Abstract
Organophosphorous compounds have been employed as pesticides and chemical warfare nerve agents. Toxicity of organophosphorous compounds is a result of excessive cholinergic stimulation through inhibition of acetyl cholinesterase. Clinical manifestations include cholinergic syndromes, central nervous system and cardiovascular disorders. Organophosphorous pesticide poisonings are common in developing worlds including Iran and Sri Lanka. Nerve agents were used during the Iraq-Iran war in 1983-1988 and in a terrorist attack in Japan in 1994-1995. Following decontamination, depending on the severity of intoxication the administration of atropine to counteract muscarinic over-stimulation, and an oxime to reactivate acetyl cholinesterase are indicated. Supportive and intensive care therapy including diazepam to control convulsions and mechanical respiration may be required. Recent investigations have revealed that intravenous infusion of sodium bicarbonate to produce mild to moderate alkalinization is effective. Gacyclidine; an antiglutamatergic compound, was also proved to be beneficial in conjunction with atropine, pralidoxime, and diazepam in nerve agent poisoning. Intravenous magnesium sulfate decreased hospitalization duration and improved outcomes in patients with organophosphorous poisoning. Bio-scavengers including fresh frozen plasma or albumin have recently been suggested as a useful therapy through clearing of free organophosphates. Hemofiltration and antioxidants are also suggested for organophosphorous poisoning. Recombinant bacterial phosphotriesterases and hydrolases that are able to transfer organophosphorous-degrading enzymes are very promising in delayed treatment of organophosphorous poisoning. Recently, encapsulation of drugs or enzymes in nanocarriers has also been proposed. Given the signs and symptoms of organophosphorous poisoning, health professionals should remain updated about the recent advances in treatment of organophosphorous poisoning poisonings.
Key Words: Organophosphorous, neurotoxicity, treatment, atropine, oxime
Introduction
Organophosphorous (OPs) compounds have been employed as pesticides, petroleum additives and chemical warfare nerve agents.1 The OPs have been used as pesticides for more than 50 years and are still used in most developing countries including Islamic Republic of Iran.2 They are also named anticholinesterase agents as they act by inhibition of acetyl cholinesterase (AChE) resulting in symptoms and signs associated with cholinergic receptor stimulation. It is believed that between 750,000 and 3,000,000 OP poisoning occur globally every year.3 Organophosphorous pesticides poisoning can result from occupational, accidental or intentional exposure. Mortality is higher in the developing countries where OP pesticides are readily available and may be used for suicide. They are estimated to cause 300,000 fatalities annually.4
For the first time, OPs were synthesized by von Hoffman. In 1873 He synthesized methyl phosphor chloride, which led to the synthesis of a number of insecticides. The OP warfare nerve agents, (commonly called ‘nerve agents’) are much more toxic than pesticides.5
The nerve agents comprise two series including G-agents and V-agents. G-agents were produced in Germany by Dr. Gerhard Schrader team in 1930s. They synthesized tabun in 1938 and then sarin. These compounds were named after him and his two co-workers (“G” means German). The G agents comprise fluorine compounds of organophosphate except for tabun (GA). The famous agents in this group are sarin (GB; 2-fluoro-methylphophoryloxypropane), soman (GD; 3-fluoro-methyl-phosphoryloxy-2, 2-dimethyl-butane), tabun (GA; ethyl N, N-dimethylphophoramidocyanidate) and cyclosarin (GF; (fluoro-methyl-phophoryloxycyclohexane). V-agents were synthesized after World War II in the United Kingdom. The V was derived from the word victory, the share of allied forces from World War II. The V agents are sulfur containing organophosphate compounds. Among these compounds VE (S-2-diethylamino ethyl O-ethylethylphophonothioate), VG (2 diethoxyphosphorylsulfanyl- N,N-diethylethanamine), VM (2-ethoxy-methylphosphoryl sulfanyl-N,N-diethylethanamine), VR (Russian VX; N,N-diethy-2-methyl-2-methylpropoxy phosphoryl sulfanylethanamine) and VX (S-2 diisopropylamino O-ethylmethylphosphonothioate) are important as warfare nerve agents. The V-agents are more toxic than the G-agents.5,6
Nerve agent tabun was used in the battlefield for the first time in 1984 by Iraqi army to achieve victory against Iran. From 1983 to 1988, Iraq used sulfur mustard and nerve agents such as sarin and tabun against Iranian combatants, and later against the civilians. Nerve agents were also used by Iraq in 1988 against Iraqi Kurdish civilians during Halabjah massacre. It was estimated that 45,000 to 100,000 individuals were poisoned by chemical weapons during the Iraq-Iran war. The poisoning, which was associated with high mortalities, was mostly caused by the nerve agents.7,8 Matsomoto (June 27, 1994) and Tokyo subway (March 20, 1995) attacks in Japan by sarin are other well-known OP nerve agent incidents with 12 deaths and more than five thousands intoxicated people.9-11
Despite the establishment of organization for prohibition of chemical weapons (OPCW), OP nerve agents are still threat to the human population. In addition, wide use of OP pesticides in most developing countries including Iran has induced health problems. Hence, it is quite logical that health professionals should increase their knowledge about all aspects of OPs, particularly on recent advances in the treatment of pesticides and nerve agent poisonings.
