Abstract
Osteogenesis imperfecta (OI) is characterized by different signs including increased bone fragility, short stature, blue sclera, abnormal tooth growth and often secondary immobility. No curative therapy has been found for this rare disease up to now, and different pharmacological substances have been tried as treatment for severe forms of OI. Promising results were seen with intravenous bisphosphonates in the treatment of patients with OI. The aim of present study was to show the effect of intravenous ibandronate therapy on bone density and bone metabolism markers. We analyzed the data of 27 patients with the diagnosis of OI who were treated off-label with intravenous ibandronate. Ibandronate was administered by intravenous infusion every three months at a dosage of 0.3–2 mg. Bone turnover markers and bone density were measured before starting therapy and every three months during treatment. Bone density was measured by using an ultrasound imaging system providing an accurate image of the calcaneus and by evaluating broadband ultrasound attenuation (BUA). Twenty-seven patients were treated with intravenous ibandronate during the observation period. 18 were female. The mean age of all patients was 23.9 years ± 19.6 (range 4–63). Seventeen patients were categorized to have OI Type I, 5 patients to have OI Type III and 5 patients to have OI Type IV. There was a statistically significant decrease in total alkaline phosphatase (P<0.0001). We detected also a statistically significant decrease in the ratio urinary deoxypyridinoline/urinary creatinine (P=0.0048) and the ratio urinary pyridinoline/urinary creatinine (P<0.0001) respectively. There was also a statistically significant increase in serum magnesium (P=0.034) and BUA (P=0.0071). No statistically significant changes were seen for total serum calcium (P=0.16), the ratio of urine calcium/urine creatinine (P=0.29), alkaline phosphatase (isoform bone) (P=0.3), procollagen-I-peptide (P=0.5), osteocalcin (P=0.9), serum phosphatase (P=0.71), parathormone (P=0.11) and the ratio urine phosphatase/urine creatinine (P=0.58) Therapy with ibandronate in patients with OI leads to a normalisation of bone turnover markers and increasing bone density. Therefore serum alkaline phosphatase and bone density are possible parameters to monitor bisphosphonate treatment in patients with OI.
Key words: osteogenesis imperfecta, bisphosphonate, ibandronate, bone density, bone turnover markers.
Introduction
Osteogenesis imperfecta (OI) is a heritable skeletal disorder characterized by different signs including increased bone fragility, short stature, blue sclera, abnormal tooth growth and often secondary immobility. A mutation in the COL1A1 or COL1A2 gene, encoding the two collagen type I chains [α1(I) and α2(I)] has been detected in individuals with the clinical diagnosis of OI.1–4
In 1979, Sillence et al. classified the OI into four major groups.4 This classification is still most often used. Recently additional types (V-XI) have been indentified. They have a similar phenotype as OI type IV but are not associated with a collagen type I mutation.1,5,6 These types are caused by a mutation in the gene encoding for cartilage-associated protein, CRTAP; prolyl-3-hydroxylase 1, LEPRE1, and cyclophilin B, PPIB.1,5,6
No curative therapy has been found for this rare disease up to now, so different pharmacological substances have been tried as treatments for severe forms of OI. Sodium fluoride, anabolic steroids, and magnesium oxide have been tried without any convincing benefit with regard to fracture risk and sustained improvement.7–11
A positive effect was seen during the application of growth hormones and (+)-cyanidanol-3.12,13 A benefit was also seen during treatment with calcitonin, but the pronounced side effects predominated.14,15
Over the last two decades promising results were seen with intravenous bisphosphonates in the treatment of patients with OI.16,17
Bipshosphonates are stable analogues of pyrophosphates with a complex effect on the bone metabolism. The predominantly inhibitory effect on osteoclasts is due to an inhibition of the farensyl pyrophosphotase synthase, which leads to osteoclast apoptosis,18–21 which may lead to an increased bone density.
Many previous studies focused on the results after treatment with pamidronate on bone density, bone turnover markers and fracture rate in patients with osteogenesis imperfect,22–29 but only a few studies have measured the effects of intravenous ibandronate in children and adults with osteogenesis imperfect.30
The aim of present study was to show the effect of intravenous ibandronate therapy on bone density and bone metabolism markers in patients with osteogenesis imperfecta. We also wanted to prove the hypothesis that there are higher levels of bone metabolism markers in these patients, indicating an increased bone turnover.
