SUMMARY
The rockpool mosquito, Georgecraigius atropalpus, is a facultatively autogenous species that produces its first egg clutch without a blood meal shortly after emergence. Several days after depositing this clutch, females must take a blood meal to produce a second egg clutch. Decapitation of females shortly after emergence or blood ingestion prevents egg maturation. Here, we report that a single injected dose of the neuropeptide ovary ecdysteroidogenic hormone (OEH) fully restored egg maturation in decapitated females in both circumstances. This neuropeptide and two insulin-like peptides (ILPs) are potent gonadotropins in the related yellow fever mosquito, Aedes aegypti. ILP3 was marginally restorative in decapitated G. atropalpus, and ILP4 had no effect. Egg maturation in non- and blood-fed G. atropalpus was dependent on the enzymatic mobilization of amino acids from stored protein or the blood meal for yolk protein (vitellogenin, VG) synthesis and uptake by oocytes. We further show that OEH stimulates serine protease activity in the fat body of newly eclosed females or in the midgut of blood-fed ones, and ecdysteroid hormone production by the ovaries of both females. In contrast, only 20-hydroxyecdysone stimulated VG synthesis in the fat body of non- and blood-fed females. Using RNA interference to knock down expression of the insulin receptor, we found that OEH still fully restored autogenous egg maturation. In summary, our results identify OEH as a primary regulator of egg maturation in both autogenous and blood-fed G. atropalpus females and suggest the shift from blood meal-dependent to blood meal-independent release of OEH is a key factor in the evolution of autogeny in this species.
KEY WORDS: neuropeptide, insulin-like peptides, ecdysteroid hormone, autogeny, anautogeny
INTRODUCTION
Mosquitoes (Diptera: Culicidae) form a monophyletic assemblage that is currently divided into two subfamilies (Anophelinae and Culicinae), 44 genera and >3500 species (Harbach and Kitching, 1998; Harbach, 2007; Bertone et al., 2008; Reidenbach et al., 2009). Typically, adult mosquitoes obtain nutrients for survival and reproduction from three sources (Clements, 1992; Foster, 1995): (1) teneral reserves from larval feeding on the microbiota and detritus in water, (2) nectar or other plant juices for energy, and (3) blood taken only by females for egg production. Mosquito species for which females require a blood meal to mature eggs are called anautogenous (Roubaud, 1929). Virtually all anautogenous species lay multiple clutches of eggs, and development of each clutch depends upon the female consuming one or more blood meals (Clements, 1992; Briegel, 2003). Some mosquito species are referred to as facultatively autogenous because the females mature a first clutch of eggs without a blood meal, but then must blood feed to produce subsequent clutches of eggs (Rioux et al., 1975; O'Meara and Lounibos, 1981; O'Meara, 1985; Attardo et al., 2005; Provost-Javier et al., 2010). A few species are obligately autogenous because females produce eggs without ever blood feeding (O'Meara et al., 1981; Masler et al., 1983).
Phylogenetic studies indicate an ancient origin for the Culicidae with divergence of the lineages leading to the Anophelinae and Culicinae occurring approximately 217 million years ago (Bertone et al., 2008; Reidenbach et al., 2009). The basal position of the Anophelinae together with nearly all known anophelines being anautogenous also strongly suggests that the common ancestor of mosquitoes had to blood feed to produce eggs. However, prior surveys (Rioux et al., 1975) as well as our more recent search of the literature indicates that facultative or obligate autogeny has arisen multiple times in the Culicinae. A key question in mosquito life history, therefore, is how do anautogenous species regulate egg formation and what changes in this regulatory program or its initiation have led to the evolution of autogeny?
Egg development among anautogenous species is best understood in the yellow fever mosquito, Aedes aegypti (Culicinae, Tribe Aedini), which transmits several pathogens that cause disease in humans including Dengue fever and Yellow fever. Upon eclosion, adult females enter a pre-vitellogenic phase where the corpora allata secrete juvenile hormone (specifically JH III) (Li et al., 2003). This programs reproductive competency by promoting the expression of the target of rapamycin (TOR) and ecdysteroid hormone (ECD) signaling pathways in the ovaries, fat body and midgut (Hansen et al., 2004; Zhu et al., 2003; Zhu et al., 2006; Clifton and Noriega, 2011). Oogenesis remains arrested, however, until a blood meal is taken, which triggers the release of insulin-like peptides (ILPs) and ovary ecdysteroidogenic hormone (OEH) from neurosecretory cells in the brain (Riehle et al., 2006; Brown et al., 1998). ILP3, ILP4 and OEH stimulate the ovaries to produce ECD (Brown et al., 1998; Brown et al., 2008; Wen et al., 2010). ILP3 together with amino acid sensing through the TOR pathway induces serine protease activity in the midgut that digests the blood meal, while ILP3, TOR and ECD interact to upregulate the expression of vitellogenin (VG) and other yolk proteins by the fat body (Hansen et al., 2005; Roy et al., 2007; Bryant et al., 2010; Roy and Raikhel, 2011; Gulia-Nuss et al., 2011). Packaging of yolk proteins into oocytes followed by chorion formation then results in the formation of 120–150 mature eggs, which females then oviposit.
The rockpool mosquito, Georgecraigius atropalpus (Coquillett 1902) (formerly Aedes and Ochlerotatus) (Culicinae, Aedini), resides in the same tribe as A. aegypti (Reinert et al., 2009) but is facultatively autogenous. Prior studies indicate that ECD production by the ovaries and yolk uptake by oocytes rise and fall within 10–50 h post-eclosion (PE) followed by oviposition at 60–72 h PE (Fuchs et al., 1980; Masler et al., 1980; Masler et al., 1981; Birnbaum et al., 1984; Kelly et al., 1981; Kelly et al., 1984). Decapitation of G. atropalpus females immediately after eclosion blocks yolk uptake into oocytes, while injection of crude head extracts from A. aegypti and 20-hydroxyecdysone (20E) partially rescue egg maturation. To mature a second clutch of eggs, G. atropalpus requires a blood meal, which females seek approximately 7 days after laying the first clutch of eggs (Hudson, 1970; Masler et al., 1983; Bowen et al., 1994). Taken together, these results suggest that, similar to A. aegypti, egg maturation by G. atropalpus depends upon neuroendocrine factors from the brain. The identity of these neuroendocrine factors, however, and whether the same cascade of events occurs in producing a blood meal-independent first clutch or blood meal-dependent second clutch is unknown. Here, we present results indicating that OEH is the key factor released from the brain that stimulates both first and second egg clutch formation in G. atropalpus.
