Abstract
Aims
This study aimed to determine the influence of carotid artery surgery on ocular functions and ocular blood flow in patients with ocular ischaemic syndrome (OIS) in the late postoperative period.
Methods
One hundred and eighty patients with OIS were operated on; 104 of them suffered from acute forms of the ischaemic disease and 76 patients had chronic forms of ocular ischaemia. Before surgery and in the course of 6 months and 12 months afterwards, all the patients were examined. Visual acuity, electrophysiological investigations (the threshold of electrical sensitivity (TES) and the level of liability of optic nerve (LON)) and blood flow in orbital vessels were assessed.
Results
After surgery visual acuity increased in patients with the acute forms of OIS (P<0.05). TES and LON also improved (P<0.01). Mean indices of blood-flow velocities in the ophthalmic artery, the central retinal artery and the posterior ciliary arteries increased at 6 and 12 months after surgery (P<0.05). There was ocular blood flow acceleration and decrease of vasoresistance in orbital arteries in both groups of patients.
Conclusions
Carotid artery surgery effectively improved ocular blood flow in patients with acute and chronic forms of OIS in the late post operative period.
Keywords: ocular ischaemic syndrome, carotid artery surgery, ocular blood flow, colour Doppler imaging
Introduction
Occlusion-stenotic lesions of the carotid arteries are one of the main causes of ischaemic stroke, leading to ischaemic lesions of retina and optic nerve in 15–46% of cases.1, 2, 3
The main reason for disrupted visual function with ocular ischaemia is severe stenosis of the extracranial segment of internal carotid artery (ICA).4, 5, 6 Ocular ischaemic syndrome (OIS) results from significantly reduced blood flow to the eye and orbit, most often as a consequence of ipsilateral ICA atherosclerotic stenosis or occlusion.7, 8 Most often, ischaemic ocular symptoms consist of acute transient monocular blindness supposedly caused by entrapment of emboli in the retinal arterial system. Less common is chronic progressive ocular ischaemia; reported frequencies range between 5 and 21% in series of patients with carotid artery stenosis or occlusion.9, 10, 11, 12
Nowadays it is known that in the case of critical stenosis of carotid arteries and ischaemic diseases of brain, carotid artery surgery is the most effective method of treatment.13, 14 Numerous prospective, randomized and multicenter studies have been designed to evaluate the efficacy and safety of carotid endarterectomy.15, 16, 17 The effect of carotid endarterectomy on embolism in the retinal circulation is well known. There is information in several papers where disappearance of amaurosis fugax, decreased neovascularization of the optic nerve head and the iris, disappearance of paresis of the pupil muscle, and improvement of blood flow in orbital vessels occurs.18, 19, 20, 21 However, no clear evidence has been found regarding the efficacy of carotid artery surgery for different clinical forms of ocular ischaemia.
The purpose of this study was to evaluate the effects of carotid artery surgery on ocular functions and ocular blood-flow in patients with OIS in late post-operative period.
Patients and methods
We retrospectively examined 180 patients (158 men, 22 women ) with OIS due to severe stenosis of ICA, who underwent carotid artery surgery at the Department of Vascular Surgery of Russian Research Centre of Surgery from September 1998 to December 2010. Their ages ranged from 58 to 73 years.
One hundred and four (58%) patients had the acute onset form of the disease. They had sudden decrease of vision and a unilateral lesion, including amaurosis fugax in 19 patients, the occlusion of central retinal artery (CRA) and its branches in 31 patients, and acute ischaemic optic neuropathy in 54 patients. The chronic forms of OIS were identified in 76 (42%) patients and differed from the acute type with the following symptoms: progressive decrease of vision, double-sided disease, including chronic ischaemic neuropathy in 41 patients and venous stasis retinopathy (early stage of chronic ocular ischaemia) in 35 patients. In each patient, the intraocular pressure was within the normal range (25 mm Hg). Patients with signs of more advanced chronic ocular ischaemia (neovascularization of the optic disc, retina, rubeosis iridis, ischaemic uveitis, and neovascular glaucoma) were excluded. Patients with severe diabetes mellitus and/or severely disabling stroke were also excluded. More details about concurrent disease and degree of stenosis are listed in Table 1.