Materials and Methods
We analyzed the data of 27 patients with the diagnosis of OI who were treated off-label with an intravenous ibandronate therapy between January 2002 and December 2007 at the Technical University of Munich Children's Hospital (Germany). All patients and their parents signed a declaration accepting the unlicensed (off-label) use of ibandronate for the treatment of osteogenesis imperfecta.
Inclusion criteria were a complete patient history and a continuous duration of treatment over at least 18 months. Demographic data of the different patients is demonstrated in Table 1.
Table 1. Demographic data of all patients.
| Patient | Sex | Age | OI-Type | Total calcium | Serum phosphate | Urinary calcium/creatinine | Magnesium | Total alkaline phosphatase | Alkaline phosphatase (isoform bone) | Parathormone | Procollagen-1-Peptide | Urinary deoxypyridinoline/creatinine | Urinary pyridinoline/creatinine | Urinary phosphate/creatinine | Osteocalcin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 12 | 4 | x | x | x | x | x | x | ||||||
| 2 | f | 7 | 4 | x | x | x | x | x | x | x | x | ||||
| 3 | f | 10 | 4 | x | x | x | x | x | x | x | x | ||||
| 4 | f | 59 | 4 | x | x | x | x | x | x | x | x | ||||
| 5 | f | 50 | 4 | x | x | x | x | x | x | x | x | ||||
| 6 | f | 13 | 1 | x | x | x | x | ||||||||
| 7 | m | 39 | 1 | x | x | x | x | x | x | x | x | ||||
| 8 | f | 48 | 1 | x | x | x | x | x | x | x | |||||
| 9 | m | 17 | 1 | x | x | x | x | x | x | x | |||||
| 10 | m | 16 | 1 | x | x | x | x | x | x | ||||||
| 11 | m | 63 | 1 | x | x | x | x | x | x | x | x | ||||
| 12 | m | 14 | 1 | x | x | x | x | x | |||||||
| 13 | f | 33 | 1 | x | x | x | x | ||||||||
| 14 | f | 4 | 1 | x | x | x | x | x | |||||||
| 15 | f | 60 | 1 | x | x | x | x | x | x | x | x | x | x | ||
| 16 | f | 5 | 1 | x | x | x | |||||||||
| 17 | f | 34 | 1 | x | x | x | x | x | x | ||||||
| 18 | f | 5 | 1 | x | x | x | x | x | x | x | |||||
| 19 | f | 4 | 1 | x | x | x | x | x | x | x | |||||
| 20 | f | 48 | 1 | x | x | x | x | x | x | x | |||||
| 21 | m | 36 | 1 | x | x | x | x | x | x | ||||||
| 22 | f | 34 | 1 | x | x | x | x | x | x | ||||||
| 23 | m | 5 | 3 | x | x | x | x | x | |||||||
| 24 | f | 7 | 3 | x | x | x | x | x | x | x | x | ||||
| 25 | f | 6 | 3 | x | x | x | x | x | x | x | x | x | x | ||
| 26 | f | 6 | 3 | x | x | x | |||||||||
| 27 | m | 11 | 3 | x | x | x | x | x | x | x |
OI, Osteogenesis imperfect; f, female; m, male; x, bone turnover marker was measured at the beginning of the therapy.
Treatment protocol
Ibandronate was administered by intravenous infusion every three months at a dosage of 0.3–2 mg (0.016 mg/kg – 0.2 mg/kg body weight). There was a dosage increase in the adult patients during treatment, whereas the children's dosage was constant during the duration of treatment.
The observation period was at least 18 months.
