Abstract
Background: Carcinomas of esophagus, mostly squamous cell carcinomas, occur throughout the world. There are a number of suspected genetic or environmental etiologies. Human papilloma virus (HPV) is said to be a major etiology in areas with high incidence of esophageal carcinoma, while it is hardly detectable in low incidence regions. This study was designed to evaluate the prevalence of HPV in esophageal squamous cell carcinoma (ESCC) cases diagnosed in Pathology Department, Medical School, Shiraz University of Medical Sciences.
Methods: DNA material for PCR amplification of HPV genome was extracted from formalin-fixed paraffin-embedded tissue blocks of 92 cases of ESCC, diagnosed during 20 years from 1982 to 2002. Polymerase chain reaction was performed for amplification and detection of common HPV and type specific HPV-16 and HPV-18 genomic sequences in the presence of positive control (HPV-18 and HPV positive biopsies of uterine exocervix) and additional internal controls i.e. beta-globin and cytotoxic T lymphocyte antigen 4 (CTLA4).
Result: Good amplification of positive control and internal controls was observed. However, no amplification of HPV genome was observed.
Conclusion: There is no association between HPV infection and the development of esophageal squamous cell carcinoma in the cases evaluated.
Key Words: Squamous cell carcinoma, esophagus, human papilloma virus, polymerase chain reaction
Introduction
Malignant esophageal tumors usually arise from epithelial layer of the esophagus. Worldwide, squamous cell carcinomas (SCC) constitute 90% of esophageal cancers although in some regions such as United States their incidence is comparable to that of adenocarcinomas.
While esophageal SCC (ESCC) occurs throughout the world, its incidence varies widely among countries and within regions of the same country. The region extending from northern Iran across central Asia to northern China exhibits annual incidence rate exceeding 100 per 100,000 with deaths from cancer of the esophagus constituting more than 20% of all cancer deaths. The death from cancer of the esophagus in this area constitutes more than 20% of all cancer deaths.1
Fars province in the south of Iran with an average annual incidence of 2.95 per 100,000 might be considered one of the low incidence areas. Esophageal carcinoma is among the most common gastrointestinal (GI) cancers in the province.2 There are significant differences in the epidemiology of ESCCs, which strongly implicate dietary and environmental factors as well as an ill-defined contribution from genetic predisposition involved in etiology and pathogenesis of esophageal carcinomas.3
It has been shown that human papilloma virus (HPV) DNA is found frequently in ESCCs from high incidence areas. Its presence is infrequent, however, in cancer-bearing patients of North America,4 and many other low incidence regions. Human papiloma virus particles are about 55 nm in diameter, and contain a circular ds DNA molecule of 7.2-8.0 Kbp. Human papiloma virus with more than 200 genotypes is implicated in the genesis of several cancers, particularly squamous cell carcinoma of the cervix, and anogenital, oral and laryngeal regions.5
Molecular analyses reveal that in benign and preneoplastic lesions, the HPV genome is maintained in an episomal (non integrated) form, whereas in cancers the viral DNA is usually integrated into the host cell genome. This suggests that the integration of viral DNA is important in malignant transformation. The site, at which the viral DNA is interrupted in the process of integration, is fairly constant. It is almost always within E1/E2 open reading frame of the viral genome. Because the E2 region of the viral DNA normally represses the transcription of the E6 and E7 early viral genes, its interruption causes over expression of the E6 and E7 proteins of the HPV-16 and HPV-18.6
Oncogenic potential of these HPVs may be related to these two early viral gene products.7,8 The E7 protein binds to the underphosphorylated form of the tumor-suppressor protein pRb and displaces the E2F transcription factors that are normally bound by pRb. The E6 protein binds to and facilitates the degradation of p53 gene product. The E6 and E7 proteins derived from high-risk HPVs (types 16, 18 and 31) bind to pRb and p53 with high affinity, whereas those of low-risk viruses (types 6 and 11) bind with low affinity. Thus, it seems that the E6 and E7 proteins of the high-risk HPVs disable two important tumor suppressor proteins that regulate the cell cycle. It has been reported that one particular allele of p53 with an arginine rather than a proline at a certain position is much more susceptible to degradation by E6. Correspondingly, individuals with the “arginine form” of p53 have a seven fold higher risk of developing cervical cancer than those who do not posses this allele of p53.8,9
Although, these observations implicate certain HPV types in the pathogenesis of human cancer, it seems most likely that infection with HPV acts as an initiating event and that additional somatic mutations are essential for full malignant transformation.