MATERIALS AND METHODS
Mosquitoes
Georgecraigius atropalpus (Bass Rock strain, founded from a colony at the University of Arizona) was maintained as described previously (Telang and Wells, 2004; Telang et al., 2006). Eggs for colony maintenance were obtained from first clutches laid by females fed a 10% sucrose solution. For mating studies, late pupae (ca. 24 h prior to adult eclosion) were sexed by size, and the larger female pupae held separately from male pupae in cages for adult emergence. Mated females were collected from cages holding the same cohort of both sexes. All females mated shortly after eclosion as confirmed by the observation of sperm in the spermatheca. Rats were anesthetized and provided to females for blood feeding in compliance with the Animal Care and Use Program at the University of Georgia.
Reagents
Aedes aegypti ILP3 (AaILP3) and ILP4 (AaILP4) were synthesized as previously described (Brown et al., 2008; Wen et al., 2010). 20E (Sigma) was solubilized in absolute ethanol. Recombinant A. aegypti OEH (AarlOEH; residues 23–149, minus the signal peptide) was produced in Escherichia coli and purified as previously outlined (Brown et al., 1998) with the exception that OEH was cloned into pET-32 instead of pET-17. A stock of the AarlOEH (100–150 pmol μl−1) in water was prepared for dilution prior to bioassay. The dose-responsive bioactivity of AarlOEH (13.3 kDa) was equivalent to that of the native truncated form (8803 kDa) isolated from head extracts, as determined by gonadotropic bioassays with A. aegypti (Brown et al., 1998).
First clutch in vivo assays
Newly eclosed females (0–6 h PE) were decapitated and injected with AarlOEH, AaILPs or 20E, in 0.5 μl of saline. Decapitated females injected with saline served as a negative control while intact (non-decapitated) females served as a positive control. Decapitated females were then held in small cages housed in humidified chambers while intact females were held similarly but with access to water. For oviposition assays, at least 10 females per treatment were kept individually in small cages lined with a moist paper towel, and eggs were counted 96–120 h PE. Tissues were dissected from females in saline and set up as triplicate samples for the assays described below. Experiments were replicated with at least three different cohorts, and the following data were collected.
Yolk deposition was determined by measuring the length of the anterior–posterior axis of 10 oocytes per ovary pair of each experimental female. ECD production by ovaries in vitro (2 pairs/dish) was assayed after incubation for 6 h (27°C) in 60 μl of saline or amino acid-rich medium (Sf-900 II SFM medium; cat. no. 10902, Gibco, Gaithersburg, MD, USA) alone or with other reagents. ECD content in each sample of medium (50 μl) was determined by radioimmunoassay (RIA) (Wen et al., 2010). Total protein was determined for abdomen walls (i.e. whole-abdomen body wall with attached fat body minus gut and ovaries) and hemolymph at different times during egg maturation. Two females were opened in 100 μl of saline, and the diffused hemolymph was transferred to a tube. The abdomen walls were dissected free from both females, homogenized in saline and centrifuged (12,000 g, 4°C). After appropriate dilution, the protein concentration of abdomen wall supernatant and hemolymph samples was determined by the Bradford assay (Pierce, Rockford, IL, USA). Serine protease activity was assayed in abdomen wall, thorax and midgut samples (Gulia-Nuss et al., 2011). To determine trypsin-like activity, 2 tissues/sample were transferred to 200 μl of 20 mmol l−1 Tris (pH 8.0 with 20 mmol l−1 CaCl2), sonicated and centrifuged (14,000 g, 2 min). Supernatants were frozen (−80°C), and later 0.05 tissue/10 l was added to 200 μl of 4 mmol l−1 N-α-benzoyl-l-arginine-p-nitroanilide (BApNA) in a well of a 96-well plate for 10 min followed by measurement of absorbance at 405 nm (Biotek plate reader, Winooski, VT, USA). Enzyme activity was then calculated from a regression line formula using trypsin standards (bovine pancreas, Sigma T1426, St Louis, MO, USA). To assay chymotrypsin-like activity (Masler et al., 1983), tissues were transferred to 200 μl of 0.1 mmol l−1 cold HCl and processed as above. Later, 0.05 tissue/sample was added to a well with 140 μl of 20 mmol l−1 Tris (pH 7.0) and 8 μl 20 mmol l−1 CaCl2, followed by 100 μl benzoyl-l-tyrosine ethyl ester (BTEE) substrate. Samples were incubated for 5 min, loaded in wells of a Greiner UV-compatible 96-well plate for measurement of absorbance at 256 nm (Biotek plate reader). Activity was quantified based on α-chymotrypsin standards (bovine pancreas, Sigma C4129).
Second clutch in vivo assays
After oviposition of the first egg clutch, females were maintained in cages and provided with a sucrose solution ad libitum. At 10–12 days PE, females were attracted to a human arm held near the cage, and only then were females given access to an anesthetized rat for blood feeding. Only females that took a full blood meal were used in the experimental procedures and assays as described above. Bioassays as above were replicated with at least three different cohorts.
In vitro assays
Females decapitated <6 h PE were held overnight in small cages in a humidified chamber (27°C). Abdomen walls (2 mosquitoes cut along one side to float flat on 100 μl) and midguts (2 in 60 μl) were incubated 4 h (27°C) in saline or Sf-900 II SFM medium alone or with AarlOEH, AaILPs or 20E and then assayed for serine protease activity or yolk deposition into oocytes, as described above. Ovaries (2 pairs in 60 μl) were similarly incubated for 6 h and assayed for ECD production. Triplicate tissue samples were prepared for each of three different cohorts.
RNA interference knockdown of the G. atropalpus insulin receptor
Total RNA was isolated from G. atropalpus abdomen walls and first strand cDNA was synthesized as previously described (Gulia-Nuss et al., 2011). A portion of the G. atropalpus insulin receptor (IR) gene was amplified from this cDNA pool using specific primers (forward 5′-TAATACGACTCACTATAGGGCCGGAGGTGAATCCAGACTA-3′ and reverse 5′-TAATACGACTCACTATAGGGCTTCTTTGCCGAAAGTACGC-3′) for the corresponding carboxyl domain (nucleotides 3001–3546) of the IR gene from A. aegypti (Brown et al., 2008). The purified PCR product (Qiaprep, Qiagen, Valencia, CA, USA) was cloned into TOPO-TA, sequenced, and determined to be 95% identical to the A. aegypti IR (CLUSTAL 2.0.12). We therefore prepared dsRNA corresponding to nucleotides 3079–3521 of the A. aegypti IR and an enhanced green fluorescent protein (eGFP) dsRNA control as previously described (Gulia-Nuss et al., 2011). Intact or decapitated G. atropalpus females (<6 h PE) were then injected with 2 μg of each dsRNA in 0.5 μl water. Knockdown of the G. atropalpus IR was then determined by RT-PCR amplification of actin and IR products from cDNA processed from abdomen walls of females 24 h post-dsRNA injection (Gulia-Nuss et al., 2011). Bioassays as above were replicated with at least three different cohorts.