Bone markers
The following bone turnover markers were measured before starting ibandronate therapy and every three months during treatment (reference intervals):
Serum calcium (2.2–2.6 mmol/L)
Serum phosphate (2.5–4.5 mg/dL)
Ratio urinary calcium/urinary creatinine (<0.5)
Serum magnesium (0.65–1.1 mmol/L)
Serum total alkaline phosphatase (60–180 UL)
Serum alkaline phosphatase isoform bone (4–21 U/L)
Parathormone (15–65 pg/mL)
Serum Prokollagen-1-Peptide (m: 50–170 ug/mL; f: 38–202 ug/mL)
Ratio urinary deoxypyridinoline/urinary cre-atinine (3–12 nmol/mmol)
Ratio urinary pyridinoline/urinary creatinine (19–51 nmol/mmol)
Ratio urinary phosphate/urinary creatinine [<2.7 mmol/mmol (age>14)]
Osteocalcin (9–42 ng/mL)
Bone density
Bone density was measured using an ultrasound imaging system providing an accurate image of the calcaneus and by evaluating broadband ultrasound attenuation (UBIS 5000; DMS group - Mauguio, France). The broadband ultrasound attenuation (BUA) was evaluated by positioning a circle in the region of interest which was located in the dorsal part of the calcaneus (Figure 1).
Figure 1.

Ultrasound imagine created by UBIS 5000. The circle marks the region of interest for measuring broadband ultrasound attenuation.
Statistical analysis
Statistical analysis was done using R 2.13.2 (R Foundation for Statistical Computing, Vienna, Austria) with packages nlme and aod.31,32 Data are presented as mean ± standard deviation (range). To analyze the longitudinal data we used a mixed effects model. To accommodate for various kinds of non-linearity in the longitudinal trends we used B-splines in the mixed model analysis. All analyses were done using a 0.05 level of significance.
Study registration and informed consent
This study protocol was reviewed and approved by the local ethics committee of the University of Tuebingen (719/2011A). Written informed consent was obtained from all patients or their parents for the unlicensed use of intravenous ibandronate.
Results
Twenty-seven patients were treated with intravenous ibandronat during the observation period: 18 were female. The mean age of all patients was 23.9 years±19.6 (range 4–63). Seventeen patients were categorized to have OI type I, 5 patients to have OI type III and 5 patients to have OI type IV. Table 1 is marking the different patients where bone turnover markers have been analysed before starting the therapy.
The mean values of bone turnover markers at the beginning of the therapy are demonstrated in Table 2.
Table 2. Levels of bone turnover markers at the beginning of therapy.
| Bone turnover marker | Material | N | Initial values (before therapy) | Reference interval |
|---|---|---|---|---|
| Total calcium | Serum | 27 | 2.3±0.1 (2.1–2.5) | 2.2–2.6 mmoL/L |
| Serum phosphate | Serum | 25 | 4.1±0.8 (2.8–5.4) | 2.5–4.5 mg/dL |
| Urinary calcium/creatinine | Urine | 15 | 1.2±0.6 (0.3–2.5) | <0.5 mmoL/mmoL |
| Magnesium | Serum | 26 | 0.8±0.1 (0.7–1.0) | 0.65–1.1 mmoL/L |
| Total alkaline phosphatase | Serum | 26 | 288.6±195.7 (76–827) | 60–180 U/L |
| Alkaline phosphatase (isoform bone) | Serum | 9 | 8.9±3.5 (5–15) | 4–21 U/L |
| Parathormone | Serum | 11 | 3.7±3.5 (0.1–13.0) | 15–65 pg/mL |
| Prokollagen-1-Peptide | Serum | 6 | 91.9±115.3 (21.7–322.4) | m: 50–170 ug/mL; f: 38–202 ug/mL |
| Urinary deoxypyridinoline/ creatinine | Urine | 6 | 31.3±32.9 (7.5–96.0) | 3–12 nmoL/mmoL |
| Urinary pyridinoline/creatinine | Urine | 7 | 189.2±210. 4 (72.3–661) | 19–51 nmoL/mmoL |
| Urinary phosphate/creatinine | Urine | 12 | 1.5±3.6 (0.1–12.8) | <2.2 mmoL/mmoL |
| Osteocalcin | Serum | 5 | 11.0±8.1 (3.3–20.9) | 9–42 ng/mL |
N, number of cases included.