There are conflicting reports on the effects of formalin fixation on DNA quality. While a number of reports indicate that the use of formalin for tissue fixation causes DNA degradation and reduces DNA solubility, a number of others suggest that formalin fixation does not have significant effect on the successful amplification of DNA.10-12
de Villiers and colleagues studied 117 samples of esophageal carcinoma originating from the high incidence areas of china, and showed that HPV DNA was present in 20 out of 117 samples (17.1%). Only three of Mucosotropic HPVs were of the high risk types (HPV-16, 18 and 33).13 Li and colleagues evaluated specimens of balloon cytology examination from volunteers in two regions with significantly different incidence of esophageal carcinoma. Specimens were evaluated using both PCR and in situ hybridization (ISH) protocols. The results of PCR showed that the prevalence of HPV-16 E6 gene in the high incidence area was 1.9 fold higher than that of low incidence area (72% and 37% respectively, P<0.01). Similar results were obtained with HPV-16 E7 gene using ISH. They suggested that HPV-16 plays a causative role in the pathogenesis of esophageal cancer, especially in the high incidence area of China.14
Si and colleagues evaluated some HPV-16 positive cases of ESCC in order to determine physical status of HPV-16 in these cases. They showed a predominance of integrated form of viral genes in HPV-16 positive ESCCs from china implying the HPV infection may play a role in the pathogenesis of ESCC.15
Shen and colleagues used a reliable model for studying the cellular and molecular mechanisms involved in carcinogenesis of esophageal carcinomas. In order to demonstrate the effect of viruses and tumor promoters on the tumorigenicity, human embryonic esophageal cells were infected with HPV-18 E6 E7-AAV in synergy with exposure to 12-o-tetradecanoyl phorbol 13-acetate (TPA). Malignant transformation of human embryonic epithelial cells was induced in vitro by HPV-18 E6E7 in synergy with TPA. This is a good evidence for the close relationship between HPV-18 as an etiologic factor and pathogenesis of esophageal carcinoma.16
In contrast to the above mentioned studies, there are several reports originating mainly from western European countries and United States of America that show the absence of HPV DNA in ESCCs. Some of these studies show only rare association of HPV DNA with ESCCs. Morgan and colleagues used PCR to examine frozen tissue from 22 cases of ESCCs for the presence of specific DNA sequences from oncogenic strains of HPV. The products of PCR were further analyzed by southern blot hybridization (SBH). No HPV sequences were detected in any tumor, suggesting it is unlikely, therefore, that HPV plays a significant role in the pathogenesis of ESCCs in the United Kingdom.17
Saegusa and colleagues examined 103 esophageal carcinomas by PCR method using two consensus (targeting either the L1 or the E6-E7 regions) and two type specific (type 16 and type 18) primer sets. However, the entire series of tumor DNA were negative for HPV sequences by PCR assays using all four primer sets.18
This study was designed to evaluate prevalence of HPV in ESCC cases diagnosed in Pathology Department, Medical School, Shiraz University of Medical Sciences.
Materials and Methods
All cases of ESCC that were reported between years 1982 to 2002 in the Pathology Department, Medical School were identified. All slides of ESCC cases (n=92) available in the departmental archive were reviewed, and the best slides and their paraffin-embedded tissue blocks were extracted. In addition, slides and paraffin-embedded tissue blocks of normal esophagus from 20 autopsy cases (15-80 years), who referred to the Department between 1996 to 2000, were extracted. To prepare DNA sample from each block one section for hematyoxyllin eosin (H&E) staining and 15 sections for DNA extraction were prepared. All sections had a thickness of 5 µm. The sections for DNA extraction placed in two microfuge tubes. Another section was prepared for H&E staining, and was used to confirm the presence of tumor tissue in all previous sections taken for DNA extraction. In order to prevent cross contamination and pick up of sectioned tissues from previous blocks, blade of microtome, working instruments, and surfaces were cleaned by Xylol and HCL 1N before starting with another block. The extraction of DNA was done by proteinase K incubation technique followed by phenol-chloroform extraction of DNA.
