Abstract
Objectives
This study examined the protective effect of salubrinal and the mechanism underlying this protection against tunicamycin (TM)- and hypoxia-induced apoptosis in rat cardiomyocytes.
Methods
Neonatal rat cardiomyocytes were cultured from the ventricles of 1-day-old Wistar rats. Cells were exposed to different concentrations of salubrinal (10, 20, and 40 µmol/L) for 30 min followed by TM treatment or hypoxia for 36 h. Apoptosis was measured by a multiparameter HCS (high content screening) apoptosis assay, TUNEL assay and flow cytometry. The phosphorylation of eukaryotic translation initiation factor 2 subunit alpha (eIF2α) and the expression of cleaved caspase-12 were determined by Western blotting. C/EBP homologous protein (CHOP) was detected by immunocytochemistry.
Results
HCS, TUNEL assays and flow cytometry showed that salubrinal protected cardiomyocytes against apoptosis induced by TM or hypoxia. Western blotting showed that salubrinal protected cardiomyocytes against apoptosis by inducing eIF2α phosphorylation and down-regulating the expression of the endoplasmic reticulum stress-mediated apoptotic proteins, CHOP and cleaved caspase-12.
Conclusions
Our study suggests that salubrinal protects rat cardiomyocytes against TM- or hypoxia-associated apoptosis via a mechanism involving the inhibition of ER stress-mediated apoptosis.
Keywords: Endoplasmic reticulum stress, Rat cardiomyocytes, Apoptosis, Salubrinal, Cell protection
1. Introduction
Cardiovascular disease is one of the leading causes of death worldwide. Hypoxia is generally associated with cardiovascular diseases, and it elicits a variety of functional responses in cardiomyocytes, including cell proliferation, cell hypertrophy and cell death.[1],[2] Hypoxia can induce cell death by increasing Ca2+ or the production of free radicals. However, various therapies that target free radicals or maintain intracellular Ca2+ homeostasis have little effect on hypoxia.[3] Thus, the development of new drugs and the discovery of novel mechanisms for treating hypoxia-induced injury are required. Evidence obtained in recent years has demonstrated that endoplasmic reticulum stress (ERS)- mediated cell apoptosis plays an important role in cardiac hypoxia injury.[4] Therefore, targeting ERS may provide a therapeutic approach for blocking the pathological process induced by heart hypoxia.
The ER is a cellular organelle that directs the folding of secretory membrane proteins.[5] Tunicamycin (TM) is an ER glycosylation inhibitor that impairs the synthesis of proteins in the ER. Similar to TM treatment, hypoxia injury also impairs key ER functions.[6] Both can activate the evolutionarily conserved unfolded protein response (UPR), which adapts efficiently to changes in the cellular environment to promote the expression of gene products that enhance protein folding within the ER and the ER-mediated removal of misfolded proteins.[7]–[9] However, prolonged ER stress triggers cell apoptosis.[10],[11] ERS-induced cell death has been shown to involve the activation of caspase-12 and C/EBP homologous protein (CHOP).[12],[13]
Salubrinal is a selective inhibitor of eIF2α dephosphorylation that was recently developed as a protective agent against ERS-mediated apoptosis.[14] eIF2α must be dephosphorylated to enable the translation of new proteins. Salubrinal attenuates unfolded or misfolded protein synthesis by inhibiting eIF2α dephosphorylation, thus rescuing cells from apoptosis. In neurons, salubrinal can reduce the load of mutant or mislocated proteins retained in the ER under conditions associated with neurodegeneration.[15],[16] In rat kidneys, salubrinal has been used to reduce cyclosporine nephrotoxicity, which can induce ERS.[17] Thus, we hypothesized that salubrinal may be capable of protecting against TM- and hypoxia-mediated apoptosis in cardiomyocytes.
In this study, we investigated the protective effect of salubrinal on rat cardiomyocytes and the mechanism underlying this protection.
2. Methods
2.1. Cell culture and treatments
Primary cultures of neonatal rat cardiomyocytes were isolated from the ventricles of 1-day-old Wistar rats (Academy of Military Medical Sciences, China). All experimental protocols complied with the recommendations of the Institutional Animal Care and Use of Laboratory Animals of Chinese PLA General Hospital. The rats were euthanized and their hearts were excised. After scalpel homogenization, hearts were digested with 0.125% trypsin and 0.1% collagenase II (4: 1) at 37°C for 10 min, and the digestion was repeated 4–5 times. Isolated cells were collected by centrifugation and resuspended in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units/mL penicillin and 100 µg/mL streptomycin. Cultures were enriched with myocytes by pre-plating for 90 min to deplete the population of non-myocytes. Non-attached cells were plated onto plastic culture dishes at an appropriate cell density. The cells were cultured at 37°C in 95% air/5% CO2 for 24 h, and half of the medium was then exchanged with DMEM containing 10% FBS and 0.1% BrdU. The culture medium was changed every 48 h. After 4 days, the culture medium was exchanged with fresh DMEM containing 1% FBS and the cells were cultured for 24 h. Subsequently, the cells were incubated in normal or a completely hypoxic (N2/CO2, 95%/5%) culture chamber. The cells were subjected to completely hypoxic conditions in the chamber at 37°C for 36 h, while the controls were left in normoxic conditions at 37°C for the same time periods. The medium was exchanged with fresh DMEM containing 1% FBS one hour before exposure to TM or hypoxia to obtain consistent ERS response data. All animal experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All reagents were from Gibco (Carlsbad, USA) unless otherwise stated.