Two patients (7%) were under the range for reference intervals of serum calcium levels at the beginning of the treatment. 8 patients (30%) were upper the range of reference intervals for serum phosphatase and 14 patients (60%) demonstrated increase levels of urinary calcium. In regard to magnesium serum levels, all patients were in the range for reference intervals. There was an increased serum total alkaline phosphatase upper the reference seen in 15 patients (57%) and one (10%) increased value for bone alkaline phosphatase. The values for parathormone were under the lower limit in 16 patients (84%) and 2 female patients (22%) were under the lower limit for procollagen-I-petide. Increased levels for urine deoxypyridinolin were seen in 8 patients (80%) and 9 patients (75%) demonstrated elevated urine pyridinolin levels. 3 patients (60%) were under the lower limit for serum osteocalcin levels.
The development of the different bone turnover markers during treatment with ibandronat is demonstrated in Figures 2–4.
Figure 2.

Changes of bone turnover markers during ibandronate therapy.
Figure 4.
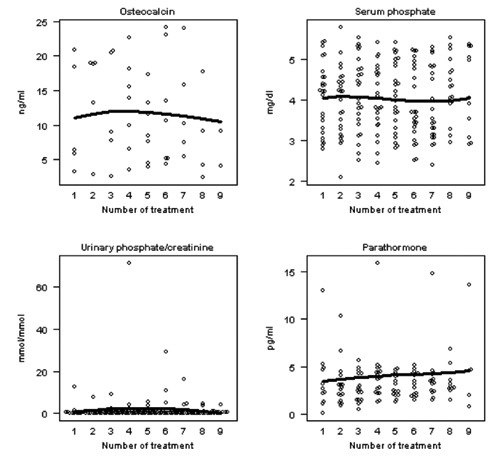
Changes of bone turnover markers during ibandronate therapy (PTH: parathormone).
Figure 3.

Changes of bone turnover markers during ibandronate therapy (DPD, deoxypyridinoline; PYP, pyridinoline).
The development of broadband ultrasound attenuation under therapy is shown in Figure 5.
Figure 5.

Changes of broadband ultrasound attenuation during ibandronate therapy.
There was a statically significant decrease in total alkaline phosphatase (P<0.001). We detected also a statistically significant decrease in the ratio urinary deoxypyridinoline/urinary creatinine (P<0.001) and the ratio urinary pyridinoline/urinary creatinine (P<0.001). There was also a statistically significant increase in serum magnesium (P=0.034) and BUA (P=0.007).
No statistical significant changes were seen for total serum calcium (P=0.158), the ratio urine calcium/urine creatinine (P=0.292), alkaline phosphatase (isoform bone) (P=0.296), procollagen-I-peptide (P=0.503), osteocalcin (P=0.903), serum phosphatase (P=0.712), parathormone (P=0.107) and the ratio urine phosphatase/urine creatinine (P=0.587).
Discussion
Osteogenesis imperfecta is a hereditary disorder that results from a genetic defect in the synthesis of type-I-collagen. This genetic defect results in a typical phenotype with increased bone fragility, short stature, blue sclera and abnormal tooth growth.1–4 Sillence et al.4 classified the OI into four different types ranging from a mild form to a lethal form, according to their appearance. OI type I is a mild form with only a few fractures in childhood, blue sclera and a good mobility. OI type II is the most serve form with multiple perinatal fractures and normally lethal course. Types III and IV are accompanied by increased bone fragility, with or without blue sclera and are clinically difficult to treat because of multiple deformities and a high rate of immobility.4,33
Bisphosphonates with their influence on bone turnover have been established in osteoporotic disorders and in the treatment of osteogenesis imperfecta. The benefits of bisphosphonate treatment, especially with pamidronate, have been demonstrated in several studies. There was an increase in bone density, a decreased fracture rate, a normalization of bone turnover markers and a subjective reduction of bone pain seen with an improvement in mobility.26–29,34–37
In the present study we retrospectively analyzed the data of 27 patients who were treated with intravenous ibandronate for at least 1.5 years. To our knowledge only one previous study also focused on the benefits of this kind of bisphosphonate in children with osteogenesis imperfect.30
Bone alkaline phosphatase and osteocalcin have been concluded to be the most valuable bone markers for bone formation, whereas urinary deoxypyridinoline (DPD) and the cross-linked telopeptides of type-I-collagen are most valuable markers for bone resorption.38 One of the main problems of the present study is that the bone turnover markers were not compared with an age-gender-matched group of healthy children. Most children with OI are smaller and lighter when compared with children of the same age and a correlation between the levels of bone turnover markers and body size and weight has been reported.39 Because of these findings and because of the mean age of our patients, we did not compare initial bone turnover markers with the few and still incompletely published paediatric reference intervals.38,40,41