Analysis of Extracted DNA Samples
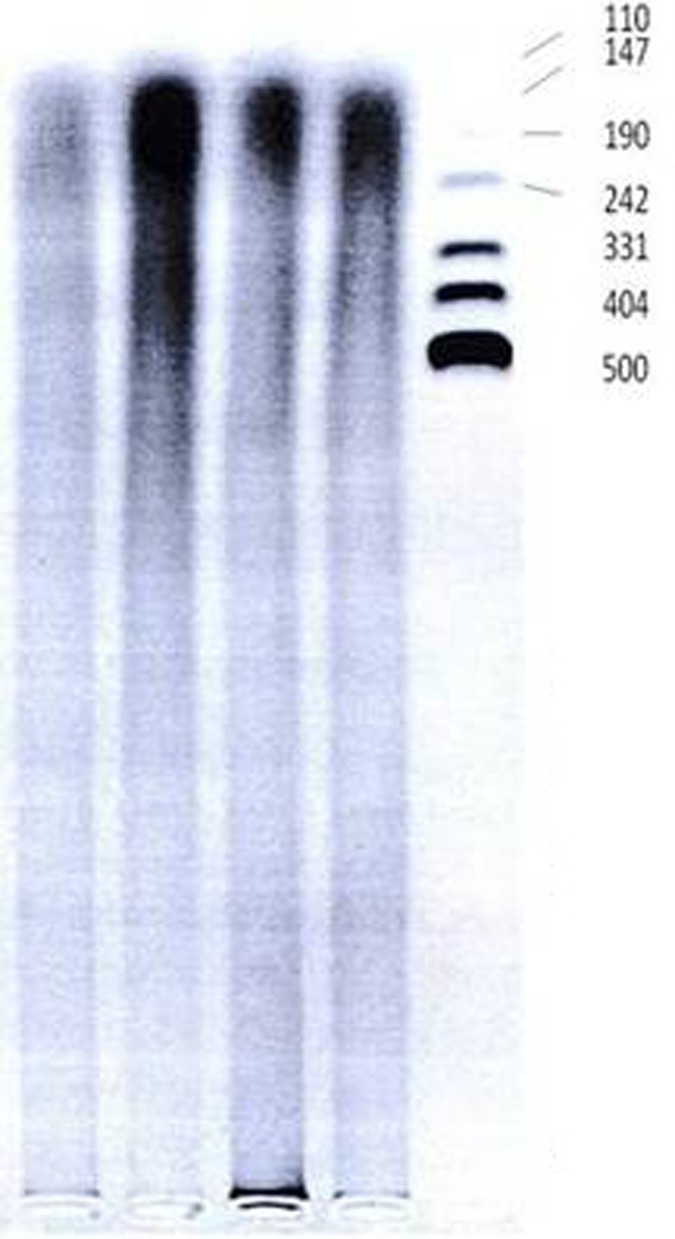
Samples of DNA were quantitatively assayed using spectrophotometery by measuring absorbance at 260 nm (A260), 280 nm (A280), and 230 nm (A230) to determine concentration of ds DNA, and to measure A260 to A280 ratio (optimal ratio is equal to 1.8 or more and less than 2) and A260 to A230 ratio (optimal ratio is greater than 2.2). A280 is an estimate of concentration of non-DNA components such as proteins, whereas A230 is measured to estimate concentration of other components such as detergents, peptides, carbohydrates, phenol and chloroform. Some randomly selected samples were assayed quantitatively by running on a 2% agarose gel electrophoresis, allowing the molecular weight of the DNA to be estimated. The electrophoresis resulted in a thick smear of DNA fragments with variable size ranging from very high molecular weight DNA to those about 100 bp in size (figure 1). Moreover, some of the samples were assayed in a PCR program using primers to amplify cytotoxic T lymphocyte antigen 4 (CTLA4) gene with a product of 162 bp in size that showed sharp specific bands on agarose gel electrophoresis indicating good quality of extracted DNA. Because, optimal concentration for PCR is about 0.1- 0.5 µg/ µl, we used 10 µl of stock DNA sample to make optimal concentration equal to 0.3 µg/µl by adding appropriate amount of deionized water to it. The DNA samples were stored in -20°C for later use.