Table 1. Characteristics of patients with ocular ischaemic syndrome, n (%).
| Acute type of OIS, n=104 | Chronic type of OIS, n=76 | |
|---|---|---|
| Age (years), (mean±SD) | 61.3±1.6 | 62.4±2.3 |
| Men | 94 (90) | 64 (84) |
| Hypercholesterolaemia | 85 (82) | 54 (72) |
| Hypertension | 87 (91) | 58 (77) |
| Peripheral vascular disease | 22 (21) | 23 (30) |
| Ischaemic heart disease | 56 (54) | 28 (37) |
| Transient ischaemic attack (TIA) | 54 (52) | 24 (32) |
| Chronic cerebral ischaemia | 15 (14) | 34 (45) |
| Stenosis of ICA 70–90% | 82 (79) | 30 (39) |
| Stenosis of ICA >90% | 22 (21) | 46 (60) |
| Stenosis 70–100% contralateral carotid artery | 45 (43) | 11 (14) |
According to the criteria of the North American Symptomatic Carotid Endarterectomy Trial,22 the grades of ICA stenosis as determined by angiography were >90% in 22 (21%) patients with acute forms and 46 (60%) patients with chronic forms of OIS. There was determined stenosis of ICA, >70%, in 82 (79%) patients with acute forms and in 30 (39%) patients with chronic forms of OIS. Indications for surgery were based on the existence of confirmed hemodynamic significant stenosis of the ICA (>70%) in the patients with unilateral neurological, ophthalmological signs; existence of impairment of blood flow in arteries of eye, and appearance in the ICA of hypoechoic, heterogeneous atherosclerotic plaques (I, II, and III types),23 with an uneven and ulcerated surface. Carotid endarterectomy was performed in 113 cases; resection of ICA with reimplantation into the common carotid artery—in 46 cases; and prosthesis of ICA—in 21 cases. Post-operative complications included stroke (one case), transient ischaemic attacks (two cases), and cranial nerve injuries (seven patients). There were no cases of death.
All patients underwent a standardized ophthalmological investigation, including measurement of visual acuity and visual field, slit lamp examination, and dilated fundoscopy. In addition, we evaluated the electrophysiological characteristics of the optic nerve: threshold of electric sensitivity (TES) and liability of the optic nerve (LON) using a device—‘Elektrophosphene' (Neuron, Ufa, Russia).24
It is well known that exposure to the eye of current pulses of several tens of microamperes (mcA) causes a sensation of light flashes called electrophosphen (EP). The minimum pulse power current at which in the eye appears EP is defined as the TES. Based on numerous experimental studies and clinical data,25, 26, 27 it was found that the threshold TES characterizes the functional state of the inner layers of the retina and layer of ganglion cells. It is known that the value of TES retina correlates with the total area of abnormal cells in the field of view: the larger the area of visual field defects, the higher threshold, and lower EP electrical excitability of the retina. The threshold current at which there are subtle flashes of light depends on the frequency of current pulses fed at a frequency of 20 Hz it requires a minimum current to cause EP in the eye.28 With continuous increase in the frequency of the current there comes a moment when an individual ceases to feel EP. This point is denoted as the critical frequency of flicker EP extinction, and is an indicator of the functional state of the axial (central) beam of the optic nerve. The critical frequency of EP disappearance called lability (with respect to the optic nerve—LON) depends on the strength of the current.