Immunoblots
To detect VG, abdomen walls were dissected in saline and transferred to sample buffer for sonication/extraction and centrifugation. Supernatants were loaded on a 4–20% Tris-HCl gel (BioRad Criterion gels, Hercules, CA, USA), transferred to nitrocellulose membrane (Protran 0.2 μm, Whatman), and probed with rabbit antiserum to A. aegypti VG (R2,1; 1:100,000 dilution) (Gulia-Nuss et al., 2011). Immunoreactive proteins were visualized with a peroxidase-conjugated goat anti-rabbit secondary antibody (1:20,000 dilution; Sigma A6667) and chemiluminescent substrate (ECL Advance kit, GE Healthcare, Wauwatosa, WI, USA). To detect OEH, heads were removed from females, and hemolymph collected as above in saline and 2× tricine sample buffer. Samples were sonicated and centrifuged, and the supernatants loaded on 16% Tris-tricine gels (BioRad Criterion gels). After transfer to nitrocellulose membrane (Protran 0.1 μm, Whatman) and drying, the blot was incubated with rabbit antiserum to A. aegypti OEH (304C, 1:10,000 dilution) (Brown and Cao, 2001).
Data analysis
All analyses were conducted using the JMP 7.0 statistical platform (SAS, Cary, NC, USA). Yolk deposition into oocytes, ecdysteroid production by ovaries, and serine protease activity were analyzed by ANOVA followed by the Tukey–Kramer multiple comparison procedure with treatment serving as the independent variable. The number of mature follicles and number of eggs laid by virgin and mated females were analyzed by t-test.
RESULTS
OEH and 20E stimulate production of the first autogenous egg clutch
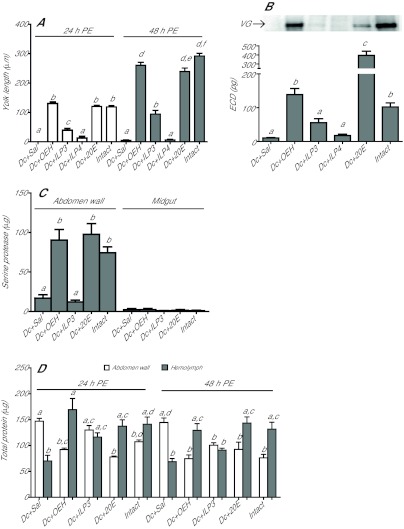
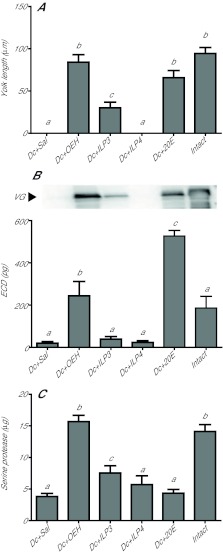
Given the roles of OEH and ILPs in egg maturation by A. aegypti, we first asked whether decapitation of newly emerged G. atropalpus blocked egg maturation and whether OEH or two ILPs from A. aegypti rescued this response. Our results showed decapitated females deposited no yolk into eggs while a single injection of AarlOEH (10 pmol) or 20E (1 μg) rescued yolk deposition to levels seen in intact females at 24 and 48 h PE (Fig. 1A). A single dose of AaILP3 (20 pmol) stimulated only half the level of yolk deposition observed in intact females, while the same dose of AaILP4 had no stimulatory effect (Fig. 1A). Higher doses of each ILP did not alter these outcomes (data not shown). AarlOEH and 20E but not ILPs also rescued VG synthesis in the fat body (abdomen wall) and ECD production by the ovaries (Fig. 1B, top and bottom panel, respectively).
Fig. 1.
Aedes aegypti recombinant long ovary ecdysteroidogenic hormone (AarlOEH) and 20-hydroxyecdysone (20E) restore autogenous egg maturation in decapitated Georgecraigius atropalpus females. (A) Decapitation (Dc) of females at 6 h post-eclosion (PE) blocks yolk deposition in oocytes (Dc+Sal, negative control), but a single injection of AarlOEH (10 pmol, OEH) or 20E (1 μg) after decapitation stimulates yolk deposition (mean ± s.e.m. yolk length/oocyte) to the same extent as in intact females at 24 and 48 h PE (F11,390=175.4, P<0.0001). Aedes aegypti insulin-like peptide 3 (AaILP3, 20 pmol, ILP3) is less effective and AaILP4 (20 pmol, ILP4) has no activity. Intact females injected with saline (Sal) served as positive controls. Different letters above bars in the graph indicate means that significantly differ; comparison of all pairs of means was made using the Tukey–Kramer procedure, α=0.05 (see Materials and methods). (B) AarlOEH stimulates vitellogenin (VG) expression in fat body and ecdysteroid hormone (ECD) production in ovaries of decapitated females to the same levels detected in intact females. Top, representative immunoblot of VG (220 kDa) expression at 24 h PE in abdomen walls (0.05 wall/lane) of females treated as above. Bottom, ECD production by ovaries (mean and s.e.m. for 2 pairs/6 h) taken at 24 h PE from females treated as above (F5,120=50.8, P<0.0001). (C) AarlOEH and 20E induce chymotrypsin-like serine protease activity (BTEE substrate; mean and s.e.m. values per abdomen) in the abdomen wall of decapitated females to the same level in intact females at 24 h PE (F4,31=26.6, P<0.0001). No chymotrypsin-like activity detected in midguts from the same females (mean and s.e.m. values per midgut). (D) AarlOEH and 20E treatment of decapitated females reduces teneral protein in abdomen walls and increases hemolymph protein at 24 and 48 h PE (mean and s.e.m. values per female). Corresponding levels in intact females indicate yolk protein secretion, but reciprocal levels in decapitated control females reflect inhibition of egg maturation (F19,173=10.2, P<0.0001).
Production of the first egg clutch by G. atropalpus is dependent on mobilization of the teneral protein reserve in the fat body by chymotrypsin-like serine protease activity, as shown in a previous study (Masler et al., 1983). Consistent with this finding, we observed that chymotrypsin-like activity increased in the fat body of intact females but not in decapitated ones by 24 h PE (Fig. 1C). Neither chymotrypsin-like activity nor trypsin-like serine protease activity was detected in the midguts of the experimental females (Fig. 1C; also not in other tissues, data not shown). AarlOEH and 20E but not AaILP3 restored this chymotrypsin-like activity in decapitated females to the level in intact females (Fig. 1C). Fully consistent with this increase in protease activity, AarlOEH and 20E treatment also reduced total protein content in the fat body and increased hemolymph protein to levels observed in intact females (Fig. 1D), thus reflecting fat body secretion of VG into hemolymph. In control decapitated females, the high protein content in fat body and low hemolymph protein indicate VG secretion is not activated (Fig. 1D).