2.2. Methylthiazoletetrazolium (MTT) assay
Cell viability was assessed by MTT (Amresco, USA) assay following treatment with TM or hypoxia. Briefly, purified neonatal rat cardiomyocytes were seeded in a 96-well plate at 104 cells per well and treated with different concentrations of salubrinal (Merck, Germany). Salubrinal was dissolved in dimethylsulfoxide (DMSO, Amresco, USA). Ten microliters of 5 mg/mL MTT were added to each well for 4 h, and the supernatants were then removed and replaced with 100 µL of DMSO. After oscillation for 10 min, the absorbance value (A) of each well was measured at 550 nm with a Universal Microplate Reader (Bio Tek Instruments, USA). The relative number of viable cells was determined by comparison to untreated cells, in which viability was assumed to be 100%.
2.3. Cell viability assay
The bioluminescence of adenosine triphosphate (ATP) was used as a marker of cell proliferation and viability. The amount of ATP present was estimated using the CellTiter- Glo® Luminescent Cell Viability Assay kit (Promega, USA) according to the manufacturer's protocol. Briefly, purified neonatal rat cardiomyocytes (104 cells/well) were seeded in a 96-well plate, treated with different concentrations of TM dissolved in DMSO, or incubated in a hypoxic culture chamber (N2/CO2, 95%/5%) for different times. Different concentrations of salubrinal (10, 20 or 40 µmol/L) were added to the cells 30 min before treatment with TM or incubation in the hypoxic culture chamber (N2/CO2, 95%/ 5%). We then added a volume of CellTiter-Glo® Reagent equal to the volume of the cell culture medium present in each well. The contents were mixed for two minutes on an orbital shaker to induce cell lysis, and then incubated at room temperature for 10 min to stabilize the luminescent signal. The absorbance value of each well was measured on a Microplate Reader (Bio Tek Instruments, USA) at 562 nm. The relative number of viable cells was determined by comparison to untreated cells, in which viability was assumed to be 100%.
2.4. TUNEL staining
Purified neonatal rat cardiomyocytes were inoculated at a concentration of 106 cells/well in a 6-well coverslip slide and treated with different concentrations of salubrinal (10, 20, 40 µmol/L) for 30 min before treatment with TM. The cells were then fixed in 4% paraformaldehyde for 15 min at room temperature. After fixation, cells were washed with phosphate buffered saline (PBS) and incubated in a 3% H2O2-methanol solution for 10 min at room temperature, and subsequently incubated with 0.1% Triton X-100 for two minutes at 4°C. Slides were rinsed twice with PBS, and the TUNEL (Roche, Switzerland) reaction mixture was then added. The slides were covered and incubated for 60 min at 37°C in a humidified chamber in the dark. Anti-fluorescein antibody was added, followed by incubation for 30 min at 37°C in a humidified chamber. The slides were rinsed three times with PBS, treated with DAB Novolink™ Polymer Detection System for 10 min at room temperature, and then stained with hematoxylin. The samples were analyzed by light microscopy.
2.5. Flow cytometry
An Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from KeyGEN Biotech (USA). Purified neonatal rat cardiomyocytes were seeded at a concentration of 106 cells/well in a 6-well plate, and the indicated concentrations of salubrinal (10, 20 or 40 µmol/L) were added 30 min before treatment with TM. Cells were washed twice with PBS and resuspended in binding buffer. FITC-Annexin V and propidium iodide were added according to the manufacturer's protocol. The mixture was incubated for 10 min in the dark at room temperature, and cellular fluorescence was then measured by FACS scan flow cytometry (Becton Dickinson and Company, Franklin Lakes, NJ, USA).
2.6. Multiparameter apoptosis evaluation
Cellomics® Multiparameter Apoptosis Kits were purchased from Thermo Scientific (USA). Purified neonatal rat cardio- myocytes were seeded at a concentration of 104 cells/well in a 96-well assay plate (a black plate with a clear bottom). Cells were treated with different concentrations of salubrinal (10, 20 or 40 µmol/L) 30 min before treatment with TM or incubation in a hypoxic culture chamber (N2/CO2, 95%/5%). Thirty minutes after the incubation was complete, MitoTracker/Hoechst Solution was added to the cells, and the cells were incubated for 30 min at 37°C. The fixation solution was added without removing the medium, and the cells were incubated in a fume hood at room temperature for 10 min. After rinsing with PBS, the cells were permeabilized with permeabilization buffer and incubated for 15 min. Cells were incubated with Alexa fluor 488 Phalloidin Solution for 30 min at room temperature in the dark. The solution was aspirated and the plate was washed three times with PBS, with the last wash being left in the wells. Nuclear fluorescence, actin cytoskeleton fluorescence and mitochondrial membrane potential were evaluated using an ArrayScan HSC Reader (General Electric Company, USA).[18] The ArrayScan HSC Reader platform was equipped with an inverted Nikon Eclipse TE2000-U microscope, a 100 W Xenon lamp, a 20 ELWD Plan Fluor objective 0.45 numerical aperture, and a 12-bit Photometrics CoolSNAP camera. Hoechst 33342 stained images were acquired using 350/40 nm excitation and 461/40 nm emission filters with a 100 ms exposure time. Alexa fluor 488 fluorescence images were acquired using 495/20 nm excitation and 519/50 nm emission filters with a 400-ms exposure time. MitoTracker Red stained images were acquired using 579/20 nm excitation and 599/50 nm emission filters with a 400 ms exposure time. The ArrayScan HSC Reader was set up to acquire five fields of view.