Chemistry and Toxicology
Organophosphorous compounds including organophosphates are chemically derived from phosphoric, phosphonic, phosphinic or thiophosphoric acids. Organophosphates are usually esters, amides, or thiol derivatives of phosphoric, phosphonic, or phosphinic acids. The general formula of organophosphates is as follows:
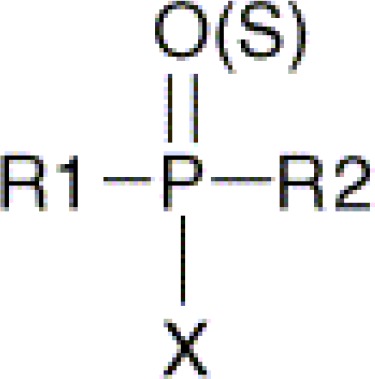
R1 and R2 are alkyl-, alkoxy-, alkylthio- or amido groups. X is the acyl residue.
Organophosphorous pesticides vary in chemical structures and toxicities. The main groups are phosphate, phosphorothioate, O-alkyl phosphorothioate and phosphorodithioate. A phosphorthioate compound such as parathion is much more toxic than a phosphorodithioate compound like Malathion. Apart from the OP pesticides and chemical warfare nerve agents, very few OP compounds such as glyphosate and merphos were used as herbicides. Organophosphorous herbicides differ from the OP pesticides structurally and their AChE–inhibiting power is much less than the other OPs.12
Although the term “nerve gas” is frequently used, all the nerve agents are liquids at standard temperature and pressure. This misunderstanding comes from the first use in World War I of CWA such as chlorine and phosgene that are true gases. These liquids are tasteless, odorless and volatile, and evaporate spontaneously at room temperature. The G agents have the density of water and evaporate at about the same rate as dose water, and have freezing points around 0°C and boiling points around 150°C. The VX, in contrast, are oily, have a consistency similar to that of motor oil, and evaporate very slowly. Thus, it will contaminate the environment for a longer period.13
Toxicokinetics
Organophosphorous compounds can easily cross the respiratory epithelial and dermal membranes because of their lipophilic structures, and thus they are formed mostly as aerosol.14,15 Gastric mucosa is also very permeable to Ops, and is a classical way of absorption in suicidal cases.16 Organophosphorous compounds are distributed in the whole body, particularly in fatty tissues, and their fast degradation usually inhibits their accumulation.