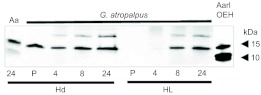
Georgecraigius atropalpus releases OEH from the brain between 4 and 8 h PE
As we used AarlOEH in the preceding assays, we assessed whether G. atropalpus produces OEH by probing immunoblots of head extract and hemolymph with our AaOEH antiserum. AaOEH (13.7 kDa processed long form) was readily detected in A. aegypti head extract, as was AarlOEH (Fig. 2). A corresponding 14 kDa band was detected in head extracts from G. atropalpus pupae and adult females at different times PE (Fig. 2). We also detected the putative native OEH in the hemolymph of adult females at 8 h and 24 h PE, which coincides with the activation of egg maturation for the first clutch (Fig. 2). It was not present in the hemolymph of pupae or females at 4 h PE, thus suggesting release from the brain neurosecretory cells occurs around 6 h PE, the latest time for decapitation to prevent autogeny.
Fig. 2.
Representative immunoblot of head extracts and hemolymph probed with AaOEH antiserum. Left side shows head extracts (Hd, 4 heads/lane) from female A. aegypti (Aa, positive control) and G. atropalpus pupae (P) and females at 4, 8 and 24 h PE. Right side shows hemolymph (HL) collected from G. atropalpus pupae and females at 4, 8 and 24 h PE (20 females/lane) and AarlOEH (5 pmol/lane, positive control). Mass markers for 10 and 15 kDa are indicated on the right.
OEH and 20E activate different tissue-specific processes in vitro
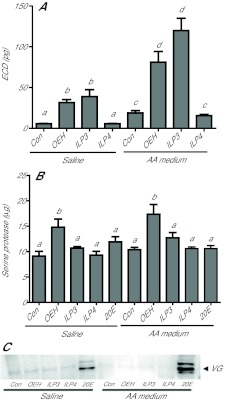
The preceding results indicated that AarlOEH was more bioactive than AaILPs in stimulating ECD production, VG synthesis and mobilization of nutrient stores. To confirm that the effects of these neuropeptides on the ovary and fat body were direct, we conducted in vitro assays using primary cultures. We also compared the effect of each neuropeptide when each tissue was cultured in saline versus amino acid-rich Sf-900 II SFM medium (AA medium) because the latter greatly enhances the in vitro response of A. aegypti tissues (Gulia-Nuss et al., 2011). Preliminary studies indicated that ovaries and fat bodies isolated at 6 h PE from G. atropalpus females were not responsive to either OEH or ILPs, but those taken from females decapitated and held overnight were fully responsive. AarlOEH and AaILP3 stimulated ovaries to produce significantly more ECD than control ovaries, whereas AaILP4 had no stimulatory effect (Fig. 3A). The absolute response to each neuropeptide, however, was also significantly higher for ovaries cultured in AA medium than in saline, suggesting a key role for amino acid sensing in this response. AarlOEH significantly increased chymotrypsin-like activity in fat bodies incubated with or without AA medium (Fig. 3B), but no increase in activity was detected in response to ILPs or 20E. In contrast, only 20E stimulated VG synthesis in vitro with a stronger response detected in fat bodies cultured in AA medium than in saline (Fig. 3C).
Fig. 3.
AarlOEH, AaILP3 and 20E activate different tissue-specific processes in vitro. Georgecraigius atropalpus females were decapitated at <6 h PE and held overnight. Dissected ovaries or abdomen walls were incubated in saline (Sal) or amino acid-rich Sf-900 II SFM medium (AA medium) alone (control, Con) and with the reagents. Statistical comparisons were performed as indicated in Fig. 1A. (A) Ovary ECD production (mean and s.e.m. for 2 pairs/6 h) is stimulated by AarlOEH (10 pmol, OEH) and AaILP3 (20 pmol, ILP3), but not AaILP4 (20 pmol, ILP4) (Saline: F3,35=12.6; AA: F3,38=22.8; P<0.0001). AA medium enhances activation of this process. (B) Chymotrypsin-like serine protease activity (BTEE substrate; mean and s.e.m. values per abdomen for 4 h) is induced by AarlOEH (15 pmol) but not by the AaILPs (30 pmol) or 20E (1.5 μg) (F9,30=6.4, P<0.0001). (C) Representative immunoblot showing induction of VG (220 kDa) synthesis in abdomen walls (0.5 wall/lane) incubated with 20E (1.5 μg) but not in samples incubated with AarlOEH (15 pmol) or the AaILPs (30 pmol).
OEH activity does not depend on the insulin receptor
OEH and ILP3 exhibit similar gonadotropic activity in A. aegypti (Brown et al., 1998; Brown et al., 2008), thus suggesting that, like ILP3, OEH may interact with the mosquito IR. Our sequencing of G. atropalpus IR revealed that it is nearly identical to that of A. aegypti (see Materials and methods). However, as AarlOEH exhibits much greater activity than AaILP3 in G. atropalpus, we reasoned this semi-autogenous species could provide interesting insights into whether OEH activity is IR dependent or independent.
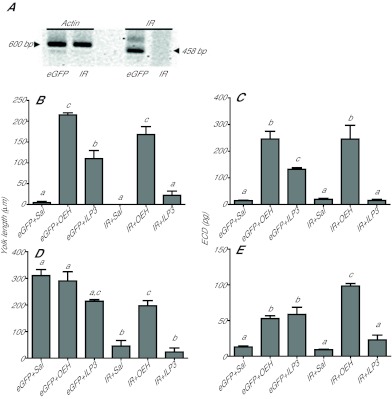
We used RNA interference (RNAi) to knock down expression of G. atropalpus IR. The absence of IR expression was confirmed by RT-PCR for decapitated and intact females 24 h after injection of IR and eGFP dsRNA (Fig. 4A). We then compared the ability of AarlOEH and AaILP3 to restore yolk deposition into oocytes and ovary ECD biosynthesis in decapitated, IR knockdown females relative to control (eGFP dsRNA pretreated), decapitated females. Strikingly, our results showed that AarlOEH rescued both responses in IR knockdown females to levels similar to those observed in control females (Fig. 4B,C). In contrast, ILP3 had essentially no effect in IR knockdown females relative to its activity in control females.
Fig. 4.