2.7. Western blot analysis
Purified neonatal rat cardiomyocytes were seeded at a concentration of 106 cells/well in a 6-well plate and treated with different concentrations of salubrinal (10, 20 or 40 µmol/L) 30 min before treatment with TM or incubation in a hypoxic culture chamber (N2/CO2, 95%/5%). Cells were lysed in whole cell lysis buffer [62.5 mmol/L Tris-HCl (pH 6.8 at 25°C], 2% w/v SDS (sodium dodecyl sulfate), 10% glycerol, 50 mmol/L DTT (dithiothreitol)), and the homoge- nates were heated at 100°C for 10 min, then centrifuged at 12,000 r/min for 10 min at 4°C. The supernatants were used as protein samples, and their concentrations were determined using the BCA (bicinchoninic acid) Protein Assay Kit (Pierce, USA). Cellular proteins were separated by electrophoresis on 10% SDS polyacrylamide gels and transferred to PVDF membranes. After blocking in 1 × PBS containing 0.1% Tween-20 and 5% w/v nonfat dry milk, the membranes were incubated with antibodies to caspase-12 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (both from Santa Cruz Biotechnology, 1: 200, rat monoclonal), phospho-eIF2α, eIF2α and β-actin (all from Cell Signaling Technology, 1: 1000, rabbit polyclonal) with gentle agitation overnight at 4°C. Membranes were then incubated with the appropriate horseradish peroxidase conjugated secondary antibodies (Zhongshan Golden Bridge Biotechnology Company, China) at a 1: 5000 dilution and incubated for 1 h at room temperature. The signal was developed with a color developing agent and the film was then scanned on an Alpha Imager 5500 (Alpha Innotech, USA) imaging densitometer. The optical density was quantified using Multi- Analyst software.
2.8. Immunocytochemistry
Purified neonatal rat cardiomyocytes were seeded at a concentration of 104 cells/well in a 96-well assay plate (a black plate with a clear bottom). Cells were treated with different concentrations of salubrinal (10, 20 or 40 µmol/L) 30 min before treatment with TM or incubation in a hypoxic culture chamber (N2/CO2, 95%/5%). Cells were fixed in 4% paraformaldehyde for 15 min at room temperature. After rinsing with PBS, cells were blocked in Blocking Buffer (1 × PBS containing 0.3% Triton X-100 and 5% normal serum from the same species as the secondary antibody) for one hour at room temperature. The blocking solution was removed and diluted CHOP primary antibody was added (Cell Signaling Technology, 1: 1600, mouse monoclonal) and incubated overnight at 4°C. The cells were then stained with Hoechst 33342 nuclear dye (3 µmol/L) and a fluorochrome-conjugated goat anti-mouse IgG secondary antibody (H&L) (DyLight™ 594; Thermo scientific, 1: 500), and incubated for one hour at room temperature in the dark. The secondary antibody was aspirated and the plate was washed three times with PBS, with the last wash being left in the wells. Hoechst and DyLight 594 were visualized using the ArrayScan HSC Reader (General Electric Company, USA). Hoechst dye was acquired using 350/40 nm excitation and 461/40 nm emission filters with a 100 ms exposure time. DyLight 594 fluorescence images were acquired using 535/20 nm excitation and 620/ 50 nm emission filters with a 400 ms exposure time. The ArrayScan HSC Reader was set up to acquire five fields of view.
2.9. Statistical analysis
Each experiment was performed on a minimum of three different cultures and was repeated at least three times; the ArrayScan immunocytochemistry results shown are from one experiment that is representative of the results from three replicate experiments, and the other results are the compiled data derived from at least three different experiments. Values are shown as the mean ± SD. Statistical analyses were performed using One-way ANOVA. P < 0.05 were considered significant.
3. Results
3.1. Effect of salubrinal on TM- and hypoxia-induced injury in rat cardiomyocytes
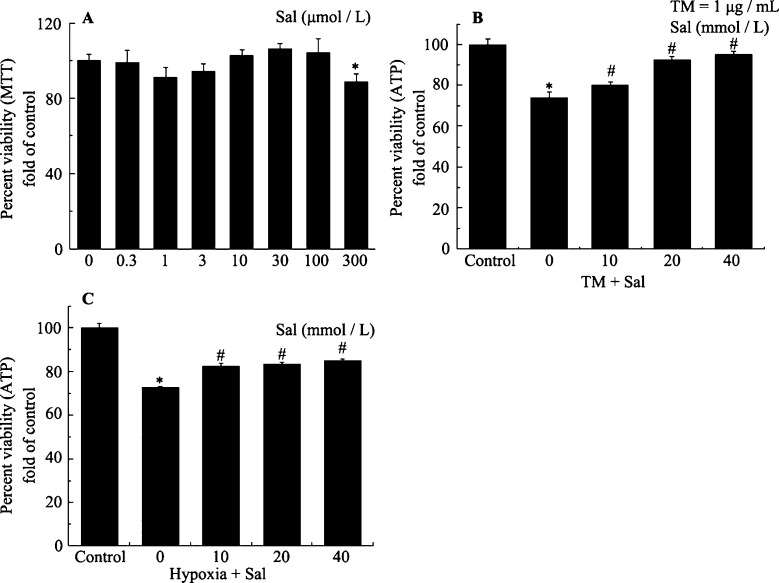
Figure 1A shows the cytotoxicity of salubrinal towards cultured rat neonatal cardiomyocytes. High concentrations of up to 100 µmol/L showed no toxicity against neonatal rat cardiomyocytes (Figure 1A). Figure 1B and 1C show the protective effects of salubrinal using the CellTiter-Glo® Luminescent Cell Viability Assay kit. The ATP signals showed that compared with TM treatment or hypoxia only, pretreatment with salubrinal (10, 20 or 40 µmol/L) markedly reduced the number of dead cells. Salubrinal provided maximum protection against TM- or hypoxia-induced toxicity at a concentration of 40 µmol/L (Figure 1B and 1C). These results showed that salubrinal can protect rat cardiac muscle cells against TM- and hypoxia-induced injury.