Nevertheless, we evaluated bone turnover markers before starting therapy with ibandronate and monitored changes during treatment. Most patients demonstrated elevated serum and urinary markers for bone formation and bone resorption, which implies that there is an increased turnover in patients with OI. We were able to detect statistically significant changes with a normalisation of the different bone markers during the therapy. There was decrease in total alkaline phosphatase (bone formation), in the ratio of urinary deoxypyridinoline/urinary creatinine (bone resorption) and the ratio of urinary pyridinoline/creatinine (bone resorption). In our opinion, these markers are suitable to detect the benefits of ibandronate therapy. These findings support the results by Aström et al., where serum alkaline phosphatase (ALP) and urinary DPD were also seen as most informative in the observation of treatment benefit, although they used pamidronate in their study.25 However Braga et al. reported serum bone ALP, urinary DPD and collagen-type I Ntelopeptides (NTX) as being the best for clinical separation.42 On the other hand there might be the possibility of a type I failure while detecting a decrease in the ratio of urinary deoxypyridinoline/urinary creatinine and the ratio of urinary pyridinoline/creatinine caused by the small sample size.
We did not detect an increased bone ALP or statistically significant changes in bone ALP which might be caused by a type II failure because of the small sample size.
Bone densitometry in infants and especially in patients with a pathological bone structure such as in patients with OI is difficult. DXA is the most commonly used method and a lot of studies have shown an increase in bone mineral density during the therapy with bisphosphonates especially with pamidronate.28,29,34,35
To our knowledge this is the first study using BUA for monitoring bone density during bisphosphonate treatment. We used BUA for measurement of bone density to avoid radiation exposure during follow-up especially in children and to minimize measuring time. Different studies have demonstrated the high sensitivity of this technique when measuring bone density.43–45 We were able to show a statistically significant increase in bone density, which is consistent with previous studies.28,29,34,35
We note several limitations of the present study. The most important limitation was that we performed a retrospective analysis of patient data. Therefore we analyzed a very heterogeneous patient collective especially in regard to the age. For the same reason there was a lack of information about different bone turnover markers such as osteocalcin. Most patients were tested for the most important bone turnover markers during therapy, such as alkaline phosphatase, deoxypyridinoline or pyridinoline and we were therefore unable to perform a statistical analysis of most parameters retrospectively. On the other hand in our opinion the patients which have been included into the study represented there age-group sufficiently.
Secondly we did not analyse clinical features such as bone fracture rate, mobility, pain during the treatment with ibandronate and have not differentiated between OI Type I/III/IV when analyzing the effects of ibandronate. Further studies should focus on these topics.
Thirdly, we analysed bone density by using broadband ultrasound attenuation and the region of interest was plotted free hand, so the interobserver quality seems to be low. However, this was only performed by one observer (author II) in a standard manner so that there was a high conformity.
Conclusions
Finally, therapy with ibandronate in patients with OI leads to a normalisation of bone turnover markers and increase in bone density. Therefore serum alkaline phosphatase and bone density are possible parameters to monitor bisphosphonate treatment in patients with OI. There seems also to be a decrease in the ratio of urinary deoxypyridinoline/urinary creatinine and the ratio of urinary pyridinoline/creatinine but because of the small sample size these parameters could only be suggested as possible parameters to monitor therapy.
