kaplan-Meier curves for the primary endpoint are shown in Figure 1A (log rank test, P = 0.04). Cox regression models confirm that, in addition to ARB or ACEI therapy, patient age (HR 1.040, 95% CI: 1.003–1.078, P = 0.034), cerebral infarction history (HR: 2.239, 95% CI: 1.257–3.988, P = 0.006), and renal dysfunction (HR: 1.423, 95% CI 1.025–1.976, P = 0.035) were independent predictors of the primary endpoint (Table 2).
Figure 1. Kaplan-Meier curves for the primary (A) and secondary (B) endpoints.
The dashed line denotes the ACEI group and the solid line denotes the ARB group. ACEI: angiotensin II converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Table 2. Cox multiple factors regression analyses for independent risk factors of the primary and secondary endpoints.
| Variables | Primary endpoint |
Secondary endpoint |
||||
| P | HR | 95% CI | P | HR | 95% CI | |
| Age | 0.034 | 1.040 | 1.003–1.078 | 0.031 | 1.028 | 1.003–1.054 |
| Body mass index | 0.506 | 0.981 | 0.926–1.039 | 1 | 1 | 0.970–1.030 |
| Smoking | 0.265 | 1.472 | 0.746–2.906 | 0.648 | 1.138 | 0.653–1.984 |
| Drug type (ARB or ACEI) | 0.037 | 2.124 | 1.048–4.306 | 0.137 | 1.454 | 0.888–2.380 |
| Coronary heart disease | 0.660 | 1.127 | 0.661–1.923 | 0.001 | 1.913 | 1.320–2.772 |
| Diabetes mellitus | 0.582 | 1.174 | 0.663–2.080 | 0.258 | 1.29 | 0.830–2.005 |
| Cerebral infarction | 0.006 | 2.239 | 1.257–3.988 | 0.778 | 0.928 | 0.552–1.560 |
| Hyperlipidemia | 0.233 | 0.560 | 0.216–1.453 | 0.156 | 0.599 | 0.295–1.215 |
| Cardiac dysfunction | 0.859 | 1.089 | 0.425–2.793 | 0.274 | 0.621 | 0.265–1.457 |
| Renal dysfunction | 0.035 | 1.423 | 1.025-1.976 | 0.416 | 0.837 | 0.545–1.286 |
ACEI: angiotensin II converting enzyme inhibitor; ARB: angiotensin receptor blocker.
3.3. Secondary endpoint
The secondary endpoint occurred in 62 patients (10.6%) in the ARB group and 22 patients (5.7%) in the ACEI group (HR: 1.138, 95% CI: 0.888–2.380, P = 0.137, ARB vs. ACEI). A non-fatal myocardial infarction occurred in 49 patients on ARB (8.4%) and 16 patients on ACEI (4.2%) therapy. A non-fatal stroke occurred in five patients on ARB (0.9%) and three patients on ACEI therapy (0.8%). A new atrial fibrillation occurred in eight patients on ARB (1.4%) and three patients on ACEI (0.8%) therapy. The Kaplan-Meier curves for the secondary endpoints are shown in Figure 1B (log rank test, P = 0.117). Cox regression models suggest that age (HR: 1.028, 95% CI: 1.003–1.054, P = 0.031) and a history of coronary heart disease (HR: 1.913, 95% CI: 1.320–2.772, P = 0.006) are independent predictors of the secondary endpoint (Table 2).
4. Discussion
A number of clinical trials have previously established the efficacy of ACEI in the management of chronic heart failure, acute myocardial infarction, post-myocardial infarction, secondary prevention of coronary heart disease, and hypertension. Conversely, ARB treatment still lacks such conclusive evidence in cardiovascular protection. Of the two classes of RAAS inhibitors, ARB are not the first choice clinically. In the VALUE trial,[1] the ARB, valsartan, produced a statistically significant and relative increase of 19% in the occurrence of myocardial infarction (fatal and non-fatal) compared to amlodipine. In the SCOPE trial,[3] candesartan was associated with a non-significant decrease in fatal plus non-fatal myocardial infarctions. In the RENAAL trial,[9] the composite of morbidity and mortality from cardiovascular causes in the losartan group was not improved compared to the placebo group.