Figure 1. Salubrinal mediates concentration-dependent protection against cytotoxicity in cardiomyocytes.
(A): Cultured neonatal rat cardiomyocytes were exposed to the indicated concentrations of salubrinal alone for 36 h. *P < 0.05 vs. control. (B): Cultured neonatal rat cardiomyocytes were exposed to TM (1 µg/mL) for 36 h in the presence or absence of the indicated concentrations of salubrinal for 30 min. The cell viability was then measured as described in Methods. *P < 0.001 vs. control; #P < 0.05 vs. TM. (C): Cultured neonatal rat cardiomyocytes were exposed to hypoxia for 36 h in the presence or absence of the indicated concentrations of salubrinal for 30 min. The cell viability was then measured as described in Materials and Methods. *P < 0.05 vs. control. #P < 0.05 vs. hypoxia. ATP: adenosine triphosphate; MTT: methylthiazoletetrazolium; Sal: salubrinal; TM: tunicamycin.
3.2. Effect of salubrinal on TM- and hypoxia-induced apoptosis in rat cardiomyocytes
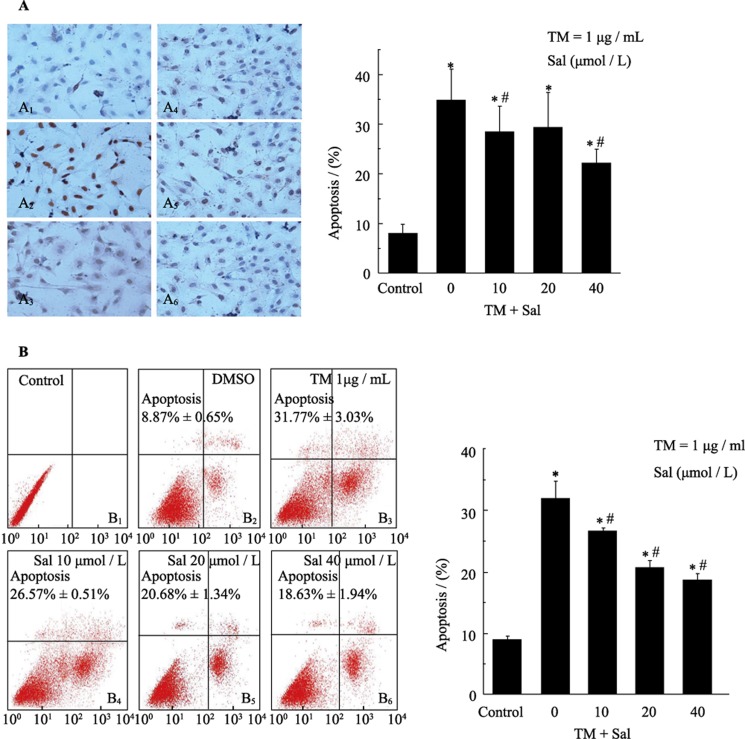
To determine whether salubrinal protected cardiomyocytes against injury via an anti-apoptotic mechanism, we investigated whether cardiomyocytes underwent apoptosis in response to TM treatment and hypoxia by TUNEL, flow cytometry and multiparameter apoptosis assays. We observed an increase in TUNEL-positive cells 36 h after exposure to 1 µg/mL TM (Figure 2A). Administration of salubrinal (10, 20 or 40 µmol/L) significantly reduced the number of TUNEL-positive cells. Among the concentrations tested, 40 µmol/L salubrinal reduced the number of TUNEL-positive cells by almost 10% compared with TM treatment alone (Figure 2B). A negligible number of TUNEL- positive cells were observed in the control cells.
Figure 2. Salubrinal reduces TM-induced apoptosis in cardiomyocytes as demonstrated by TUNEL and flow cytometry assays.
(A): Cardiomyocytes were treated with tunicamycin (TM) (1 µg/mL) in the presence (A4: 10 µmol/L, A5: 20 µmol/L, A6: 40 µmol/L) or absence (A3) of salubrinal for 36 h. The negative control (A1) and positive control (A2) cells were analyzed by TUNEL assay. The results are presented as the means ± SE of three experiments. *P < 0.001 vs. control. #P < 0.05 vs. TM. (B): Cardiomyocytes treated with TM (1 µg/mL) in the presence (B4: 10 µmol/L, B5: 20 µmol/L, B6: 40 µmol/L) or absence (B3) of different concentrations of salubrinal for 24 h. Cells were not treated with either TM or salubrinal in (B1, B2). Apoptosis was assessed by flow cytometry. The results are the means ± SE of three experiments. *P < 0.001 vs. control; *P < 0.05 vs. TM. DMSO: Dimethylsulfoxide; Sal: salubrinal; TM: tunicamycin.
Flow cytometry showed that compared with DMSO treatment, there was a significant increase in apoptosis 24 h after exposure to 1 µg/mL TM (Figure 2C). Administration of salubrinal (10, 20 or 40 µmol/L) significantly suppressed apoptosis, which was similar to the results of the TUNEL assay; 40 µmol/L salubrinal reduced the number of apoptotic cells by more than 10% compared with TM treatment alone (Figure 2D).