Some OPs are eliminated without considerable metabolism. However, they usually become degraded and eliminated in urine, feces and exhaled air. Most OP insecticides are activated through oxidation in the liver by enzymes of cytochrome P450 system and flavin-containing monooxygenases. Soman, sarin and other nerve agents are inherently active. The main enzymatic systems involved in the detoxification of OPs are phosphotriesterases, carboxylesterases and glutathione-S-transferases. A main detoxification pathway of OPs is hydrolysis by esterases called ‘phosphotriesterases’ (PTEs). The products of the reaction display no phosphorylating capability, and therefore the hydrolysis of OPs by PTEs is considered a detoxification. The most known PTEs is human serum paraoxonases.17
Compared to G-agents, VX has several particular characteristics. The anticholinesterase properties of VX are as a result of the oxo (O) group, and partly the presence of alkyl substituents. The VX is present in blood as a protonated amine. It is hydrolyzed at a slower rate than G-agents, and reacts more slowly with CarbE and A-esterases. The VX is also metabolized by other pathways like oxidation reactions at nitrogen and/or sulfur.18
Shih et al. studied the excretion pattern of alkyl methylphosphonic acids from sarin, soman and cyclosarin in the rat following subcutaneous administration with a dose of 0.075 mg/kg.19 Urinary excretion over the first 24 h constituted nearly 90% of the sarin and cyclosarin. Soman was eliminated with a slower and biphasic elimination curve. Approximately 50% was excreted within the first 24 h. The initial hydrolysis of tabun produces ethyl N, N-dimethylphosphoramidic acid and ethyl phosphorocyanidic acid, that are unstable and hydrolyze further to ethyl phosphoric acid and then slowly to phosphate. But the problem is that the background of ethyl phosphoric acid in the general population is quite variable, presumably from pesticides and plasticizers.
The Remnant of unbound OP in patients depends on the chemical properties and activity of OP hydrolyzing enzymes, like paraoxonases. Lipophilic OP compounds such as parathion and its active form paraoxon, may distribute widely in the body resulting in long-term toxic plasma levels.20
Mechanism of Toxicity
Toxicity of OPs is the result of excessive cholinergic stimulation through inhibition of acetyl cholinesterase (AChE). Muscarinic and nicotinic acetylcholine (ACh) receptors are found in the central and peripheral nervous system. Acetylcholine is a neurotransmitter that contributes to nerve conduction following its release in autonomic ganglia at sympathetic preganglionic synapses, at parasympathetic postganglionic synapses, and at neuromuscular junctions of the skeletal muscle. The actions of ACh are removed by hydrolysis by AChE enzyme.
In human body there are different types of cholinesterases, which differ in their location in tissues, substrate affinity, and physiological function. Two main types of cholinesterases include: 1-Acetyl cholinesterase (AChE) or true cholinesterase and 2-Butyrylcholinesterase (BChE) or pseudecholinesterase. Acetyl cholinesterase is the principal form that is found in neurons, neuromuscular junctions and erythrocyte membranes. Another form of AChE, which is known as serum cholinesterase (ChE), is a group of enzymes present in plasma, liver, cerebrospinal fluid and glial cells. It is a circulating plasma glycoprotein synthesized in the liver, and does not serve any known physiological function. Butyrylcholinesterase acts as a stoichiometric scavenger of nerve agents and its inhibition appears to have no significant physiological effects in the absence of other toxicants.21 It has been proposed that BChE may have a role in cholinergic neurotransmission, and is involved in other nervous system functions. It is also important as a biomarker of exposure to OPs.22
Nerve agents react rapidly with a serine hydroxyl group in the active site of AChE and form a phosphate or phosphonate ester. The G-agents are anticholinesterase OP nerve agents that at sufficient concentrations can be toxic or fatal by any route of exposure. Phosphorylated AChE is not able to hydrolyze ACh, and regenerates very slowly, thus, the enzyme will remain inhibited until new enzyme is generated, or until an enzyme reactivator (oxime) is used.23 In addition, binding reactions of nerve agents to esterases such as AChE, BChE, carboxylesterases (CarbE) and other proteins occur. It has also been reported that at very high doses of nerve gases, they can activate AChE receptors. Both OP pesticides and nerve agents lose their acyl radicals when they react with AChE, BChE and CarbE. After binding to AChE and BChE the phosphoryl residues of soman, sarin, tabun and VX undergo an intramolecular rearrangement with subsequent loss of one phosphoryl group. This reaction is known as aging (The time between OP exposure and the irreversible phosphorylation), and defined as non-enzymatic time-dependent loss of one alkyl group bound to the phosphorus, which leads to a stable non-receivable form of phosphorylated AChE that is resistant to both spontaneous and oxime-induced reactivation. The aging varies from a few minutes (soman) to 22 hours (cyclosarin).24