Figure 1:
Photograph (negative version) of ethidium bromide-stained agarose gel after electrophoresis of extracted DNA from some randomly selected cases. (5 µl of stock DNA mixed with 4µl of loading dye applied for electrophoresis under 70 volts for 6 hours)
Positive Controls
Human papilloma virus-18 full length genome, cloned in pBR322 (ATTC 45152), was extracted from transformed E. Coli using the technique of alkaline lysis / phenolic extraction of DNA. It was used as a positive control in each run of PCR assay using general HPV or HPV-18 primer sets. Several HPV-positive biopsies of uterine exocervix from previous studies on cervical biopsies were also used in randomly-selected runs as a positive control.
Polymerase Chain Reaction
In order to detect HPV DNA sequence in our cases, including tumor and non-tumor autopsy cases, we performed PCR assay using a pair of general HPV primer which could anneal with and amplify target DNA sequence in the L1 open reading frame of many types of HPV especially the types 6b, 11, 16, 18, 31 and 33. The sequence of forward and reverse general primers as well as the size of expected amplification product is shown in table 1.
Table 1:
The sequence of primer sets for human papilloma virus (HPV) and beta globin gene used in a touch down PCR program
| General HPV primer set | Product size | |
| Forward | 5-TTT GTT ACT GTG GTA GAT AC | 140 bp |
| Reverse | 5-GAA AAA TAA ACT GTA AAT CA | |
| Beta globin gene specific primer set | Product size | |
| Forward | 5-ACA CAA CTG TGT TCA CTA GC | 100 bp |
| Reverse | 5-CAA CTT CAT CCA CGT TCA CC | |
| PCR Program | ||
| -Initial denaturation | 94 C for 3 min | |
| -16 cycles with | -denaturation at | 94C for 30 seconds |
| -anealing at | 65C to 50C for 2 minutes | |
| -extension at | 72C for 30 seconds | |
| -14 cycles with | -denaturation at | 94C for 30 seconds |
| -anealing at | 50C for 1 minutes | |
| -extension at | 72C for 30 seconds | |
| -10 cycles with | -denaturation at | 94C for 30 seconds |
| -anealing at | 49C to 40C for 1 minutes | |
| -extension at | 72C for 30 seconds | |
| -Final extension | 72C for 5 minutes | |
The PCR assay using general HPV primer set was performed with and without internal control. Internal control was a 100 bp sequence of beta globin gene (as an endogenous target), and was amplified and detected in all of cases using a beta globin gene specific primer set. The sequences of forward and reverse beta globin gene primers are shown in table 1.
Briefly, PCR was performed in a total volume of 25 µl prepared by adding appropriate amounts of reaction components into a 0.5 ml Eppendorf microtube. The reaction mixture contained 2.5 µl of 10x buffer, 1.5 mM MgCl2, 0.3 mM of each dNTP, 20 pM of each general HPV primer and 5 pM of each beta globin primer, two unit of Taq DNA polymerase and 300 ng of template DNA sample extracted from the cases under study or 10 ng of p HPV (recombinant plasmid) as positive control. A negative control or blank (all reactants minus target DNA) was also used in every run of PCR. The mixture was overlaid with 40 µl of mineral oil and subjected to 40 cycles of PCR amplification using an Eppendorf thermocycler and a touch down PCR program shown in table 1.
At the end of the PCR, tubes were transferred to refrigerator for later use in agarose gel electrophoresis, and analysis of the results. We also examined half of tumoral cases in a heteroduplex PCR using both HPV-16 and HPV-18 specific primer sets.