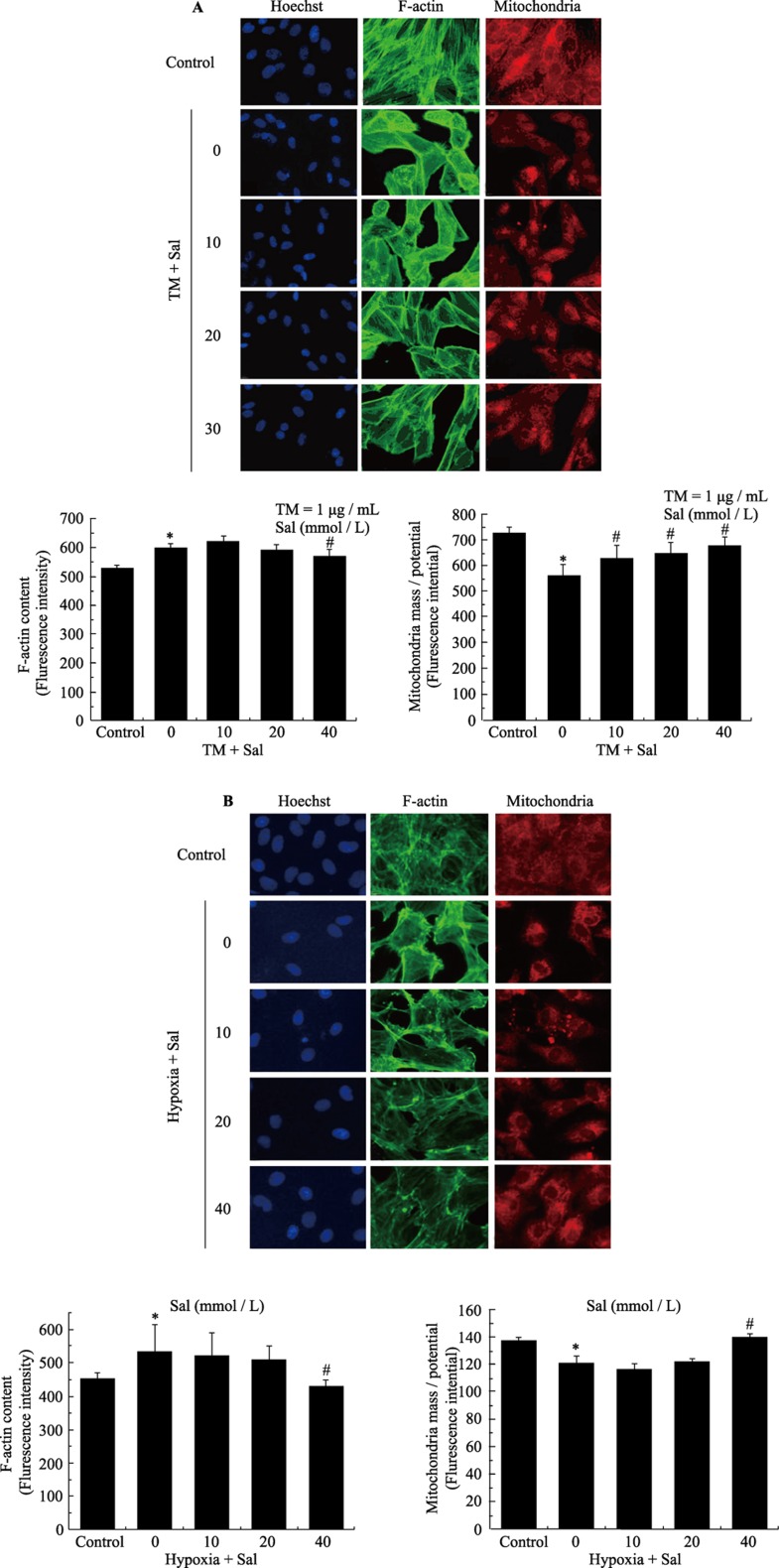
The multi-parameter apoptosis assay included the use of Hoechst dye, Alexa fluor 488-conjugated phalloidin and MitoTracker dye. The multiparameter apoptosis assay showed that compared with DMSO treatment, there was a significant increase in nuclear condensation, a decrease in the mitochondrial membrane potential and an increase in actin cytoskeleton fluorescence in cells 36 h after exposure to 1 µg/mL TM. Administration of salubrinal (10, 20 or 40 µmol/L) signifycantly suppressed these apoptotic changes. We observed that the condensation of nuclei lessened, the mitochondrial membrane potential increased and the actin cytoskeleton fluorescence decreased, which was similar to the results of the TUNEL and flow cytometry assays (Figure 3).
Figure 3. Salubrinal reduces TM- and hypoxia-induced apoptosis in cardiomyocytes as demonstrated by a multi-parameter HCS apoptosis assay.
(A): Analysis of apoptosis in response to TM-treatment of cardiomyocytes using the ArrayScan HCS Reader. Cardiomyocytes were exposed to TM (1 µg/mL) in the presence or absence of salubrinal. The cells were then fixed and stained with Hoechst 33342 to analyze nuclear morphology, Alexa fluor 488-phalloidin to analyze F-actin content and MitoTracker Red to analyze mitochondrial mass/potential. Apoptotic parameters were quantified by analyzing the stained cells on a Thermo Scientific ArrayScan HCS Reader. Dose-response plots for F-actin content and mitochondrial mass/potential. *P < 0.05 vs. control; #P < 0.05 vs. TM. (B): Measurement of apoptosis in response to hypoxia in cardiomyocytes on the ArrayScan HCS Reader. The cardiomyocytes were exposed to hypoxia in the presence or absence of salubrinal. The cells were then fixed and stained with Hoechst 33342 to analyze nuclear morphology, Alexa fluor 488-phalloidin to analyze F-actin content and MitoTracker Red to analyze mitochondrial mass/potential. Apoptotic parameters were quantified by analyzing the stained cells on a Thermo Scientific ArrayScan HSC Reader. Dose-response plots for F-actin content and mitochondrial mass/potential. *P < 0.05 vs. control. #P < 0.05 vs. hypoxia. HCS: high content screening; Sal: salubrinal; TM: tunicamycin.