As inhibitors of AChE, Organophosphorous compounds, may act directly or indirectly. Direct inhibitors, such as dichlorvos, are useful without additional metabolic modification following absorption, and thus cause rapid symptoms and signs during or after exposure. To be effective, indirect inhibitors such as malathion need to be transformed. All thiono OP pesticides containing a P=S bond need activation by oxidation of the P=S to the P=O group. The symptoms and signs of these compounds appear later, and last longer. In addition, due to the reversibility of the binding reaction of sarin and soman to CarbE, it appears that CarbE is involved in metabolic detoxification of these agents to their corresponding non-toxic metabolites isopropyl methylphosphonic acid (IMPA) and pinacolyl methylphosphonic acid (PMPA).25,26
Organophosphorous compounds poisoning can be diagnosed based on a history of exposure via intentional or accidental oral OP pesticide ingestion, occupational or chemical warfare assult, and clinical manifestations. The enzymes inhibited by OPs provide specific biomarkers of exposure, until the turnover of the enzyme in favorable cases. Accessible AChE is found in red blood cells, and BChE in the plasma.27 Butyrylcholinesterase is usually preferred as an early biomarker due to its higher presence and sensitivity than AChE, however, is not as specific as AChE. Screening the red blood cell concentrations of AChE in individuals who are exposed to these agents is essential. Although screening has several limitations, it can also be used for suspected individuals with nerve agent poisoning. Due to interindividual variations, it does not provide a reliable evidence for low levels of organophosphate exposure at low levels due to interindividual variations. Moreover, control activity levels are often not available.28 Finally, it is less suitable for retrospective detection of exposure because of new synthesis of enzyme. However, measurement of AChE inhibition is still the most widely used method for the assessment of exposure to nerve agents.26 Grading of OP poisoning severity based on different types of cholinesterases are presented in table 1.
Table 1:
Severity grading of organophosphorus poisoning based on the cholinesterase inhibition and atropine dose required for atropinization
| Grade | Butyrylcholinesterase activity (%) | Acetylcholinesterase activity (%) | Atropine dose for atropinization |
|---|---|---|---|
| Mild | 40-50 | 50-90 | <2mg |
| Moderate | 10-40 | 10-50 | <2-10mg |
| Severe | <10 | <10 | >10mg |
Urine metabolites, or adducts to proteins and DNA can also be used as biomarkers for detecting nerve agent exposure. The main metabolites of nerve agents are alkyl methylphosphonic acids that are found rapidly in the urine, and can be detected up to one week after exposure depending on the extent of eposure.29-33 Polhuijs et al described the fluoride reactivation technique, which implies the release of the nerve agent upon incubation with a large concentration of fluoride ions followed by analysis of the generated phosphofluoridate with gas chromatograpy-mass spectrophotometry (GC–MS), which is based on reactivation of the phosphorylated enzyme with fluoride ions.34 In this way both the origin and extent of the organophosphate poisoning can be determined. Fidder and co-workers developed a procedure that is based on straightforward isolation of adducted BChE from plasma by means of affinity chromatography with a procainamide column, followed by pepsin digestion and LC/electrospray tandem MS analysis of a specific nonapeptide, containing the phosphorylated active site serine-198 residue.35 The VX hydrolysis product, O-ethyl methylphosphonic acid, has been determined by GC–MS in serum. Recently, an LC/tandem MS method was developed for quantitative determination of IMPA in blood and urine. High levels of IMPA appeared to correlate with low levels of residual BuChE activity in the plasma.36 Diagnosis of a certain nerve agent requires toxicological analyses of the environmental and/or blood samples for the nerve agents. A biosensor which is a potentiometer enzyme electrode has been developed to determine OP nerve agents directly.37 A fiber optic enzyme biosensor for the direct measurement of OP nerve agents is also introduced. Using the kinetic response, concentrations as low as 2 μM can be measured in less than two minutes.38
Albumin is another target following nerve agent exposure.36-40 Moreover, α-glucoronidase in liver has been proposed as a biomarker of exposure to Ops.41 Diagnosis of the delayed neurotoxic effects can be made by estimation of NTE, although it is not probable to occur following the nerve agents poisoning.