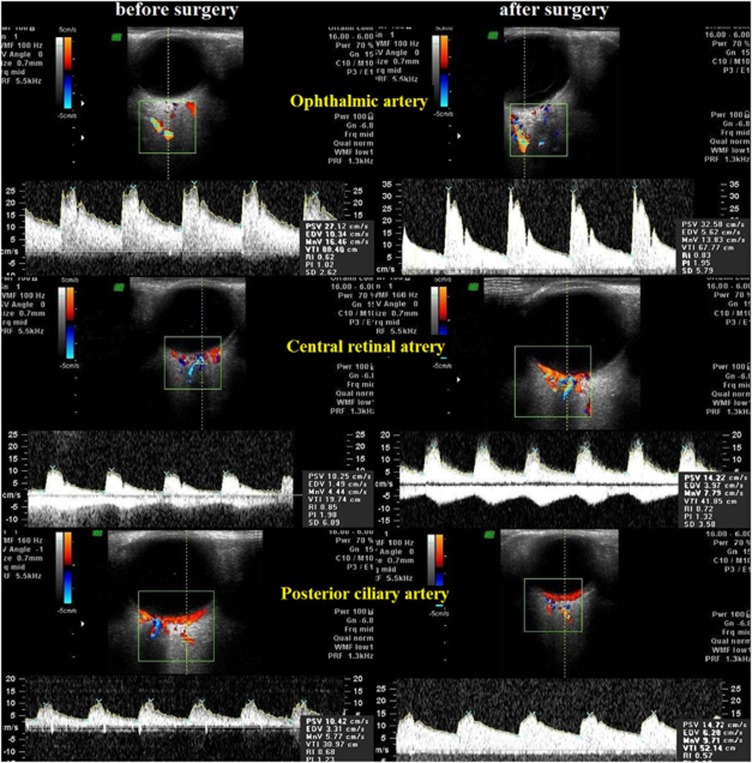
The methods of investigation of the blood flow in orbital vessels included high frequency duplex ultrasonography, Colour Doppler Imaging (CDI), and spectral Doppler analysis. The ultrasound examinations were performed with VOLUSON 730 Pro ultrasound system (GE Medical Systems Kretztechnik GmbH and Co OHG, Zipf, Austria) and SP 10–16 transducer. With the patient in supine position, sterile ophthalmic gel was applied as a coupling to the closed eyelid, and the probe was positioned gently with minimal pressure. Application of grey-scale ultrasound enabled us to obtain the image of the globe and orbit. The CDI method was used to display directly the fine orbital vessels, including the ophthalmic artery (OA) and its branches, the CRA, and the posterior ciliary arteries (PCAs). It was done according to the expected anatomical position of the vessels and its red colour code. The blood flow in the OA was evaluated at the depth of 35 mm. The CRA blood flow was examined in the canal of the optic nerve at the distance of 5–6 mm from the posterior wall of the globe. The PCAs were identified on either side of the optic nerve, about the same distance from the fundus as the CRA and vein. We measured the blood-flow spectrum of vessels and its main indices the peak systolic velocity (Vsyst) and end-diastolic velocity (Vdiast) and resistance index (RI), which is indicative of the downstream resistance of the end organ's vascular bed (Figure 1). Patients who had reversed OA blood flow were excluded from the analysis of the hemodynamic parameters in this artery. The control group included 40 healthy subjects of the appropriate age group.
Figure 1.
Colour Doppler sonograms show the vessels and the corresponding spectral waveforms for the ophthalmic artery, central retinal artery and vein, posterior ciliary arteries before and after carotid surgery.
Methods of investigation of the blood flow in carotid arteries included high frequency duplex ultrasonography, CDI and spectral Doppler analysis, and 3D-mode and 4D real-time sonography. We evaluated the passability of vessels (passable, occlusive), the direction of pathway of the vessels (deformity, pathological curling); the diameter of the vessels; the intima-media thickness; pathological changes inside of vessel (arteriosclerotic plaque, its size, and structure), the state of perivascular tissue.
The examinations were carried out before, and at 6 and 12 months after carotid artery surgery on the carotid arteries.
Statistical analysis included the Student's t-test for paired data and the analysis of variance for repeated readings. The P values of <0.05 were considered significant.