OEH-mediated yolk deposition and ECD production does not require the insulin receptor (IR) in G. atropalpus. Females were injected with IR or enhanced green fluorescent protein (eGFP, control) dsRNA at <6 h PE, then decapitated or left intact, held overnight and injected with saline (control), AarlOEH (10 pmol) or AaILP3 (20 pmol). After 24 h or approximately 48 h PE, tissues were assayed as below. Statistical comparisons were performed as indicated in Fig. 1A. (A) Georgecraigius atropalpus IR expression is silenced by IR RNAi. RT-PCR of actin and IR products amplified from cDNA processed from abdomen walls of females 24 h post-dsRNA injection. (B) Oocyte yolk deposition (mean and s.e.m. yolk length/oocyte) in decapitated females (F5,46=56.7, P<0.0001). (C) ECD production by ovaries (mean and s.e.m. for 2 pairs/6 h) taken from decapitated females (F5,33=24.0, P<0.0001). (D) Oocyte yolk deposition (as above) in intact females (F5,47=23.5, P<0.0001). (E) ECD production by ovaries (as above) taken from intact females (F5,26=39.3, P<0.0001).
We also examined the effect of IR knockdown on the same processes in intact G. atropalpus females. Control (eGFP dsRNA pre-treated) females injected with saline deposited the same amount of yolk in oocytes as normal, intact G. atropalpus (Fig. 4D; see Fig. 1A for intact level). Injection of AarlOEH or AaILP3 into cohort control females also did not alter this process (Fig. 4B,C), and presumably did not interfere with the release of endogenous OEH and ILPs from their brains. Ovary ECD production was low in the control females injected with saline (Fig. 4E) because this process is normally completed by 48 h PE (the assay time), but injection of AarlOEH and AaILP3 extended ECD production in the control females (Fig. 4E). In contrast, the IR knockdown females injected with saline or AaILP3 deposited almost no yolk into oocytes and exhibited low ECD production (Fig. 4D,E), but injection of AarlOEH into these females restored both processes to levels similar to those in the control females injected with the two peptides (Fig. 4D,E). Taken together, these data strongly suggested that AarlOEH activity in G. atropalpus does not depend on the IR, whereas AaILP3 activity does.
Maturation of eggs in the first clutch does not depend upon mating
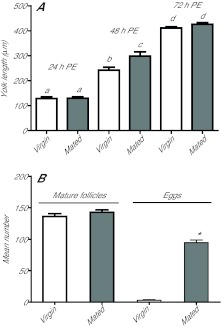
As G. atropalpus produces a first clutch without blood feeding, we asked whether mating served as an alternative signal for stimulating the release of OEH and ILPs from the brain of newly emerged females. Our results showed that yolk deposition into oocytes did not differ greatly between mated and virgin females at 24, 48 or 72 h PE (Fig. 5A). The number of follicles (~60/ovary) with mature oocytes also did not differ (data not shown). However, mating status was crucial for oviposition as mated females laid more than 100 eggs by 120 h PE, whereas virgin females laid almost none (Fig. 5B). Subsequent observations found that virgin females retained mature oocytes for at least 10 days.
Fig. 5.
Mating affects egg laying by G. atropalpus but not autogenous egg maturation. (A) Oocyte yolk deposition (mean and s.e.m. yolk length/oocyte) in virgin and mated females at 24, 48 and 72 h PE (F5,97=188.9, P<0.0001). Statistical comparisons performed as indicated in Fig. 1A. (B) Mature follicles (observed at 72 h PE) and eggs oviposited by virgin and mated females (mean and s.e.m. number/female). No difference was observed in follicle numbers, but mated females laid significantly more eggs (*) than virgin females (t-tests, α≤0.01, N=16–20 females per treatment).
OEH and 20E also stimulate production of a second egg clutch
As G. atropalpus fully depends on blood feeding to produce a second egg clutch, we assessed whether the stimulatory activity of AarlOEH, AaILPs and 20E in egg maturation remained similar to or differed from the first clutch. The females of our G. atropalpus colony are not attracted to a human host for approximately 7 days after ovipositing the first clutch of eggs. Thereafter, females would orient to a human host and feed from an anesthetized rat. Decapitation of females within 1 h post-blood meal blocked egg maturation, while a single injection of AarlOEH (10 pmol/female) or 20E (1 μg) rescued yolk deposition into oocytes, VG synthesis by the fat body, and ECD production by the ovaries to levels observed in intact females at 24 h post-blood meal (Fig. 6A,B). Similar to outcomes in producing a first clutch, AarlOEH stimulated more yolk deposition and VG synthesis than AaILP3, while AaILP4 exhibited no activity. Also similar to production of a first clutch, AarlOEH and 20E stimulated ECD production by the ovaries but neither ILP exhibited any activity.
Fig. 6.
AarlOEH and 20E restore egg maturation in blood-fed, decapitated female G. atropalpus. At 12 days PE, females were blood fed, decapitated (Dc) after 1 h, and given a single injection of saline (Sal, negative control), AarlOEH (10 pmol, OEH), AaILP3 (20 pmol, ILP3), AaILP4 (20 pmol, ILP4) or 20E (1 μg). Intact females were injected with saline as a positive control. At 24 h post-blood meal, females were processed for the following assays. Statistical comparisons were performed as indicated in Fig. 1A. (A) AarlOEH and 20E stimulate yolk deposition (mean and s.e.m. yolk length/oocyte) in decapitated females to levels observed in intact females (F5,159=37.6, P<0.0001). (B) AarlOEH and 20E stimulate VG expression in fat body and ECD production in ovaries of decapitated females. Top, representative immunoblot of VG (220 kDa) in abdomen walls (0.05 wall/lane). Bottom, ECD production by ovaries (mean and s.e.m. for 2 pairs/6 h) (F5,53=16.9, P<0.0001). (C) AarlOEH induces trypsin-like serine protease activity (BApNA substrate; mean and and s.e.m. values per midgut) in the midgut of decapitated females to the same level as that in intact females (F5,127=30.1, P<0.0001).
Whereas chymotrypsin-like activity rises in the fat body during first-clutch formation, an earlier study found that trypsin-like serine protease activity is predominant in the whole body of G. atropalpus 12–48 h post-blood feeding (Masler et al., 1983). Our assays revealed that the trypsin-like activity is restricted to the midgut of intact blood-fed females (Fig. 6C; data for other tissues not shown), and no chymotrypsin-like activity is detected in the midgut or other tissues of these females (data not presented). Decapitation after blood feeding blocked the increase in trypsin-like activity observed in intact females at 24 h post-blood meal, but a single dose of AarlOEH restored this to the same level of activity in decapitated females (Fig. 6C). In contrast, neither AaILPs or 20E had any rescue effect (Fig. 6C).