Detection and Analysis of the Reaction Products
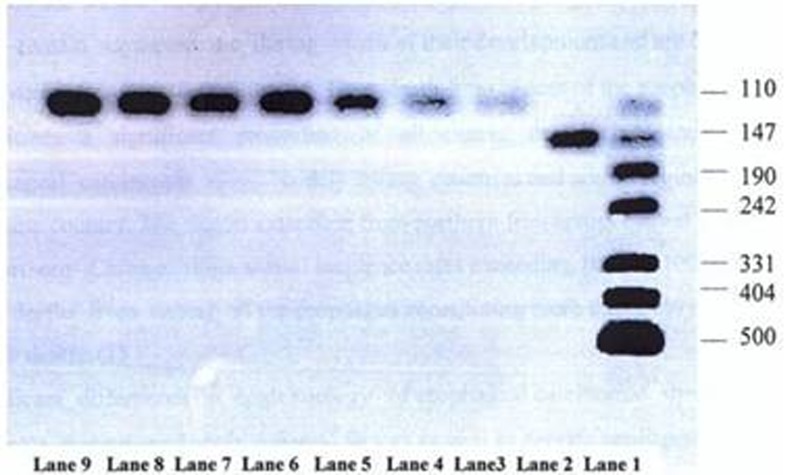
5 µl of each PCR product was mixed well with 4 µl of loading dye (Bromophenol blue, EDTA, Glycerol) on a clean surface, and the mixtures were then transferred into the wells created within agarose gel. Along with cases under study, both negative and positive controls as well as DNA size marker were loaded onto gel simultaneously for electrophoresis in a tank containing Tris/Acetic acid/ EDTA (TAE) buffer and little amount of ethidium bromide as intercalating dye under 70 volts for 2 hours. After electrophoresis, the gel was transferred onto UV transilluminator to visualize PCR products, if there was any positive reaction (figure 2).
Figure 2:
Photograph (negative version) of ethidium-bromide stained agarose gel On UV transilluminator showing results of PCR assay using general human papilloma virus Primer set with internal control. Lane 1: DNA size marker (pUC 19 DNA/M spI); Lane 2: Positive control; Lane 3 to9: Samples negative for HPV DNA but showing amplified Beta-globin gene as internal control
Results
No HPV genomic sequence was detected in any of 92 cases of ESCC. In other words no specific band was seen on agarose gel after electrophoresis except for a sharp band equal to 100 bp representing amplified beta globin gene sequence used as internal control. Similarly, none of 20 autopsy cases showed evidence of HPV infection in PCR assay. Because PCR assay with internal control showed no amplified HPV DNA sequence in any of the cases, second PCR assay using general HPV primer set was performed, but no internal control was included in the reaction. Thus, the possibility of inhibitory effect of competition between two primer sets (general HPV primer set and beta globin gene specific primer set) on each other as a possible cause of negative results in previous PCR assay was ruled out. In addition, heteroduplex PCR assay using both HPV-16 and HPV-18 specific primer sets was performed for half of tumor cases, and no evidence of infection by these high risk types of HPV was found.
Discussion
Esophageal squamous cell carcinoma is one of the most common causes of cancer death worldwide. In western countries, where the risk of ESCC is generally low, consumption of tobacco and alcohol could explain great majority of the cases of ESCC. However, in regions such as northern Iran, which has a high rate of ESCC, only a small proportion of ESCC cases could be attributed to smoking or alcohol consumption. So, other risk factors must be responsible for the high incidence of ESCC in these areas.19 According to the results of previous studies in Iran, the main suspected risk factors include low intake of fruits and vegetables, drinking hot tea, consumption of opium products and tobacco, Helicobacter pylori infection of the stomach, drinking contaminated water from cisterns, and genetic susceptibility.19 The main suspected mutagens are polycyclic aromatic hydrocarbons and N-nitroso compounds.20
Microbial agents, especially HPV may also be one of the factors that could explain part of such a high incidence rate in northern Iran as was shown by Farhadi and colleagues.19 They showed a prevalence of 36.8% for HPV infection in ESCC cases. The prevalence of HPV16 was significantly higher in ESCC cases (13.2%) than that in controls (0%) (P=0.05), but there was no statistically significant difference in the prevalence of HPV18 between cases and controls. They concluded that only HPV16, but not HPV18, may be considered as a risk factor for ESCC in northern Iran. While northern Iran is considered a high incidence area, remaining parts of Iran including southern Iran appear to have much lower annual incidence of ESCCs. Although, epidemiologic study and cancer registry of ESCC cases show that esophageal cancer is among the ten most prevalent cancers in Fars province, its annual incidence rate is 1.3 to 2.8 per 100,000. However, in northern Iran, annual incidence of ESCC is much higher (108 per 100,000) in Mazandaran and even higher in Torkamansahra (108.8 per 100,000 in men and 174 per 100,000 in women).