3.3. Effect of salubrinal on TM- and hypoxia- induced ERS signaling proteins and ERS-mediated apoptotic signals
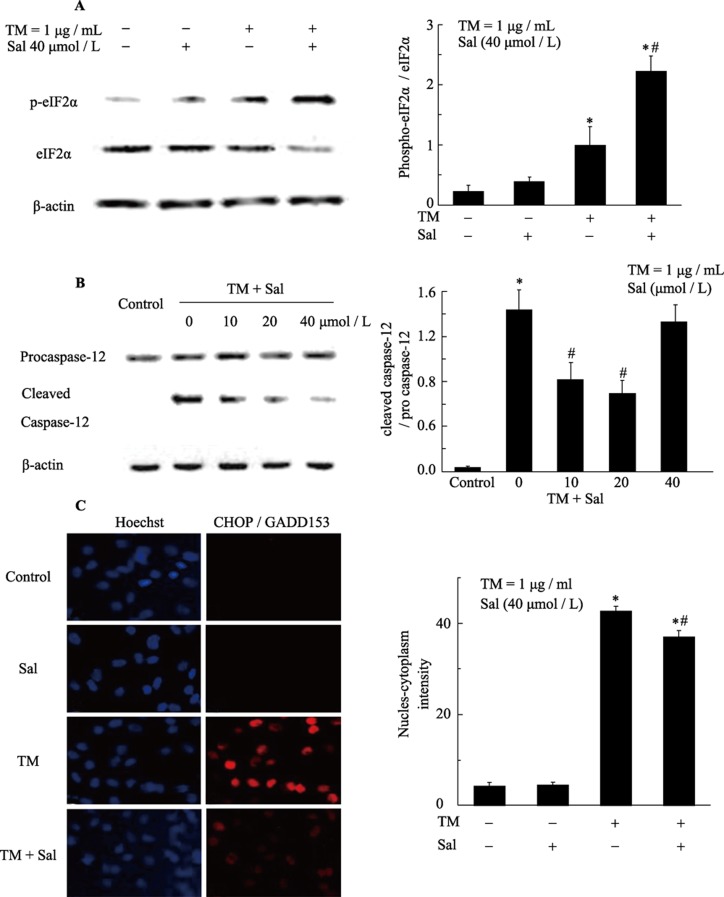
Based on reports showing that TM and hypoxia are associated with the induction of ERS signaling, this study examined whether such an association exists and whether salubrinal plays a role in protecting cells from ERS. Phosphorylated eIF2α mediates a transient decrease in protein translation, and salubrinal selectively engages the translational control branch of the UPR by inducing eIF2α phosphorylation. In our previous study, we found that caspase-12 is most highly expressed four hours after hypoxia treatment. CHOP expression occurs downstream of caspase-12 and is most highly expressed eight hours after the induction of hypoxia.
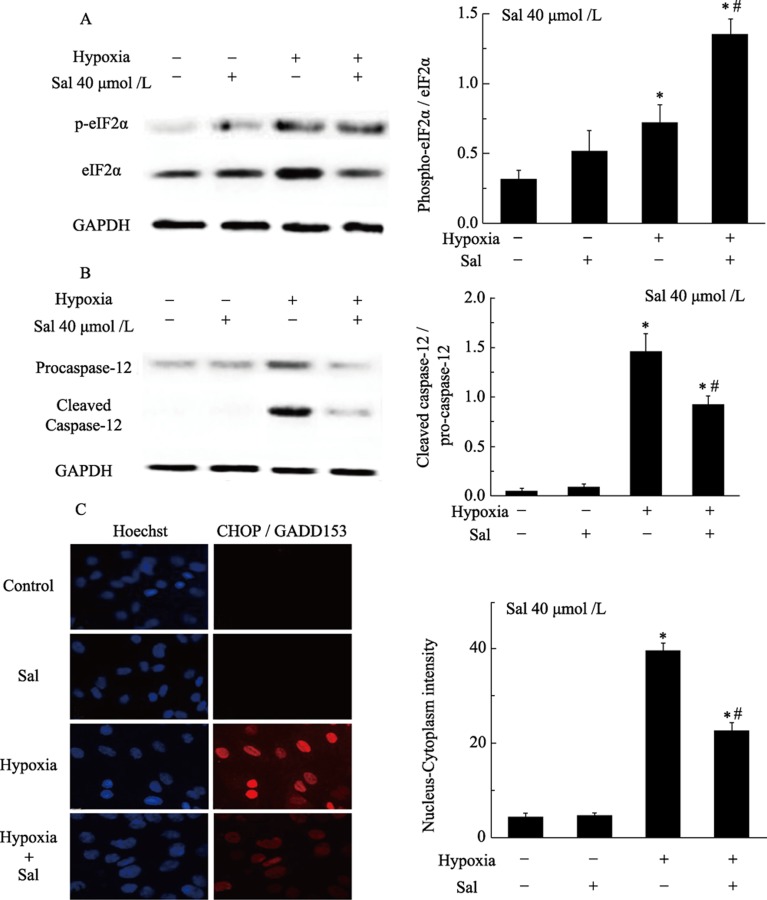
We first assessed whether salubrinal could affect the phosphorylation of eIF2α, which has also been demonstrated to induce CHOP transcription. The levels of p-eIF2α in TM-treated and hypoxia-exposed cells were slightly increased. The p-eIF2α/eIF2α ratio was markedly higher in the presence of salubrinal (Figure 4A, 5A), suggesting that salubrinal regulates p-eIF2α processing, inhibits translation and protects cells from ERS.