DISCUSSION
Vertebrate blood provides a rich source of nutrients for egg production by mosquitoes, but acquisition of this resource also has costs that include the time required to find a suitable host and the risk of mortality while feeding. The ability to produce eggs without blood feeding obviously eliminates these costs, but the physiological and/or regulatory changes that underlie the evolution of autogeny largely remain unclear. In the case of G. atropalpus, females always produce their first clutch of eggs autogenously while a second clutch requires a blood meal (Hudson, 1970; Masler et al., 1983; Bowen et al., 1994). The amount of food a female acquires during the larval stage does not alter this pattern, although teneral reserves and sugar feeding as an adult do affect how many eggs a female produces in her first clutch (i.e. clutch size) (Telang and Wells, 2004; Telang et al., 2006). Genetic analysis indicates that autogeny is monofactoral and not sex linked (O'Meara and Craig, 1969; Gwadz, 1970; O'Meara and Krasnick, 1970; O'Meara, 1972; Masler et al., 1981). Prior studies also clearly establish that maturation of a first clutch depends on the release of neurohormones from the brain, which stimulates ECD production by the ovaries, mobilization of teneral nutrient reserves in the fat body, and VG production (Van Handel, 1976; Fuchs et al., 1980; Masler et al., 1980; Masler et al., 1981; Masler et al., 1983; Birnbaum et al., 1984; Kelly et al., 1981; Kelly et al., 1984; Ma et al., 1984).
The central question of this study was whether OEH and ILPs, which regulate egg maturation in A. aegypti, also regulate egg production in G. atropalpus. Our results show that a single dose of AarlOEH stimulates maturation of both a first and a second clutch in decapitated females by restoring ovary ECD production and protein mobilization, so that vitellogenesis and yolk deposition can proceed. AaILP3, in contrast, is less effective in rescuing maturation of either clutch, while AaILP4 exhibits no activity in G. atropalpus. Both AarlOEH and AaILP3 directly stimulate ovaries of G. atropalpus to produce ECD with the activity of each being greater in medium containing amino acids. This finding is very similar to previous studies with A. aegypti which showed that ILP/insulin signaling interact with amino acid sensing through the TOR pathway to activate ovary ECD production and VG synthesis (Hansen et al., 2005; Roy et al., 2007; Roy and Raikhel, 2011; Gulia Nuss et al., 2011). Prior studies show that G. atropalus ovaries predominantly produce ecdysone, which is converted to 20E in target tissues, and that 20E stimulates egg maturation in decapitated autogenous females (Masler et al., 1980; Birnbaum et al., 1984). Our study confirmed that 20E activates maturation not only of the first egg clutch but also of the second one in decapitated G. atropalpus females, and it alone stimulates VG synthesis in isolated G. atropalpus fat body. These findings are fully shared with A. aegypti (Attardo et al., 2005; Roy et al., 2007) in that ECD signaling is a potent promoter of VG synthesis. Finally, our results strongly suggest that AarlOEH mimics a native OEH detected in the brains of G. atropalpus females and released into the hemolymph at the initiation of autogenous egg maturation.
Taken together, our results indicate that, as in A. aegypti, OEH, ILP, ECD and amino acid/TOR signaling control egg maturation in G. atropalpus during formation of both an autogenous first clutch and anautogenous second clutch. The crucial difference is that G. atropalpus produces an autogenous first clutch because it releases OEH and likely ILPs within the first 6–12 h after eclosion without blood feeding, whereas OEH and ILP release in A. aegypti is blood meal dependent. Transplantation experiments conducted with autogenous and anautogenous strains of the salt marsh mosquito, Aedes taeniorhynchus, suggest that blood meal-independent release of neurohormones from the brain also underlies autogenous production of eggs (Lea, 1970). Notably, OEH/ILP release by G. atropalpus for production of a second clutch is blood meal dependent, suggesting that regulation of OEH/ILP release changes in females over time. Other key transitions to enable blood feeding include the shift from fat body to midgut for enzymatic mobilization of amino acids for VG synthesis and the acquisition of host-seeking behavior (Bowen et al., 1994). The regulation of these changes may involve some combination of these signaling elements and novel ones that similarly underpin variations in autogeny/anautogeny observed in mosquitoes (O'Meara, 1985).
Given that all mosquito genomes examined to date (A. aegypti, Anopheles gambiae and Culex pipiens) encode an OEH and multiple ILP genes (Marquez et al., 2011; Antonova et al., 2012), we think it likely that all anautogenous species rely on the release of one or both of these neuroendocrine factors to stimulate egg maturation. We further suggest the likely key factor in the evolution of autogeny by G. atropalpus and other species is the shift from blood meal-dependent to blood meal-independent regulation of OEH and/or ILP release. How OEH and ILP release is regulated in contrast is unknown in any species. In A. aegypti, the medial neurosecretory cells in the female brain are the primary site of OEH and ILP synthesis, while storage and release of these peptides occurs in the corpus cardiacum, which is innervated by the medial neurosecretory cell axons (Brown and Cao, 2001; Riehle et al., 2006). Studies with A. aegypti indicate that JH released from the corpora allata after eclosion but prior to a blood meal establishes tissue competency to respond to OEH/ILP signaling by promoting expression of the TOR and ECD signaling pathways (Li et al., 2003; Shiao et al., 2008; Clifton and Noriega, 2011). The results of our in vitro assays likewise suggest that tissues from G. atropalpus must acquire competency to respond to OEH. However, JH has no known role in stimulating the synthesis or the release of OEH and ILPs in A. aegypti or any other mosquito.
Other factors suggested to affect autogenous egg production include teneral nutrient reserves, sugar feeding and mating (Hudson, 1970; O'Meara and Krasnick, 1970; Kalpage and Brust, 1974; O'Meara, 1985; Wheeler and Buck, 1996; Telang and Wells, 2004; Telang et al., 2006). As previously noted, differences in teneral reserves and sugar intake clearly affect first clutch size by determining the amount of VG and other yolk proteins a female can produce. Suboptimal teneral reserves or sugar intake, however, never affect the ability of G. atropalpus females to always produce their first clutch autogenously. These findings collectively suggest nutrient availability is not the sole determinant of OEH/ILP release, although it is possible that nutrient stores or a particular level of some factor, like amino acids, plays a role in this process. In contrast, our results strongly indicate that mating status does not affect OEH/ILP release because virgin and mated females mature a first clutch of eggs at exactly the same time after emerging. In contrast, mating does profoundly affect oviposition by G. atropalus given that mated females lay most or all of the eggs they mature while virgin females lay almost none. Clearly then a key goal for future studies will be to identify the factor(s) that triggers the release of OEH and ILPs in anautogenous species like A. aegypti. Another will be to determine whether autogenous species like G. atropalpus rely on the same factor(s) but control its release independently of blood feeding or regulate OEH/ILP release entirely differently.