Shiraz Medical Centers in the past 2-3 decades have been the major referral centers for referral of patients from large areas of south and south west of Iran. Therefore, registered cases of ESCC may be used to estimate the incidence of ESCC in such areas. Therefore, Fars province and neighboring provinces in south of Iran can be considered as low incidence areas. Human papilloma virus DNA has been found frequently in ESCCs from high incidence areas.13,14,15,21 As mentioned earlier, de Villier and colleagues demonstrated presence of HPV DNA in 17.1% of the ESCC cases.13 Li and colleagues evaluated specimens of balloon cytology examination from volunteers in two regions with significantly different incidence of esophageal carcinoma. PCR results showed that prevalence of HPV-16 E6 gene in a high incidence area was 1.9 fold higher than that of low incidence area (72% and 37%, respectively, P<0.01). Similar results were obtained with HPV-16 E7 gene using ISH. They suggested that HPV-16 plays a causative role in the pathogenesis of esophageal cancer, especially in the high incidence area of China.14
Si and colleagues evaluated some HPV-16 positive cases of ESCC to determine physical status of HPV-16 in these cases. They showed a predominance of integrated form of viral genes in HPV-16 positive ESCCs from china implying that the HPV infection may play a role in the pathogenesis of ESCC.15 In contrast, HPV infection was infrequent in cancer-bearing patients of north America,4 and even undetectable in many other low incidence regions.17,18
Our study shows that there was no association between HPV infection and development of esophageal squamous cell carcinoma. Thus, the absence of HPV DNA in this region is similar to that in most of other low incidence areas, and indicates that HPV infection is not likely to be involved in esophageal tumorigenesis in this region. However, there are some other factors such as dietary and environmental factors as well as an ill-defined contribution from genetic predisposition that may be involved in pathogenesis of esophageal carcinomas.2,3 Esophageal cancers in south of Iran may be attributed to dietary deficiencies of vitamins and essential metals, presence of carcinogens such as fungus-contaminated and nitrosamine-containing food stuffs, drink of hot tea, chronic esophagitis, and tobacco usage. Although alcoholic drinks and their carcinogenic components such as poly cyclic hydrocarbons, fusel oil and nitrosamine along with other mutagenic compounds and associated nutritional deficiencies are considered to play a significant role in some low incidence areas such as Europe and United States, they may not a risk factor in southern parts of Iran.2
The possibility of any technical error in reaching negative results in PCR assay is not appreciable. Good quantity and quality of extracted DNA confirmed by spectrophotometery and agarose gel electrophoresis, strong positive reaction in positive controls as well as successful amplification of beta globin gene, as internal control in all cases, clearly rule out the possibility of any technical error.
Negative results in PCR assay with and without internal control in tumoral and non-tumoral cases also rule out the possibility of competitive inhibition of general HPV primer set by beta globin gene specific primer set. Formalin, which is the most usual fixative, has profound effects on nucleic acids. However, its effects depend on concentration, pH, temperature, duration of fixation as well as type of tissue storage.22 Obviously, DNA extracted from tissue left in formalin solution for a long period is more likely to be damaged by formalin compared to that extracted from tissue embedded in paraffin blocks after initial fixation only for one to two days.23 However, several studies have shown that formalin fixed tissues, even those left in formalin for a long duration, are quantitatively and qualitatively good enough to yield DNA to succeed in PCR assays.24 However, few studies have reported yield of high molecular weight DNA from formalin fixed tissues.13
Thus, the effect of formalin on DNA as a probable cause of negative results in PCR assay is ruled out. On the other hand, several studies mostly from low incidence areas used extracted DNA from non fixed, frozen tissues as well as cell lines of ESCC (not exposed to formalin) and similarly showed negative results in PCR or southern blot hybridization techniques.18, 25 Moreover, using the same materials, primers and instruments, a PCR assay of cases with uterine cervical carcinoma in this center successfully revealed the presence of common and type specific HPV DNA sequences in many of the cases. So, negative findings in our study can’t be attributable to a sensitivity problem in the PCR assay.