Acute and Chronic Clinical Manifestations
Acute Effects
Clinical effects after OP exposure can be divided into acute and chronic manifestations. The acute effects of OPs depend on the site of exposure, which can be following inhalation, skin or eye contact, or ingestion. However, large doses all exposure routes cause similar effects.36 For most OP pesticides, dermal exposure and subsequent absorption through the skin is the most common way of poisoning in occupational exposure. Percutaneous absorption of OPs varies according to the exposed site and the ambient temperature. The VX was absorbed nearly eight times more rapid from facial skin than from the volar forearm, and the absorption increased considerably as the temperature rose from 18 to 46°C in the site. Initial local effects of liquid include muscular fasciculation’s and sweating at the site, malaise and weakness. The initiation of these effects is immediate, usually after an interval of 10 to 30 min.42
Although occupational and accidental ingestion may occur in children and work settings, the oral route of entry is important in intentional OP pesticide poisoning. Nausea, vomiting, abdominal pain, and diarrhea as well as cholinergic syndrome, central nervous system and cardiovascular problems may occur in moderate to severe oral OP poisoning.43
Exposure to low-vapor levels can involve the eyes, nose, and airways. Visual disturbances, rhinorhea, and/or dyspnea can develop seconds to several minutes after exposure. Eye contact with vapor causes miosis that may be accompanied by deep eye pain, conjunctival irritation, and visual disturbances. Inhalaltion of high-vapor concentration can induce consciousness within one or two minutes and then cause seizures, flaccid paralysis, and apnea and the victims may die within 30 minutes in the absence of immediate medical care.44
Cholinergic clinical manifestations of OPs are as a result of excessive ACh receptors (muscarinic and nicotinic) stimulation, which appear during the first few hours after exposure.28 Muscarinic receptors include dizziness, nausea, vomiting, abdominal pain, diarrhea, miosis, blurred vision, salivation, lacrimation, urination and respiratory dysfunctions. Major effects on the respiratory system include bronchoconstriction and increased bronchial secretion leading to respiratory failure, which is the main cause of mortality.
Nicotinic effects include easy fatigue, weakness, muscle cramp, fasciculations, skeletal muscle twitching, convulsions and flaccid paralysis. Central nervous system effects include: irritability, nervousness, giddiness, ataxia, fatigue, and generalized weakness, depression of respiratory and circulatory centers with dyspnea, cyanosis, hypoventilation and hypotension, lethargy, impairment of memory, confusion, convulsions, coma and respiratory depression.45-48 Consequently, depression of respiratory and vasomotor centers in the brain can occur and deteriorate the clinical picture.49 With moderate to large doses of OPs, nicotinic and central stimulation predominates over most muscarinic effects. Death is usually due to respiratory and cardiovascular failure.50 The most life-threatening complication is respiratory failure, that is the most severe result of the nerve agents.51,52 One of the remarkable problems in the nerve agent poisoning is hypoxia, which may lead to cerebral edema, convulsions, and histopathological brain damage.
Cardiovascular complications are sometimes severe and life threatening.53,54 Acute OP poisoning is associated with ventricular arrhythmias, tachycardia or bradycardia, and mild myocardial ischemia.55 Exaggerated cholinergic stimulation increases the vagal nerve influence on heart rate and induces bradycardia and slowed cardiac conduction, leading to a decrease in cardiac output. However, in practice, tachycardia is usually observed as a result of fear and anxiety. Electrocardiogram abnormalities consist of idioventricular dysrhythmias, atrial fibrillation, multiform ventricular extra systoles, ventricular fibrillation, and complete heart block.52,56-58 Intermittent ST-T wave alterations and second-degree atrioventricular heart block also occurs. The nerve agents; tabun, sarin, soman, or VX at 5 to 10 times the LD50 in guinea pigs induced circulatory arrest a few minutes after apnea in nontreated animals. Histopathological changes compatible with toxic myocarditis were observed following sarin and soman in animal experiments, but it has not been reported in humans.58
Intermediate Syndrome
The intermediate syndrome, which occurs on 1-4 days after acute poisoning, consists of marked weakness in the proximal skeletal muscles, respiratory muscles, and cranial nerve palsies.59,60 Intermediate syndrome is due to cholinergic over activity at the neuromuscular junction, and a connection has been made between the intermediate syndrome and OP-induced myopathy. Studies conducted in the 1990s have shown that intermediate syndrome is associated with an excretion of cholinesterase inhibitor metabolites in the urine and by a severe depression in cholinesterase levels. It has been reported following exposure to specific OP pesticides with a dimethyl phosphate moiety such as fenthion, dimethoate, dichlorvos and methylparathion, but has also been observed following parathion exposure.60,61 It was suggested that the condition might reflect the recirculation of lipid soluble cholinesterase inhibitors from body fat compartments or gastric fluids.62 The intermediate syndrome has not been reported after nerve agents poisoning.