Results
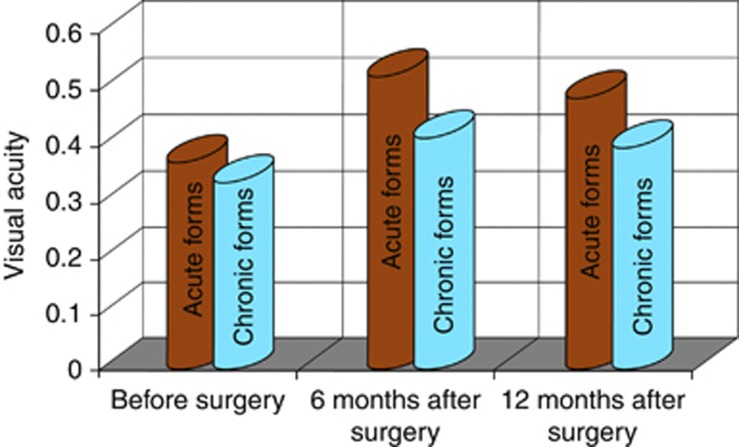
After carotid artery surgery the improvement of visual functions of the affected eye was determined. During the follow-up period, there were no patient complaints of amaurosis fugax or blurred vision. The data on visual acuity in the patients with different types of OIS are presented in Figure 2. A significant increase in visual acuity after operation on the involved side was established in the patients with acute forms of the OIS (before surgery: 0.37±0.05; 6 months after surgery: 0.52±0.07; and 12 months after surgery 0.48±0.03, P<0.05). In chronic forms of the OIS, the positive time course of changes in visual acuity was noted only in 12 (16%) of operated patients.
Figure 2.
Mean parameters of visual acuity in patients with different clinical forms of ocular ischaemic syndrome.
After surgery the examination of the visual fields revealed the expansion of its margins by 10° and more in 42.3% of the patients with acute forms and only in 7.8% of cases with chronic OIS. The perimetry demonstrated the disappearance of visual field defects in 33.7% of patients with acute forms.
There were positive dynamics of TES and LON in the patients with acute forms of OIS (Table 2). In the control group, the TES was 52.7±2.6 mcA, the LON: 43.4±3.2 Hz. The average TES in patients with acute type of OIS were 150.8±26.1 mcA at 6 months and 74.8±19.8 mcA at 12 months after carotid surgery. The average LON in this group of patients rose significantly at 12 months (<0.001). The electrophysiological parameters were found to be affected to a greater measure in acute forms of OIS. However, in the post-operative period, this patient group showed the positive time course of changes in optic nerve functions: a significant decrease in the TES and a rise in the LON as compared with the pre-operative findings. In the group of patients with chronic forms these values did not show a significant change during the follow-up period.
Table 2. Mean parameters of the threshold of electrical sensitivity and the level of liability of optic nerve in OIS patients (mean±SD).
| OIS types |
Before surgery |
After surgery |
||||
| TES, mcA | LON, Hz |
6 months |
12 months |
|||
| |
|
|
TES, mcA |
LON, Hz |
TES, mcA |
LON, Hz |
| Acute type | 276.8±38.1 | 28.5±2.1 | 150.8±26.1 | 34.9±2.2 | 74.8±19.8 | 36.2±2.8 |
| P | <0.01 | <0.01 | <0.001 | <0.001 | ||
| Chronic type | 230.1±36.2 | 29.6±4.1 | 180.6±41.6 | 32.5±5.2 | 174.3±38.5 | 30.7±4.2 |
| P | >0.05 | >0.05 | >0.05 | >0.05 | ||
Note: Р, significance relative to the parameters recorded in the appropriate group before surgery.
After surgery patients with acute OIS showed resolution of the ophthalmoscopic findings.
According to CDI-mode examination before surgery, 36 (20%) patients had retrograde blood flow in the OA, while in one case there was no blood flow at that vessel at all. In 14 (8%) patients blood flow of retinal arteries wasn't registered because of vascular occlusion.