We recognize the lesser activity of AaILP3 in G. atropalpus may be that it suboptimally mimics the endogenous ILP(s) in G. atropalpus, given that all insects including mosquitoes encode multiple ILP family members (Marquez et al., 2011; Antonova et al., 2012). Overall though, our results suggest that OEH is a more potent regulator of egg development in G. atropalpus. Sequence analysis indicates that mosquito OEH is a member of the neuroparsin family of insect neuropeptides, which share some homology with mammalian insulin growth factor binding proteins. This interaction was demonstrated for a neuroparsin and ILP from Locusta migratoria (Badisco et al., 2008), but its functional significance in this insect is not resolved. The primary gonadotropic activity of OEH in two related mosquito species combined with our lack of knowledge of how OEH and other neuroparsins function led us to examine whether OEH activity depends upon the IR. Our RNAi results strongly indicate that the activity of AaILP3 and endogenous ILPs is fully IR dependent, whereas the activity of OEH is not. Previous receptor-binding studies in A. aegypti indicate that ILP3 binds with high affinity to the IR (Brown et al., 2008; Wen et al., 2010). In contrast, our preliminary results indicate that OEH does not bind to either the IR or ILPs, which together with the results of this study suggests OEH activity does not depend on direct interactions with either ILPs or their receptor. Aedes aegypti is likely the better model for identifying how OEH interacts with the ovary, fat body and potentially other tissues to regulate egg development. Once a candidate receptor and signaling pathway is identified though, studies with G. atropalpus could help advance our understanding of OEH function.
ACKNOWLEDGEMENTS
We thank Sarah Robertson, Anne Robertson and Amy Johnson for their assistance in maintaining the mosquito colony.
LIST OF ABBREVIATIONS
- 20E
20-hydroxyecdysone
- AaILP
Aedes aegypti ILP
- AarlOEH
A. aegypti recombinant long OEH
- ECD
ecdysteroid hormone
- ILP
insulin-like peptide
- IR
insulin receptor
- JH
juvenile hormone
- OEH
ovary ecdysteroidogenic hormone
- PE
post-eclosion
- RIA
radioimmunoassay
- RNAi
RNA interference
- TOR
target of rapamycin
- VG
vitellogenin
FOOTNOTES
FUNDING
This work was supported by the National Institutes of Health (NIH) [grant AI33108] to M.R.S. and M.R.B. Deposited in PMC for release after 12 months.
REFERENCES
- Antonova Y., Arik A. J., Moore W., Riehle M. R., Brown M. R. (2012). Insulin-like peptides: structure, signaling, and function. In Insect Endocrinology (ed. Gilbert L. I.), pp. 63-92 New York, NY: Elsevier, Academic Press; [Google Scholar]
- Attardo G. M., Hansen I. A., Raikhel A. S. (2005). Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem. Mol. Biol. 35, 661-675 [DOI] [PubMed] [Google Scholar]
- Badisco L., Claeys I., Van Hiel M., Clynen E., Huybrechts J., Vandersmissen T., Van Soest S., Vanden Bosch L., Simonet G., Vanden Broeck J. (2008). Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J. Mol. Endocrinol. 40, 137-150 [DOI] [PubMed] [Google Scholar]
- Bertone M. A., Courtney G. W., Wiegmann B. M. (2008). Phylogenetics and temporal diversification of the earliest true flies (Insecta: Diptera) based on multiple nuclear genes. Syst. Entomol. 33, 668-687 [Google Scholar]
- Birnbaum M. J., Kelly T. J., Woods C. W., Imberski R. B. (1984). Hormonal regulation of ovarian ecdysteroid production in the autogenous mosquito, Aedes atropalpus. Gen. Comp. Endocrinol. 56, 9-18 [DOI] [PubMed] [Google Scholar]
- Bowen M. F., Davis E. E., Haggart D., Romo J. (1994). Host-seeking behavior in the autogenous mosquito Aedes atropalpus. J. Insect Physiol. 40, 511-517 [Google Scholar]
- Briegel H. (2003). Physiological bases of mosquito ecology. J. Vector Ecol. 28, 1-11 [PubMed] [Google Scholar]
- Brown M. R., Cao C. (2001). Distribution of ovary ecdysteroidogenic hormone I in the nervous system and gut of mosquitoes. J. Insect Sci. 1, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Graf R., Swiderek K. M., Fendley D., Stracker T. H., Champagne D. E., Lea A. O. (1998). Identification of a steroidogenic neurohormone in female mosquitoes. J. Biol. Chem. 273, 3967-3971 [DOI] [PubMed] [Google Scholar]
- Brown M. R., Clark K. D., Gulia M., Zhao Z., Garczynski S. F., Crim J. W., Suderman R. J., Strand M. R. (2008). An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 105, 5716-5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant B., Macdonald W., Raikhel A. S. (2010). microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 107, 22391-22398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A. N. (1992). The Biology of Mosquitoes, Vol. 1 London, UK: Chapman & Hall; [Google Scholar]
- Clifton M. E., Noriega F. G. (2011). Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 57, 1274-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster W. A. (1995). Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443-474 [DOI] [PubMed] [Google Scholar]
- Fuchs M. S., Sundland B. R., Kang S. H. (1980). In vivo induction of ovarian development in Aedes atropalpus by a head extract from Aedes aegypti. Int. J. Invert. Repro. 2, 121-129 [Google Scholar]
- Gulia-Nuss M., Robertson A. E., Brown M. R., Strand M. R. (2011). Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS ONE 6, e20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz R. W. (1970). Monofactorial inheritance of early sexual receptivity in the mosquito, Aedes atropalpus. Anim. Behav. 18, 358-361 [DOI] [PubMed] [Google Scholar]
- Hansen I. A., Attardo G. M., Park J. H., Peng Q., Raikhel A. S. (2004). Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 101, 10626-10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I. A., Attardo G. M., Roy S. G., Raikhel A. S. (2005). Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 280, 20565-20572 [DOI] [PubMed] [Google Scholar]
- Harbach R. E. (2007). The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. In Linnaeus Tercentenary: Progress in Invertebrate Taxonomy, Vol. 1668 (ed. Zhang Z.-Q., Shear W. A.), pp. 591-638 New Zealand: Zootaxa, Magnolia Press; [Google Scholar]
- Harbach R. E., Kitching I. J. (1998). Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 23, 327-370 [Google Scholar]