Conclusion
The findings of the present study indicate that there is no association between HPV infection and development of esophageal squamous cell carcinoma in the cases evaluated.
Acknowledgment
The authors would like to thank Mr. Abdul-Mohammad Pezeshki for his great cooperation and technical support. This study was supported by a grant (number; 81-1528) from Vice Chancellor for Research, Shiraz University of Medical Sciences.
Conflict of interest: None declared.
References
- 1.Crawford JM. The gastrointestinal tract. In: Cotran RS, Kumar V, Collins T, editors. Robbins pathologic basis of disease. 6th ed. Philadelphia: W.B. Saunders; 1999. pp. 776–87. [Google Scholar]
- 2.Bagheri LankaraniK, Mowla A, Asadian F, Tabei SZ, Panjeshahin MR. Changing epidemiology of esophageal cancer in Fars province, Iran. Iran J Med Sci. 2002;27:4–10. [Google Scholar]
- 3.Riddell RH. Early detection of neoplasia of the esophagus and gastroesophageal junction. Am J Gastroenterol. 1996;91:853–63. PubMed PMID: 8633572. [PubMed] [Google Scholar]
- 4.Turner JR, Shen LH, Crum CP, Dean PJ, Odze RD. Low prevalence of human papillomavirus infection in esophageal squamous cell carcinomas from North America: analysis by a highly sensitive and specific polymerase chain reaction-based approach. Hum Pathol. 1997;28:174–8. doi: 10.1016/s0046-8177(97)90102-7. doi: 10.1016/S0046-8177(97)90102-7. PubMed PMID: 9023398. [DOI] [PubMed] [Google Scholar]
- 5.Zur HausenH. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996;1288:55–78. doi: 10.1016/0304-419x(96)00020-0. PubMed PMID: 8876633. [DOI] [PubMed] [Google Scholar]
- 6.Vousden K. Interactions of human papillomavirus transforming proteins with the products of tumor suppressor genes. FASEB J. 1993;7:872–9. doi: 10.1096/fasebj.7.10.8393818. [DOI] [PubMed] [Google Scholar]
- 7.Huibregtse JM, Beaudenon SL. Mechanism of HPV E6 proteins in cellular transformation. Semin Cancer Biol. 1996;7:317–26. doi: 10.1006/scbi.1996.0041. doi: 10.1006/scbi.1996.0041. PubMed PMID: 9284524. [DOI] [PubMed] [Google Scholar]
- 8.Zur HausenH. Papillomavirus and p53. Nature. 1998;393:217. doi: 10.1038/30363. PubMed PMID: 9607756. [DOI] [PubMed] [Google Scholar]
- 9.Miller OJ, Therman E. Human chromosomes. 4th ed. New York: Springer-Verlag; 2001. [Google Scholar]
- 10.Yagi N, Satonaka K, Horio M, Shimogaki H, Tokuda Y, Maeda S. The role of DNase and EDTA on DNA degradation in formaldehyde fixed tissues. Biotech Histochem. 1996;71:123–9. doi: 10.3109/10520299609117148. doi: 10.3109/10520299609117148. PubMed PMID: 8724437. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Furuya S, Takeuchi T, Hirohashi S. Modified formalin and methanol fixation methods for molecular biological and morphological analyses. Pathol Int. 1997;47:685–91. doi: 10.1111/j.1440-1827.1997.tb04442.x. doi: 10.1111/j.1440-1827.1997.tb04442.x. PubMed PMID: 9361102. [DOI] [PubMed] [Google Scholar]
- 12.Díaz-Cano SJ, Brady SP. DNA extraction from formalin-fixed, paraffin-embedded tissues: protein digestion as a limiting step for retrieval of high-quality DNA. Diagn Mol Pathol. 1997;6:342–6. doi: 10.1097/00019606-199712000-00006. PubMed PMID: 9559294. [DOI] [PubMed] [Google Scholar]