In comparison studies of ACEI and ARB in the ELTTE II study, Pitt et al.[10] found that losartan and captopril both improved the outcomes of elderly heart failure patients. However, in the OPTIMAAL trial,[11] losartan was not superior to the ACE inhibitor, captopril, in decreasing all-cause mortality in high-risk patients after an acute myocardial infarction. In fact, there were more cardiovascular deaths in the losartan group. In the ONTARGET trial,[12] telmisartan was no less favorable than ramipril in reducing cardiovascular mortality, myocardial infarction, stroke, and congestive heart failure. Additionally, telmisartan was better tolerated than ramipril. In the TRANSCEND trial,[13] one of the secondary outcomes—a composite of cardiovascular death, myocardial infarction, or stroke—occurred in 384 (13.0%) patients on telmisartan compared to 440 (14.8%) on placebo (HR: 0.87, 95% CI: 0.76–1.00, P = 0.048 unadjusted). However, after adjustment for multiplicity of comparisons and overlap with the primary outcome, there were no significant differences (P = 0.068).
In elderly patients with hypertension, our cross-sectional study demonstrated that age, drug type, history of cerebral infarction, or renal dysfunction were independent predictors of the primary endpoint, namely the composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. The risk of a primary endpoint was higher in the ARB group than the ACEI group (RR: 2.124, 95% CI: 1.048–4.306, P = 0.037).
Both ACEI and ARB target the RAAS via different mechanisms of action. ACEI prevents the conversion of Ang I to Ang II, whereas ARB selectively prevents the binding of Ang II to the AT1R. Both classes of drugs block the detrimental effects of Ang II overproduction on the cardiovascular system. However, ACEI has some advantages over ARB in its pharmacological mechanism of action. Research suggests that ACEI increases Ang (1–7) levels 10-25 fold,[14] which is one of the important biological active substances in the RAAS. As a substrate for ACE, Ang (1–7) competes with Ang I and bradykinin (BK) for degradation, thereby inhibiting Ang II formation and augmenting BK activity. It is generally accepted that ARB preferentially blocks AT1R, and stimulates angiotensin type 2 (AT2R) and angiotensin IV receptors (AT4R).[15]–[19] In contrast to the AT1R, the signal pathways promoted by AT2R are far from being resolved. Studies found that AT2R stimulation engenders an autacoid vasodilator cascade composed of BK, nitric oxide (NO), and guanosine cyclic 3′,5′-monophosphate (cGMP).[20]–[22] Therefore, it is generally assumed that AT2R activation exerts opposite effects to those of AT1R activation.[23],[24] Thus, stimulation of the AT2R during AT1 blockade with an ARB would result in beneficial responses. Unfortunately, data suggest that AT2R stimulation may be less beneficial than previously proposed and may even be harmful under certain circumstances through the mediation of proatherogenic and proinflammatory effects. Levy[16] reported that multiple hormonal, cytokine, and metabolic factors are involved in up-regulating the AT2R density on cell surfaces. Evidence in human myocytes suggests that Ang II may promote plaque rupture by augmenting matrix metalloproteinase-1 in an AT2-dependent fashion.[25] Ruiz-Ortega et al.[26] demonstrated that Ang II infusion for 72 h increases the number of glomerular and interstitial inflammatory cells. Interestingly, an AT2R antagonist, but not losartan, significantly reduces this inflammatory upregulation. Moreover, the activation of AT4R promotes the release of prothrombotic PAI-1, which can be linked to the occurrence of acute coronary syndromes.[27]
In the present study, in addition to drug type, we identified age, a history of cerebral infarction, and renal dysfunction as the independent predictors of the primary endpoint. Furthermore, age and a history of coronary heart disease were identified as independent predictors of secondary endpoints. These findings suggest that older patients who have vascular lesions in their vital organs are more susceptible to cardiovascular and cerebrovascular events. These results corroborate those of the ROADMAP trial.[28] In that trial, although the ARB (olmesartan) produced a significant reduction in the risk of micro-albuminuria in subjects with type 2 diabetes, fatal cardiovascular events were higher among patients with pre-existing coronary heart disease. Chronic kidney dysfunction (CKD) is also likely a risk factor of atherosclerosis. There is a linear relationship between decreases in glomerular filtration rate and risk of cardiovascular events. Takahashi et al. [29] found that renal dysfunction was related to cerebrovascular disease. Atherosclerotic lesions were significantly more frequent in subjects with CKD stage 3 than in stage 1 or 2. Yiu et al.[30] suggested that moderate CKD was associated with cardiovascular events.