References
- 1.Rauch F, Lalic L, Roughley P, Glorieux FH. Relationschip between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfect. J Bone Min Res. 2010;25:1367–74. doi: 10.1359/jbmr.091109. [DOI] [PubMed] [Google Scholar]
- 2.Rauch F, Glorieux FH. Osteogenesis imperfect. Lancet. 2004;24(363):1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MM., Jr. The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 4.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–16. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dijk FS, Pals G, Van Rijn RR, et al. Classification of osteogenesis imperfecta revisited. Eur J Med Genet. 2010;53:1–5. doi: 10.1016/j.ejmg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro JR, Sponsellor PD. Osteogenesis imperfecta: questions and answers. Curr Opin Pediatr. 2009;21:709–16. doi: 10.1097/MOP.0b013e328332c68f. [DOI] [PubMed] [Google Scholar]
- 7.Spencer H, Kramer L, Wiatrowski E, Lender M. Fluorid therapy in metabolic bone disease. Isr J Med Sci. 1984;76:373–80. [PubMed] [Google Scholar]
- 8.Cattell HS, Clayton B. Failure of anabolic steroids in the therapy of osteogenesis imperfecta: a clinical, metabolic, and biochemical study. J Bone Joint Surg Am. 1968;50:123–41. [Google Scholar]
- 9.Albright JA, Grunt JA. Studies of patients with osteogenesis imperfecta. J Bone Joint Surg Am. 1971;53:1415–25. [PubMed] [Google Scholar]
- 10.Castells S. New approaches to treatment of osteogenesis imperfecta. Clin Orthop. 1973;93:239–49. doi: 10.1097/00003086-197306000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen U, Charles P, Hansen HH, Elbrond O. Lack of effects of human calcitonin in osteogenesis imperfecta. Acta Orthop Scan. 1985;56:260–4. doi: 10.3109/17453678508993009. [DOI] [PubMed] [Google Scholar]
- 12.Wright NM. Just taller or more bone? The impact of growth hormone on osteogenesis imperfecta and idiopathic juveline osteoporosis. J Pediatr Endocrinol Metab. 2000;13:999–1002. [PubMed] [Google Scholar]
- 13.Pontz BF, Krieg T, Müller PK. (+)-Cyanidanol-3 changes functionel properties of collagen. Biochem Pharmacol. 1982;22:3581–9. doi: 10.1016/0006-2952(82)90579-2. [DOI] [PubMed] [Google Scholar]
- 14.Castells S, Colbert C, Chakrabarti C, et al. Therapy of osteogenesis imperfecta with synthetic salmon calcitonin. J Pediatr. 1979;95:807–11. doi: 10.1016/s0022-3476(79)80741-6. [DOI] [PubMed] [Google Scholar]
- 15.Nishi Y, Hamamoto K, Kajiyama M, et al. Effect of long-term calcitonin therapy by injection and nasal spray on the incidence of fractures in osteogenesis imperfecta. J Pediatr. 1992;121:477–80. doi: 10.1016/s0022-3476(05)81809-8. [DOI] [PubMed] [Google Scholar]
- 16.Castillo H, Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51:17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 17.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD005088.pub2.CD005088 [DOI] [PubMed] [Google Scholar]
- 18.Lindsay R. Modeling the benefits of pamidronat in children with osteogenesis imperfecta. J Clin Invest. 2002;110:1239–41. doi: 10.1172/JCI17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–42. [PubMed] [Google Scholar]
- 20.Fleisch H. Bisphosphonates: mechanism of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 21.Allgrove J. Bisphosphonates. Arch Dis Child. 1997;76:73–5. doi: 10.1136/adc.76.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung MS, Glorieux FH. Intravenous pamidronate in osteogenesis imperfecta Type VII. Calcif Tissue Int. 2009;84:203–9. doi: 10.1007/s00223-008-9211-9. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro JR, Thompson CB, Wu Y, et al. Bone mineral density and fracture rate response to intravenous and oral bisphosphonates in adult osteogenesis imperfect. Calcif Tissue Int. 2010;87:120–9. doi: 10.1007/s00223-010-9383-y. [DOI] [PubMed] [Google Scholar]