Spectral Doppler analysis in normal OA, CRA, and PCAs revealed pulsing arterial blood flow with a high peak of systolic wave. Before surgery the shape of the peak systolic wave of blood-flow spectrum was attenuated, while after the surgery the peak magnitude was increased (Figure 1). The data on blood flow parameters in the ipsilateral OA before surgery and at 6 and 12 months after surgery are presented in Table 3. The patients who had retrograde OA flow before surgery were excluded from the analysis of the hemodynamic parameters in this artery and showed forward OA flow after carotid surgery. In the post-operative period all patients showed the rise of the Vsyst, the Vdiast blood-flow velocities and decrease RI, evidencing an improvement of blood-flow supply to the globe.
Table 3. Mean hemodynamic parameters in the orbital arteries before the surgery and at 6 and 12 months after carotid surgery (mean±SD).
| Parameter |
The orbital arteries |
|||||
|---|---|---|---|---|---|---|
|
OA |
CRA |
PCAs |
||||
| Acute forms | Chronic forms | Acute forms | Chronic forms | Acute forms | Chronic forms | |
| Before surgery | ||||||
| Vsyst, sm/s | 20.7±0.9 | 21.1±0.5 | 10.4±0.5 | 10.2±0.2 | 7.2±0.4 | 8.1±0.3 |
| Vdiast, sm/s | 1.7±0.3 | 3.5±0.6 | 1.6±0.2 | 1.9±0.09 | 0.22±0.09 | 0.73±0.1 |
| RI | 0.92±0.03 | 0.83±0.04 | 0.85±0.03 | 0.84±0.03 | 0.97±0.08 | 0.91±0.02 |
| After 6 months | ||||||
| Vsyst, sm/s | 29.5±0.4* | 28.8±0.1* | 13.4±0.3* | 13.9±0.2* | 14.8±0.3* | 12.8±0.3* |
| Vdiast, sm/s | 7.7±0.3** | 8.3±0.3** | 3.8±0.1** | 4.2±0.1** | 4.7±0.1** | 4.3±0.1** |
| RI | 0.78±0.05* | 0.7±0.03* | 0.73±0.03* | 0.7±0.07* | 0.68±0.09* | 0.71±0.04* |
| After 12 months | ||||||
| Vsyst, sm/s | 38.9±1.3* | 29.8±0.7* | 13.9±0.2* | 14.4±0.3* | 14.6±0.6* | 13.6±0.2* |
| Vdiast, sm/s | 9.1±0.2** | 4.7±0.2* | 4.8±0.1** | 4.3±0.1** | 4.9±0.5** | 4.6±0.1** |
| RI | 0.69±0.09* | 0.68±0.02* | 0.70±0.04* | 0.69±0.09* | 0.66±0.09* | 0.66±0.09* |
Abbreviations: CRA, central retinal artery; OA, ophthalmic artery; PCAs, posterior ciliary arteries (medial and lateral); RI, resistance index; Vsyst, sm/s, peak systolic velocity; Vdiast, sm/s, end-diastolic velocity.
Note: *P<0.05 and **P<0.01; Р, significance relative to the parameters recorded in the appropriate group before surgery.
Table 3 displays hemodynamic parameters in the CRA and the PCAs before surgery and at 6 and 12 months after carotid surgery in the ipsilateral orbit. Mean Vsyst and Vdiast in CRA increased significantly after surgery (P<0.05 and P<0.001, respectively). Post-operative mean resistance indices were significantly lower than those measured before surgery (P<0.05) (Figure 1). Mean peak systolic blood-flow velocity and Vdiast in the short PCAs increased significantly at 6 and 12 months after surgery (P<0.05 and P<0.001, respectively) (Figure 1). Mean RI decreased at 12 months after surgery (P<0.05).