In summary, ACEI appears to have more protective effects than ARB in elderly populations. In the aging vasculature, increased arterial wall thickening and vascular inflammation contribute to vascular dysfunction, which accelerate the progression of coronary heart disease and stroke. Additionally, the heterogenicity of the two types of drugs is a very important consideration. For instance, ACEI is known to increase Ang (1–7) levels and inhibit the degradation of BK, which may result in a more beneficial response than ARB.
However, both drugs have advantages and disadvantages. The use of ACEI is limited, particularly in elderly patients, due to their poor tolerance to its side effects. Thus, the replacement frequency of ACEI is much higher than that of ARB. However, in our cross-sectional study, we found that elderly hypertensive patients were more susceptible to the primary endpoint on ARB therapy. Thus, not only is there biological plausibility, but also the available clinical evidence suggests that ARB are indeed inferior to ACEI with respect to cardiovascular protection. When clinicians are faced with the decision of choosing either an ACEI or ARB in high-risk patients, they should be cognizant of the unique differences between these two medications. Accordingly, ACEI should be the first choice for a spectrum of cardiovascular risks.
There are a few limitations in this study that need to be addressed. First, this was a retrospective cohort study, and a more rigorous prospective, controlled, randomized study needs to be designed to compare the two classes of drugs. Second, we focused on cardiovascular and cerebrovascular morbidity and mortality, and changes in blood pressure were not included as an effective index in reducing the outcomes. Last, to determine and compare the effects of age-related factors with these treatment modalities, a study on a younger cohort is warranted.
Acknowledgments
This work was supported by the Fund of the Ministry of Science and Technology of China (2009BAI86B04). All authors had full access to the data and take full responsibility for their integrity. All authors agree and approve the content of the manuscript.
References
- 1.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 3.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui H, Matsushima S, Kinugawa S, et al. Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Hypertens Res. 2007;30:439–449. doi: 10.1291/hypres.30.439. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, Solomon SD. Angiotensin II receptor blockade and ventricular remodelling. J Renin Angiotensin Aldosterone Syst. 2005;6:43–48. doi: 10.3317/jraas.2005.006. [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 8.Thone-Reineke C, Steckelings UM, Unger T. Angiotensin receptor blockers and cerebral protection in stroke. J Hypertens Suppl. 2006;24:S115–S121. doi: 10.1097/01.hjh.0000220416.07235.37. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial-the Losartan Heart Failure Survival study (ELITE II) Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. OPTIMAAL randomized trial. Optimal Trial in Myocardial infarction with Angiotensin II antagonist Losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 12.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Kladis A, Duncan AM. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- 15.Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 2002;1:621–636. doi: 10.1038/nrd873. [DOI] [PubMed] [Google Scholar]
- 16.Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin system. Circulation. 2004;109:8–13. doi: 10.1161/01.CIR.0000096609.73772.C5. [DOI] [PubMed] [Google Scholar]
- 17.Mogi M, Iwai M, Horiuchi M. Emerging concept of adipogenesis regulation by the renin-angiotensin system. Hypertension. 2006;48:1020–1022. doi: 10.1161/01.HYP.0000248196.14826.31. [DOI] [PubMed] [Google Scholar]
- 18.Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 19.Volpe M, Tocci G, Savoia C. Angiotensin II receptor blockers and coronary artery disease: ‘presumed innocents’. Eur Heart J. 2006;27:1506–1507. doi: 10.1093/eurheartj/ehi892. [DOI] [PubMed] [Google Scholar]
- 20.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siragy HM, Jaffa AA, Margolius HS, et al. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol. 1996;271:R1090–R1095. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 22.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 24.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 25.Kim MP, Zhou M, Wahl LM. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collet JP, Montalescot G, Vicaut E, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108:391–394. doi: 10.1161/01.CIR.0000083471.33820.3C. [DOI] [PubMed] [Google Scholar]
- 28.Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or preventionof microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi W, Tsukamoto Y, Ohnuki T, et al. Is mild renal dysfunction a risk factor for carotid atherosclerosis in apparently healthy adults? Intern Med. 2011;50:2285–2289. doi: 10.2169/internalmedicine.50.5725. [DOI] [PubMed] [Google Scholar]
- 30.Yiu KH, de Graaf FR, Schuijf JD, et al. Prognostic value of renal dysfunction for the prediction of outcome versus results of computed tomographic coronary angiography. Am J Cardiol. 2011;108:968–972. doi: 10.1016/j.amjcard.2011.05.031. [DOI] [PubMed] [Google Scholar]