Figure 4. Salubrinal activates eIF-2 expression and attenuates TM induced CHOP and caspase-12 expression in cardiomyocytes.
(A): Salubrinal maintains eIF-2α phosphorylation. Cardiomyocytes were exposed to tunicamycin (TM) (1 µg/mL) in the presence or absence of salubrinal for four hours. The expression of eIF2α and p-eIF2α was analyzed by Western Blotting using anti-eIF2α and anti-p-eIF2α antibodies. *P < 0.001 vs. control; #P < 0.01 vs. TM. (B): Salubrinal regulates the expression of caspase-12. Cardiomyocytes were exposed to TM (1 µg/mL) in the presence or absence of salubrinal for eight hours. Caspase-12 protein expression was analyzed by western blotting with anti-caspase-12 antibodies. *P < 0.001 vs. control. #P < 0.01 vs. TM. (C): Measurement of CHOP expression in cardiomyocytes with the ArrayScan HSC Reader. The cardiomyocytes were exposed to TM (1 µg/mL) in the presence or absence of salubrinal and then fixed. The cells were then stained with Hoechst 33342 to analyze nuclear morphology, and with CHOP antibody followed by a DyLight 488 secondary antibody to quantify CHOP expression. *P < 0.05 vs. control; #P < 0.05 vs. hypoxia. CHOP: C/EBP homologous protein; Sal: salubrinal; TM: tunicamycin.
Figure 5. Salubrinal activates eIF-2α expression and attenuates hypoxia induced CHOP and caspase-12 expression in cardiomyocytes.
(A): Salubrinal maintains eIF-2α phosphorylation. Cardiomyocytes were exposed to hypoxia in the presence or absence of salubrinal for four hours. The expression of eIF2α and p-eIF2α was analyzed by Western Blotting using anti-eIF2α, anti-p-eIF2α antibodies. *P < 0.001 vs. control; #P < 0.01 vs. hypoxia. (B): Salubrinal regulates the expression of caspase-12. Cardiomyocytes were exposed to hypoxia in the presence or absence of salubrinal for eight hours. Caspase-12 protein expression was analyzed by Western Blotting using anti-caspase-12 antibodies. *P < 0.001 vs. control. #P < 0.01 vs. hypoxia. (C): Measurement of CHOP expression in cardiomyocytes with the ArrayScan HCS Reader. The cardiomyocytes were exposed to hypoxia in the presence or absence of salubrinal. The cells were then fixed and stained with Hoechst 33342 to analyze nuclear morphology, and with CHOP antibody followed by a DyLight 488 secondary antibody to quantify CHOP expression. *P < 0.05 vs. control; #P < 0.05 vs. hypoxia. CHOP: C/EBP homologous protein; HCS: high content screening; Sal: salubrinal; TM: tunicamycin.
In our study, we observed that CHOP was significantly up-regulated in TM-treated or hypoxia-exposed neonatal rat cardiomyocytes. Quantification revealed a notable increase in the protein levels of CHOP in TM-treated or hypoxia-exposed neonatal rat cardiomyocytes compared with control cells. However, injection of salubrinal down-regulated CHOP protein levels compared with the TM-treated or hypoxia-exposed groups (Figure 4C, 5C).
Furthermore, Western Blotting showed that TM and hypoxia led to the activation of caspase-12, as demonstrated by an increase in the levels of cleaved caspase/procaspase-12. The activation of caspase-12 was largely restored by salubrinal. No difference in the activation of caspase-12 was observed between the control and the salubrinal only-treated groups of cultured neonatal rat cardiomyocytes (Figure 4B, 5B).
4. Discussion
The present study is the first to demonstrate that salubrinal protects cardiomyocytes against hypoxic injury and apoptosis. We showed that administration of salubrinal protected cardio- myocytes from injury induced by TM and hypoxia, as demonstrated by increased cell viability and reduced apoptosis. Enhanced eIF2α phosphorylation was also observed in salubrinal-treated cells. These findings indicate that the protective effect of salubrinal on hypoxia-induced injury was mediated by the restoration of ER dysfunction.
The ER-dependent apoptotic pathway is one of the mechanisms by which hypoxic injury is induced in cardio- myocytes.[4] Salubrinal is a selective inhibitor of eukaryotic translation initiation factor 2α dephosphorylation. In the rat pheochromocytoma cell line PC-12, salubrinal in concentrations ranging from 10 µmol/L to 75 µmol/L protects cells against death induced by TM-mediated ERS.[15] Salubrinal has also been used to reduce nephrotoxicity induced by cyclosporine- mediated ERS in rats.[18] Cardiomyocytes are considered to be physiologically different from pheochromocytoma and renal cells. We therefore hypothesized that salubrinal may protect cardiomyocytes from ERS-induced cell death.