Discussion
Severe carotid artery stenosis causes decreased or reversed OA flow and causes ocular complications, such as transient monocular blindness, retinal artery occlusion, and ocular ischaemia.10, 29, 30 OIS is the clinical manifestation of ocular hypoperfusion.31 There is a highly significant correlation between ICA stenosis and OA peak systolic flow velocity.21, 32
The effects of carotid artery surgery on the clinical findings in patients with OIS have been investigated by many authors. Rubin et al17 described 18 patients with OIS and severe carotid stenosis who showed an improvement of visual acuity and normalization of the photo-stress test in 87.5% of the cases, as well as a resolution of the ophthalmoscopic changes in 93.3% of the patients after endarterectomy. All our patients with acute forms of OIS showed an increase in visual acuity and resolution of ophthalmoscopic findings after carotid surgery. Electrophysiological parameters of optic nerve function (TES and LON) improved in patients with acute OIS.
There have been some reports that carotid endarterectomy resulted in significantly increased flow in the OA and that it also corrected reversed OA flow.21, 32, 33 Riiheläinen et al21 studied the effects of carotid endarterectomy on blood-flow velocities in the OA and CRA of 17 patients. Similarly to our study the authors reported that the Vsyst and Vdiast increased significantly in both arteries after carotid surgery. However, blood flow in the PCAs was not measured.
Costa et al33, 34, 35 reported about 17 patients with severe occlusive carotid artery disease and neurological symptoms that showed improvement in retrobulbar blood flow after carotid endarterectomy. There was a significant increase in the mean Vsyst and Vdiast at two post-operative intervals in OA, CRA, and PCAs analysed. The significant reduction of the mean resistance indices in the CRA and PCAs was observed 1 week and 1 month after carotid surgery.
Carotid artery surgery is effective for the treatment of OIS and is most beneficial if performed early, before the onset of irreversible neovascular glaucoma, or irreversible ischaemia to the retina.17, 21
The presence of iris rubeosis most likely implies a greater degree of ischaemia in the globe and increased total ocular damage. It was considered an indicator of poor visual prognosis.8 In our study, patients displayed a good visual outcome after carotid surgery. We performed carotid operations on patients without iris rubeosis and with normal intraocular pressure and with the early stage of chronic ischaemic retinopathy or venous stasis retinopathy.
In our study, all patients with OIS had hemodynamic significant stenosis of the ipsilateral ICA (>70%). The ICA stenosis (>90%) was mainly in patients with chronic forms of OIS (60% of cases) compared with acute forms (21 of cases). It should be noted that in all the patients had marked impairment of hemodynamics in ocular vessels independently from percentage of degree of hemodynamic significant ICA stenosis. The improvement of blood flow in OA, CRA, and short PCA was noticed in all patients with OIS in the late post-operative period.
We conclude that carotid artery surgery is an effective treatment for OIS. It allows improving visual functions (visual acuity and visual fields) and electrophysiological characteristics of the visual system in patients with acute forms of OIS. The indices of TES and LON did not change significantly in patients with chronic OIS. This is because of long-lasting ocular ischaemia. Visual acuity improved in only 16% of the patients operated on.
Carotid artery surgery results in ocular blood flow acceleration in all patients presenting with OIS. Modern ultrasound methods including CDI enable us to evaluate the effectiveness of treatment of ischaemic disease of the globe.
The authors declare no conflict of interest.
Footnotes
This work was presented in part at the 23rd World Congress of the International Union of Angiology, 21–25 June 2008 and at the World Ophthalmology Congress, 5–9 June 2010.