- 24.Andiran N, Alikasifoglu A, Gonc N, et al. Cyclic pamidronat therapy in children with osteogenesis imperfecta: results of treatment and follow-up after discontinuation. J Pediatr Endocrinol Metab. 2008;21:63–72. doi: 10.1515/jpem.2008.21.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Aström E, Magnusson P, Eksborg S, Söderhäll S. Biochemical bone markers in the assessement and pamidronat treatment of children with osteogenesis imperfecta. Acta Paediatr. 2010;99:1834–40. doi: 10.1111/j.1651-2227.2010.01968.x. [DOI] [PubMed] [Google Scholar]
- 26.Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight developement during four years of therapy with cyclical intravenous pamidronate in children with osteogenesis imperfecta types I, III, and IV. Pediatrica. 2003;111:1030–6. doi: 10.1542/peds.111.5.1030. [DOI] [PubMed] [Google Scholar]
- 27.Montpetit K, Plotkin H, Rauch F, et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics. 2003;111:601–3. doi: 10.1542/peds.111.5.e601. [DOI] [PubMed] [Google Scholar]
- 28.Di Meglio LA, Ford L, McClintock C, Paecock M. Intravenous pamidronate treatment of children under 36 months age with osteogenesis imperfecta. Bone. 2004;35:1038–45. doi: 10.1016/j.bone.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Munns CF, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Min Res. 2005;20:1235–43. doi: 10.1359/JBMR.050213. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Xia W, Xing X, et al. Benefit of infusions with ibandronate treatment in children with osteogenesis imperfecta. Chin Med J. 2011;124:3049–53. [PubMed] [Google Scholar]
- 31.Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–102. Available from: http://cran.r-project.org/web/packages/nlme/nlme.pdf.
- 32.Lesnoff M., Lancelot R. aod: analysis of overdispersed data. R package version 1.2. 2010 Available from: http://cran.r-project.org/package=aod. [Google Scholar]
- 33.Andersen PE, Jr, Hauge M. Osteogenesis imperfecta: a genetic, radiological, and epidemilogical study. Clin Genet. 1989;36:250–5. doi: 10.1111/j.1399-0004.1989.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 34.Aström E, Sonderhall S. Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatr. 1998;87:64–8. doi: 10.1080/08035259850157895. [DOI] [PubMed] [Google Scholar]
- 35.Plotkin H, Rauch F, Bishop NJ, et al. Pamidronate treatment of servere osteogenesis imperfecta in children und 3 years of age. J. Clin Endocrinol Metab. 2000;85:1846–50. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 36.Aström E, Jorulf H, Söderhäll S. Intravenous pamidronate treatment to infants with severe osteogenesis imperfecta. Arch Dis Child. 2007;92:332–8. doi: 10.1136/adc.2006.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Pediatr. 2006;149:174–9. doi: 10.1359/jbmr.2003.18.4.610. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Grey V. Pediatric reference intervals for bone markers. Clin Biochem. 2006;39:561–8. doi: 10.1016/j.clinbiochem.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92:443–9. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- 40.Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–94. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- 41.Tuchman S, Thayu M, Shults J, et al. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153:484–90. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braga V, Gatti D, Rossini M, et al. Bone turnover markers in patients with osteogenesis imperfecta. Bone. 2004;34:1013–6. doi: 10.1016/j.bone.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Hans D, Alekxandrova I, Njeh C, et al. Appropriateness of internal digital phantoms for monitoring the stability of the UBIS 5000 quantitative ultrasound device in clinical trials. Osteoporos Int. 2005;4:435–45. doi: 10.1007/s00198-004-1683-5. [DOI] [PubMed] [Google Scholar]
- 44.Du YC, Chen YF, Lin CJ, et al. The application of quantitative ultrasound (QUS) on study of aging effects of achilles tendons. Conf Proc IEEE Eng Med Biol Soc. 2005;6:6344–7. doi: 10.1109/IEMBS.2005.1615948. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen TV, Chu J, Sathiakumar C, Pocock NA. Reproducibility and concordance in quantitative ultrasound measurements between densitometers: a comparative study. J Clin Densitom. 2003;6:337–44. doi: 10.1385/jcd:6:4:337. [DOI] [PubMed] [Google Scholar]