When TM and hypoxia were induced in cardiomyocytes for 36 h, decreased cell viability and induction of apoptosis were observed by TUNEL, flow cytometry and HCS assays. The results indicated that TM and hypoxia caused severe cell injury and triggered apoptosis. In contrast, pretreatment with salubrinal significantly inhibited apoptosis and increased cell viability. Therefore, salubrinal protects cardiomyocytes against apoptosis related cell death. Recently, Dalal et al.[19] demonstrated that salubrinal could protect cardiomyocytes from βzeceptor stimulation-induced apoptosis. Here, we demonstrated that salubrinal could protect cardiomyocytes from ERS-mediated apoptosis induced by hypoxia. These results indicate that salubrinal significantly inhibits apoptosis in several cell types, including cardiomyocytes.
ERS-specific apoptotic signaling is involved in hypoxic injury of cardiomyocytes. Of the apoptotic pathways, two are known to be ER-specific pathways. The first pathway involves transcriptional induction of the gene encoding CHOP. Overexpression of CHOP promotes apoptosis, while CHOP deficiency can protect cells from ERS-induced apoptosis, suggesting that CHOP is involved in the ERS-mediated cell death pathway.[20] The second pathway involves the activation of caspase-12, which is known to be an important regulator of ERS-induced apoptosis because caspase-12 deficient cells exhibit reduced sensitivity to apoptosis induced by the ERS agent tunicamycin.[21],[22] In the present study, induction of CHOP and cleavage of caspase 12 increased in cardiomyocytes after exposure to TM or hypoxia for eight hours (Figure 1A, 1B and 3A). However, pretreatment with salubrinal significantly attenuated ER-specific death pathways during TM and hypoxia. Moreover, pretreatment with salubrinal significantly decreased the rate of protein synthesis by maintaining eIF-2α phosphorylation. Given the present findings, we speculate that eIF2α phosphorylation plays an important role in mediating the cardioprotective effects against ER death pathways during TM-treatment and hypoxia. Therefore, the protective effects of salubrinal against injury caused by TM or hypoxia may be due to inhibition of ERS and the subsequent apoptotic signaling pathway. Recent studies by Scheuner et al.[23] suggest that a subtle increase in rate of protein synthesis via a reduction in eIF2α phosphorylation leads to cell failure and type 2 diabetes when an animal is exposed to ERS. Our results match well with this previous work, suggesting that salubrinal protects several cell types from apoptosis by attenuating ERS associated apoptosis via a decreased rate of protein synthesis.
In the present study, we speculate that salubrinal widely suppresses ERS-mediated apoptotic signaling caused by severe ER dysfunction.[24],[25] Such apoptotic signaling contributes to the pathogenesis of heart hypoxia, myocarditis, myocardial infarction and congestive heart failure. However, certain issues remain to be resolved. It is poorly understood for how long or at what levels sustained inactivation of the eIF2α phosphorylation would be beneficial, and the long-term effects of sustained eIF2α phosphorylation need to be further evaluated.
In summary, the data presented here are of clinical significance because apoptosis is a common feature of heart hypoxia injury. Inhibition of apoptosis in myocytes with salubrinal suggests that the balance between phosphorylated and unphosphorylated eIF-2α may be necessary for myocyte survival. Targeting the ERS may provide a therapeutic approach for increasing the protection against heart hypoxia injury. We propose that the therapeutic potential of salubrinal may extend to other ERS-related cardiovascular diseases, but further studies are needed to confirm this.
Acknowledgments
This study was supported by the Ministry Science Foundation of the Chinese People's Liberation Army during the 12th Five-Year Plan Period (No.BWS12J048).
References
- 1.Chiu CZ, Wang BW, Chung TH, et al. Angiotensin II and the ERK pathway mediate the induction of myocardin by hypoxia in cultured rat neonatal cardiomyocytes. Clin Sci (Lond) 2010;119:273–282. doi: 10.1042/CS20100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calmettes G, Deschodt-Arsac V, Gouspillou G, et al. Improved energy supply regulation in chronic hypoxic mouse counteracts hypoxia-induced altered cardiac energetics. PLoS One. 2010;5:e9306. doi: 10.1371/journal.pone.0009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajiri S, Oyadomari S, Yano S, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 4.Terai K, Hiramoto Y, Masaki M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 6.Qi X, Hosoi T, Okuma Y, et al. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky JL, Skach WR. Protein folding and quality control in the endoplasmic reticulum: Recent lessons from yeast and mammalian cell systems. Curr Opin Cell Biol. 2011;23:464–475. doi: 10.1016/j.ceb.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 11.Faitova J, Krekac D, Hrstka R, et al. Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett. 2006;11:488–505. doi: 10.2478/s11658-006-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morishima N, Nakanishi K, Takenouchi H, et al. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 13.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 14.Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 15.Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34:365–383. doi: 10.1016/s0143-4160(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 16.Sokka AL, Putkonen N, Mudo G, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallet N, Bouvier N, Bendjallabah A, et al. Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am J Transplant. 2008;8:2283–2296. doi: 10.1111/j.1600-6143.2008.02396.x. [DOI] [PubMed] [Google Scholar]
- 18.Gasparetto M, Gentry T, Sebti S, et al. Identification of compounds that enhance the anti-lymphoma activity of rituximab using flow cytometric high-content screening. J Immunol Methods. 2004;292:59–71. doi: 10.1016/j.jim.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Dalal S, Foster CR, Das BC, et al. Beta-adrenergic receptor stimulation induces endoplasmic reticulum stress in adult cardiac myocytes: role in apoptosis. Mol Cell Biochem. 2012;364:59–70. doi: 10.1007/s11010-011-1205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morishima N, Nakanishi K, Takenouchi H, et al. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 23.Scheuner D, Vander MD, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 24.Toth A, Jeffers JR, Nickson P, et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–H60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 25.Anversa P, Cheng W, Liu Y, et al. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93:8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]