Abstract
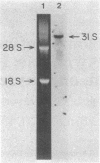
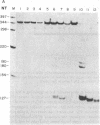
We have isolated cDNA clones from thyrotoxic (pMHC alpha) and normal (pMHC beta) adult rabbit hearts. Restriction map analysis and DNA sequence analyses show that, although there is strong homology between overlapping regions of the two clones, they are distinctly different. The two clones exhibited 78-83% homology between the derived amino acid sequences and those determined by direct amino acid sequence analysis of rabbit fast skeletal muscle myosin heavy chains. The clones specify a segment of the myosin heavy chain corresponding to subfragment 2 and the COOH-terminal portions of subfragment 1. Nuclease S1 mapping was used to compare transcription of the two clones with expression of the alpha and beta forms of myosin heavy chains in the ventricles of thyrotoxic, hypothyroid (propylthiouracil-treated), and normal rabbits. Thyrotoxic ventricles contained only pMHC alpha transcripts whereas hypothyroid ventricles contained exclusively pMHC beta transcripts. These data correlate well with the presence of alpha- and beta-form myosin heavy chains. In the normal young adult rabbit, pMHC beta transcripts predominate, agreeing with the known beta form/alpha form ratio of 4:1. We therefore conclude that pMHC alpha and pMHC beta contain sequences of the alpha- and beta-form myosin heavy chain genes, respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Morkin E. Actin-activated adenosine triphosphatase activity of native and N-ethylmaleimide-modified cardiac myosin from normal and thyrotoxic rabbits. Circ Res. 1977 Nov;41(5):630–634. doi: 10.1161/01.res.41.5.630. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R. A., Everett A. W., Clark W. A., Jakovcic S., Rabinowitz M., Zak R. Isolation and characterization of two molecular variants of myosin heavy chain from rabbit ventricle. Change in their content during normal growth and after treatment with thyroid hormone. J Biol Chem. 1982 Feb 25;257(4):2056–2065. [PubMed] [Google Scholar]
- Flink I. L., Rader J. H., Morkin E. Thyroid hormone stimulates synthesis of a cardiac myosin isozyme. Comparison of the two-two-dimensional electrophoretic patterns of the cyanogen bromide peptides of cardiac myosin heavy chains from euthyroid and thyrotoxic rabbits. J Biol Chem. 1979 Apr 25;254(8):3105–3110. [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Benfield P. A., Hobbs A. W. Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol. 1982 Feb;92(2):471–484. doi: 10.1083/jcb.92.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979 Apr;81(1):10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L., Pauletto P., Pessina A. C., Sartore S., Schiaffino S. Isomyosin distribution in normal and pressure-overloaded rat ventricular myocardium. An immunohistochemical study. Circ Res. 1981 Oct;49(4):1003–1009. doi: 10.1161/01.res.49.4.1003. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F. Light chain distribution of chicken skeletal muscle myosin isoenzymes. FEBS Lett. 1978 Jun 15;90(2):297–300. doi: 10.1016/0014-5793(78)80390-1. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Yeoh G. P., Thomas M. A., Higginbottom L. Structural differences in the heavy chains of rat ventricular myosin isoenzymes. FEBS Lett. 1979 Jan 15;97(2):330–334. doi: 10.1016/0014-5793(79)80115-5. [DOI] [PubMed] [Google Scholar]
- Huszar G. Developmental changes of the primary structure and histidine methylation in rabbit skeletal muscle myosin. Nat New Biol. 1972 Dec 27;240(104):260–264. doi: 10.1038/newbio240260a0. [DOI] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. F., Pagani E. D., Solaro R. J. Thyroxine-induced redistribution of isoenzymes of rabbit ventricular myosin. Circ Res. 1982 Jan;50(1):117–124. doi: 10.1161/01.res.50.1.117. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yoshizaki C. Differentiation of myosin in chick embryos. J Biochem. 1974 Jul;76(1):123–131. doi: 10.1093/oxfordjournals.jbchem.a130536. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Carmon Y., Zevin-Sonkin D., Levi Z., Shaul Y., Shani M., Yaffe D. Identification of recombinant phages containing sequences from different rat myosin heavy chain genes. Nucleic Acids Res. 1980 May 24;8(10):2133–2146. doi: 10.1093/nar/8.10.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Cozens P. J., Cato A. C., Jost J. P. Recombinant plasmids containing avian vitellogenin structural gene sequences derived from complementary DNA. Biochim Biophys Acta. 1980;606(1):34–46. doi: 10.1016/0005-2787(80)90095-7. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. The duplicated human alpha-globin genes: their relative expression as measured by RNA analysis. Cell. 1981 May;24(2):345–351. doi: 10.1016/0092-8674(81)90324-x. [DOI] [PubMed] [Google Scholar]
- Pope B., Hoh J. F., Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980 Sep 8;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Robbins J., Freyer G. A., Chisholm D., Gilliam T. C. Isolation of multiple genomic sequences coding for chicken myosin heavy chain protein. J Biol Chem. 1982 Jan 10;257(1):549–556. [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N., Mabuchi K., Pepe F., Salmons S., Gergely J., Sreter F. Use of type-specific antimyosins to demonstrate the transformation of individual fibers in chronically stimulated rabbit fast muscles. J Cell Biol. 1978 Oct;79(1):252–261. doi: 10.1083/jcb.79.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushbrook J. I., Stracher A. Comparison of adult, embryonic, and dystrophic myosin heavy chains from chicken muscle by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and peptide mapping. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4331–4334. doi: 10.1073/pnas.76.9.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S., Gorza L., Pierobon Bormioli S., Dalla Libera L., Schiaffino S. Myosin types and fiber types in cardiac muscle. I. Ventricular myocardium. J Cell Biol. 1981 Jan;88(1):226–233. doi: 10.1083/jcb.88.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R., Offer G. Polarity of the myosin molecule. J Mol Biol. 1973 Nov 25;81(1):17–31. doi: 10.1016/0022-2836(73)90244-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Umeda P. K., Sinha A. M., Jakovcic S., Merten S., Hsu H. J., Subramanian K. N., Zak R., Rabinowitz M. Molecular cloning of two fast myosin heavy chain cDNAs from chicken embryo skeletal muscle. Proc Natl Acad Sci U S A. 1981 May;78(5):2843–2847. doi: 10.1073/pnas.78.5.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda P. K., Zak R., Rabinowitz M. Purification of messenger ribonucleic acids for fast and slow myosin heavy chains by indirect immunoprecipitation of polysomes from embryonic chick skeletal muscle. Biochemistry. 1980 Apr 29;19(9):1955–1965. doi: 10.1021/bi00550a035. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Schwartz K., Bouveret P., Sell S. M., Gros F. Contractile protein isozymes in muscle development: identification of an embryonic form of myosin heavy chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5197–5201. doi: 10.1073/pnas.76.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Zweig S. E. The muscle specificity and structure of two closely related fast-twitch white muscle myosin heavy chain isozymes. J Biol Chem. 1981 Nov 25;256(22):11847–11853. [PubMed] [Google Scholar]