- Hudson A. (1970). Factors affecting egg maturation and oviposition by autogenous Aedes atropalpus (Diptera: Culicidae). Can. Entomol. 102, 939-950 [Google Scholar]
- Kalpage K. S. P., Brust R. A. (1974). Studies on diapause and female fecundity in Aedes atropalpus. Environ. Entomol. 3, 139-145 [Google Scholar]
- Kelly T. J., Fuchs M. S., Kang S. H. (1981). Induction of ovarian development in autogenous Aedes atropalpus by juvenile hormone and 20-hydroxyecdysone. Int. J. Invert. Repro. 3, 101-112 [Google Scholar]
- Kelly T. J., Birnbaum M. J., Woods C. W., Borkovec A. B. (1984). Effects of house fly oostatic hormone on egg development neurosecretory hormone action in Aedes atropalpus. J. Exp. Zool. 229, 491-496 [Google Scholar]
- Lea A. O. (1970). Endocrinology of egg maturation in autogenous and anautogenous Aedes taeniorhychus. J. Insect Physiol. 16, 1689-1696 [DOI] [PubMed] [Google Scholar]
- Li Y., Hernandez-Martinez S., Unnithan G. C., Feyereisen R., Noriega F. G. (2003). Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochem. Mol. Biol. 33, 1307-1315 [DOI] [PubMed] [Google Scholar]
- Ma M., Newton P. B., He G., Kelly T. J., Hsu H. T., Masler E. P., Borkovec A. B. (1984). Development of monoclonal antibodies for monitoring Aedes atropalpus vitellogenesis. J. Insect Physiol. 30, 529-536 [Google Scholar]
- Marquez A. G., Pietri J. E., Smithers H. M., Nuss A., Antonova Y., Drexler A. L., Riehle M. A., Brown M. R., Luckhart S. (2011). Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 173, 303-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masler E. P., Fuchs M. S., Sage B., O'Connor J. D. (1980). Endocrine regulation of ovarian development in the autogenous mosquito, Aedes atropalpus. Gen. Comp. Endocrinol. 41, 250-259 [DOI] [PubMed] [Google Scholar]
- Masler E. P., Fuchs M. S., Sage B., O'Connor J. D. (1981). A positive correlation between oocyte production and ecdysteroid levels in adult Aedes. Physiol. Entomol. 6, 45-49 [Google Scholar]
- Masler E. P., Whisenton L. V. R., Schlaeger D. A., Kang S. H., Fuchs M. S. (1983). Chymotrypsin and trypsin levels in adult Aedes atropalpus and Toxorhynchites brevipalpus (Theobald). Comp. Biochem. Physiol. 75B, 435-440 [Google Scholar]
- O'Meara G. F. (1972). Polygenic regulation of fecundity in autogenous Aedes atropalpus. Ento. Exp. Applicata 15, 81-89 [Google Scholar]
- O'Meara G. F. (1985). Gonotrophic interactions in mosquitoes – kicking the blood-feeding habit. Flor. Entomol. 68, 122-133 [Google Scholar]
- O'Meara G. F., Craig G. B. (1969). Monofactoral inheritance of autogeny in Aedes atropalpus. Mosq. News 29, 14-22 [Google Scholar]
- O'Meara G. F., Krasnick G. J. (1970). Dietary and genetic control of the expression of autogenous reproduction in Aedes atropalpus (Coq.) (Diptera: Culicidae). J. Med. Entomol. 7, 328-334 [DOI] [PubMed] [Google Scholar]
- O'Meara G. F., Lounibos L. P. (1981). Reproductive maturation in the pitcher plant mosquito, Wyeomyia smithii. Physiol. Entomol. 6, 437-443 [Google Scholar]
- O'Meara G. F., Lounibos L. P., Brust R. A. (1981). Repeated egg clutches without blood in the pitcher plant mosquito. Ann. Entomol. Soc. Am. 74, 68-72 [Google Scholar]
- Provost-Javier K. N., Chen S., Rasgon J. L. (2010). Vitellogenin gene expression in autogenous Culex tarsalis. Insect Mol. Biol. 19, 423-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidenbach K. R., Cook S., Bertone M. A., Harbach R. E., Wiegmann B. M., Besansky N. J. (2009). Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 9, 298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert J. F., Harbach R. E., Kitching I. J. (2009). Phylogeny and classification of tribe Aedini (Diptera: Culicidae). Zool. J. Linn. Soc. 157, 700-794 [Google Scholar]
- Riehle M. A., Fan Y., Cao C., Brown M. R. (2006). Molecular characterization and developmental expression of insulin-like peptides in the yellow fever mosquito, Aedes aegypti. Peptides 27, 2547-2560 [DOI] [PubMed] [Google Scholar]
- Rioux J. A., Croset H., PechPérières J., Guilvard E., Belmonte A. (1975). Autogenesis in the Diptera Culicides. Synoptic table of autogenic species. Ann. Parasitol. Hum. Comp. 50, 134-140 [PubMed] [Google Scholar]
- Roubaud E. (1929). Cycle autogene d'attente generations hivernales suractives inaparentes chez le moustique commun Culex pipiens. C. R. Acad. Sci., Paris 180, 735-738 [Google Scholar]
- Roy S. G., Raikhel A. S. (2011). The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development. Insect Biochem. Mol. Biol. 41, 62-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. G., Hansen I. A., Raikhel A. S. (2007). Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 37, 1317-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao S. H., Hansen I. A., Zhu J., Sieglaff D. H., Raikhel A. S. (2008). Juvenile hormone connects larval nutrition with target of rapamycin signaling in the mosquito Aedes aegypti. J. Insect Physiol. 54, 231-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang A., Wells M. A. (2004). The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 50, 677-685 [DOI] [PubMed] [Google Scholar]
- Telang A., Li Y. P., Noriega F. G., Brown M. R. (2006). Effects of larval nutrition on the endocrinology of mosquito egg development. J. Exp. Biol. 209, 645-655 [DOI] [PubMed] [Google Scholar]
- Van Handel E. (1976). The chemistry of egg maturation in the unfed mosquito Aedes atropalpus. J. Insect Physiol. 22, 521-522 [DOI] [PubMed] [Google Scholar]
- Wen Z., Gulia M., Clark K. D., Dhara A., Crim J. W., Strand M. R., Brown M. R. (2010). Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol. Cell. Endocrinol. 328, 47-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. E., Buck N. A. (1996). A role for storage proteins in autogenous reproduction in Aedes atropalpus. J. Insect Physiol. 42, 961-966 [Google Scholar]
- Zhu J., Chen L., Raikhel A. S. (2003). Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 100, 13338-13343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Chen L., Sun G., Raikhel A. S. (2006). The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol. Cell. Biol. 26, 9402-9412 [DOI] [PMC free article] [PubMed] [Google Scholar]