- 13.de VilliersEM, Lavergne D, Chang F, Syrjänen K, Tosi P, Cintorino M, et al. An interlaboratory study to determine the presence of human papillomavirus DNA in esophageal carcinoma from China. Int J Cancer. 1999;81:225–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<225::aid-ijc10>3.0.co;2-0. doi: 10.1002/(SICI)1097-0215(19990412)81:2<225::AID-IJC10>3.3.CO;2-S. PubMed PMID: 10188723. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, et al. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929–34. doi: 10.1093/carcin/22.6.929. doi: 10.1093/carcin/22.6.929. PubMed PMID: 11375901. [DOI] [PubMed] [Google Scholar]
- 15.Si HX, Tsao SW, Poon CS, Wong YC, Cheung AL. Physical status of HPV-16 in esophageal squamous cell carcinoma. J Clin Virol. 2005;32:19–23. doi: 10.1016/j.jcv.2004.04.004. PubMed PMID: 15572001. [DOI] [PubMed] [Google Scholar]
- 16.Shen Z, Cen S, Shen J, Cai W, Xu J, Teng Z, et al. Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol. 2000;126:589–94. doi: 10.1007/PL00008469. doi: 10.1007/PL00008469. PubMed PMID: 11043396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RJ, Perry AC, Newcomb PV, Hardwick RH, Alderson D. Human papillomavirus and oesophageal squamous cell carcinoma in the UK. Eur J Surg Oncol. 1997;23:513–7. doi: 10.1016/s0748-7983(97)92981-4. doi: 10.1016/S0748-7983(97)92981-4. PubMed PMID: 9484921. [DOI] [PubMed] [Google Scholar]
- 18.Saegusa M, Hashimura M, Takano Y, Ohbu M, Okayasu I. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in oesophageal and gastric carcinomas in Japan. Mol Pathol. 1997;50:101–4. doi: 10.1136/mp.50.2.101. doi: 10.1136/mp.50.2.101. PubMed PMID: 9231159; PubMed Central PMCID: PMC379592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200–3. doi: 10.3748/wjg.v11.i8.1200. PubMed PMID: 15754405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadjadi AR, Marjani H, Semnani Sh, Nasseri-Moghaddam S. Esophageal cancer in Iran: a review. Middle East J Cancer. 2010;1:5–14. [Google Scholar]
- 21.Shuyama K, Castillo A, Aquayo F, Sun Q, Khan N, Koriyama C, et al. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. Br J Cancer. 2007;96:1554–9. doi: 10.1038/sj.bjc.6603765. doi: 10.1038/sj.bjc.6603765. PubMed PMID: 17453003; PubMed Central PMCID: PMC2359949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGhee JD, von HipplePH. Formaldehyde as a probe of DNA structure. 3. Equilibrium denaturation of DNA and synthetic polynucleotides. Biochemistry. 1977;16:3267–76. doi: 10.1021/bi00634a001. PubMed PMID: 560859. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–71. doi: 10.1016/S0002-9440(10)64472-0. doi: 10.1016/S0002-9440(10)64472-0. PubMed PMID: 12466110; PubMed Central PMCID: PMC1850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitta Y, Tanaka H, Masuda Y, Hoshi M. The quality of DNA recovered from the archival tissues of atomic bomb survivors is good enough for the single nucleotide polymorphism analysis in spite of the decade-long preservation in formalin. J Radiat Res. 2002;43:65–75. doi: 10.1269/jrr.43.65. doi: 10.1269/jrr.43.65. PubMed PMID: 12056331. [DOI] [PubMed] [Google Scholar]
- 25.Mizobuchi S, Sakamoto H, Tachimori Y, Kato H, Watanabe H, Terada M. Absence of human papillomavirus-16 and -18 DNA and Epstein-Barr virus DNA in esophageal squamous cell carcinoma. Jpn J Clin Oncol. 1997;27:1–5. doi: 10.1093/jjco/27.1.1. doi: 10.1093/jjco/27.1.1. PubMed PMID: 9070332. [DOI] [PubMed] [Google Scholar]