References
- Katsnelson LA, Forofonova ТI, Bunin AY.Vascular Diseases of Eye Meditsina: Moscow; 1990. p272 [Google Scholar]
- Atebara NH, Brown GC.Ocular ischemic syndromeIn: Tasman W, Jaeger EA (eds).Duane's Clinical Ophthalmology3rd Vol.Lippincott Williams &Wilkins: Philadelphia, PA; 19981–19.(Chapter 12. [Google Scholar]
- Brown GC, Magarcal LE, Simeone FA, Goldberg RE, Federman JL, Benson WE. Arterial obstruction and ocular neovascularisation. Ophthalmology. 1982;2:139–146. doi: 10.1016/s0161-6420(82)34837-x. [DOI] [PubMed] [Google Scholar]
- Fox GM, Sivalingham A, Brown GC.Ocular ischemic syndromeIn: Yanoff M, Duker J (eds).Ophthalmology Mosby: St Louis, MO; 1999 [Google Scholar]
- Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–864. doi: 10.1016/s0161-6420(97)30221-8. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, Schachat AP, Murphy RP, Patz A.In: Ryan SJ (ed). The Ocular Ischemic Syndrome.Retina 1989II547–559.
- Hovland PG, Ip MS.Ocular ischemic syndromeIn: Huang D, Kaiser PK (eds).Retinal Imaging Mosby: Philadelphia, PA; 2006276–282.(Chapter 27. [Google Scholar]
- Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome: Part III—Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15–20. doi: 10.1007/BF00150974. [DOI] [PubMed] [Google Scholar]
- Kearns TP, Hollenhorst RW. Venous-stasis retinopathy of occlusive disease of the carotid artery. Mayo Clin Proc. 1963;38:304–312. [PubMed] [Google Scholar]
- Kearns TP, Siekert RG, Sundt TM. The ocular aspects of bypass surgery of the carotid artery. Mayo Clin Proc. 1979;54:3–11. [PubMed] [Google Scholar]
- Sarkies NJC, Schilling JS, Ross Russell RW. Fluorescein angiography in carotid disease. Trans Ophthalmol Soc UK. 1986;105:489–493. [PubMed] [Google Scholar]
- Kersemakers P, Beintema M, Lodder J. Venous stasis retinopathy unlikely results from internal carotid artery obstruction alone. Cerebrovasc Dis. 1992;2:305–307. [Google Scholar]
- Hankey GJ. The effect of treating people with reversible ischemic attacks of the brain and eye on the insidence of stroke in Australia. Aust NZ J Med. 1997;27 (4:420–430. doi: 10.1111/j.1445-5994.1997.tb02201.x. [DOI] [PubMed] [Google Scholar]
- Callow AD.Carotid endarterectomy: indications and techniquesIn: Chang JB (ed).Modern Vascular Surgery 5th Vol.Springer-Verlag: NY, Budapest; 199243–51. [Google Scholar]
- Yates GN, Bergamini TM, George SM, Yates GN, Bergamini TM, George SM, Kentucky Vascular Surgery Society Study Group et al. Carotid endarterectomy results from a state vascular society. Am J Surg. 1997;173 (4:342–344. doi: 10.1016/s0002-9610(96)00396-0. [DOI] [PubMed] [Google Scholar]
- Jones CE, Jescovitch AJ, Kahn A, Jones CE, Jescovitch AJ, Kahn A, et al. Technical results from eversion technique of carotid endarterectomy. Am Surg. 1996;62 (5:361–365. [PubMed] [Google Scholar]
- Rubin JR, McIntyre KM, Lukens MC, Rubin JR, McIntyre KM, Lukens MC, et al. Carotid endarterectomy for chronic retinal ischemia. Surg Gynecol Obster. 1990;171:497–501. [PubMed] [Google Scholar]
- Balcer LJ, Galetta SL, Yousem DM, Golden MA, Asbury AK. Pupil-involving third-nerve palsy and carotid stenosis: rapid recovery following endarterectomy. Ann Neurol. 1997;41 (2:273–276. doi: 10.1002/ana.410410221. [DOI] [PubMed] [Google Scholar]
- Geroulakos G. Carotid surgery and ocular ischemia. Eur J Vasc Endovasc Surg. 1997;14 (5:417. doi: 10.1016/s1078-5884(97)80301-2. [DOI] [PubMed] [Google Scholar]
- Hejcmanova D, Jebava R, Kunc P, Hejcmanová D, Jebavá R, Kunc P, et al. Treatment of internal carotid artery occlusion and the ocular ischemic syndrome. Cesk Slov Oftalmol. 1998;54 (6:362–367. [PubMed] [Google Scholar]
- Riihelainen K, Paivansalo M, Suramo I, Laatikainen L. The effect of carotid endarterectomy on ocular blood velocity. Ophthalmology. 1997;104 (4:672–675. doi: 10.1016/s0161-6420(97)30253-x. [DOI] [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- Geroulacos G, Hobson RW, Nicolaides AN.Ultrasonic carotid plaque morphologyIn: Caplan L, Shifrin EG, Nicolaides AN, Moore WS (eds).Cerebrovascular Ischemia Investigation & Management 199625–32.
- Tarasova LN, Kiseleva TN, Fokin AA.Ocular Ischemic Syndrome M. Mditsina: Moscow; 2003. p173 [Google Scholar]
- Delbeke J, Parrini S, Andrien A, Oozer M, Legat V, Veraart C.Modeling activation of visual structures through eyelid surface electrodes preliminary result Pfluegers Arch Eur J Physiol 2000440R4 (abstract no. 5). [Google Scholar]
- Humayun MS, Dejuan E, Dagnetic G, Greenberg RJ, Propst RH, Phillips DH. Visual perception elicited by electrical stimulation of retina in blind humas. Arch Ophthalmol. 1996;114:40–46. doi: 10.1001/archopht.1996.01100130038006. [DOI] [PubMed] [Google Scholar]
- Shahin ME, Rizzo JF, Wyatt J, Loewenstein J.Evaluation of external elektrical stimulation of the eye as a screening test for acute intraocular retinal stimulation studies (ARVO Abstract) Invest Ophthalmol Vis Sci 20004S 860(Abstract no. 4570). [Google Scholar]
- Potts AM, Jnoue J. The electrically evoked response of the visual system (EER). 3. Further contribution to the origin of the EER. Invest Ophthalmol. 1970;9:814–819. [PubMed] [Google Scholar]
- Mawn LA, Hedges TR, Rand W, Heggerick PA. Orbital color Doppler imaging in carotid occlusive disease. Arch Ophthalmol. 1997;115:492–496. doi: 10.1001/archopht.1997.01100150494007. [DOI] [PubMed] [Google Scholar]
- Young LH, Appen RE. Ischemic oculopathy: A manifestation of carotid artery disease. Arch Neurol. 1981;38:358–361. doi: 10.1001/archneur.1981.00510060060009. [DOI] [PubMed] [Google Scholar]
- Sturrock GD, Mueller HR. Chronic ocular ischaemia. Br J Ophthalmol. 1984;68:716–723. doi: 10.1136/bjo.68.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S, Okuno S, Sakaki T, Nishikawa N. Effect of carotid endarterectomy on chronic ocular ischemic syndrome due to internal carotid artery stenosis. Neurosurgery. 2001;48 (2:328–333. doi: 10.1097/00006123-200102000-00016. [DOI] [PubMed] [Google Scholar]
- Costa VP, Kuzniec S, Molnar LJ, Cerri GG, Puech-Leão P, Carvalho CA. Clinical findings and hemodynamic changes associated with severe occlusive carotid artery disease. Ophthalmology. 1997;104:1994–2001. doi: 10.1016/s0161-6420(97)30066-9. [DOI] [PubMed] [Google Scholar]
- Cost VP, Kuzniec S, Molnar LJ, Cerri GG, Puech-Leão P, Carvalho CA. Collateral blood supply through the ophthalmic artery: a steal phenomenon analyzed by color Doppler imaging. Ophthalmology. 1998;105:689–693. doi: 10.1016/S0161-6420(98)94025-8. [DOI] [PubMed] [Google Scholar]
- Costa VP, Kuzniec S, Molnar LJ, Cerri GG, Puech-Leão P, Carvalho CA. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. Opthalmology. 1999;106:306–310. doi: 10.1016/S0161-6420(99)90086-6. [DOI] [PubMed] [Google Scholar]