Clinical severity grading of OP poisoning as mild, moderate, severe and fatal are summarized in table 2.
Table 2:
The grading of clinical severity of organophosphate poisoning
| Grade | Symptoms | Signs |
|---|---|---|
| Mild | Dizziness, anxiety, headache, tightness of breath | Rhinorrhea, sweating, salivation, nausea, weakness, coughing, lacrimation, mild bradycardia and Hypotension |
| Moderate | Restlessness, confusion, dyspnea, disorientation, abdominal pain, vomiting, diarrhea, drowsiness | Pallor, meiosis/mydriasis*, bradycardia/ tachycardia, hypertension/hypertension*, muscle twitching, fasciculation, respiratory depression, bronchorrhea, bronchospasm, loss of consciousness |
| Severe | ___ | Convulsions, respiratory failure, pulmonary edema, flaccid paralysis, involuntary micturation/defecation Cyanosis, deep coma |
| Fatal | ___ | Coma, convulsions, hyper secretions and apnea within a few minute after exposure |
*Depending on the muscarinic or nicotinic syndrome domination, miosis or mydriasis, bradycardia or tachycardia, hypertension or hypertension may occur. Moderate: Worsening of the above features plus the followings; Severe: Worsening of the above features plus the followings
Chronic Effects
Chronic poisoning may occur in workers (mainly agricultural workers) with daily exposure to OP compounds. Some OP pesticides are able to induce organophosphate-induced delayed neuropathy (OPIDN). It is a symmetrical sensorimotor axonopathy, which is most severe in long axons, and occurs seven to 14 days following exposure. Organophosphate-induced delayed neuropathy is initiated by phosphorylation and subsequent aging of >70% of the functional neuropathy target esterase (NTE) in peripheral nerves. The mechanism is believed to be via inhibition of NTE or a trophic factor such as depletion of ornithine decarboxylase in spinal cord.63 A case of sensory polyneuropathy seven months after sarin poisoning has been reported.64
Chronic OPIND
This occurs without cholinergic symptoms and apparently is not dependent on AChE inhibition. It is a symmetrical sensorimotor axonopathy, tending to be most severe in long axons and occurring after several exposures.65,66 The most common symptoms of Chronic OPIND (COPIND) include cognitive deficit (impairment in memory, concentration and learning, problems with attention, information processing, eye-hand coordination and reaction time), mood changes (anxiety, depression, psychotic symptoms, and emotional labiality), chronic fatigue, autonomic dysfunction, peripheral neuropathy and extrapyramidal symptoms such as dystonia, resting tremor, bradikynesia, postural instability and rigidity of face muscles. Diagnostic criteria for COPIND include: 1-repeated exposure to organophosphates; 2-at least four of the following: (a) personality change and destabilization of mood, (b) impairment of concentration, (c) impaired exercise tolerance, (d) reduced tolerance to alcohol, (e) heightened sensitivity to organophosphates; 3-at least three of the following: (a) exacerbation of “dippers flu”, (b) impulsive suicidal thinking, (c) language disorder, (d) heightened sense of smell, (e) deterioration of handwriting”.61 Tan et al, recently hypothesized that COPIND could be derived from withdrawal of OP pesticide after chronic low-level exposure or acute exposure.67
The most significant long-term neurologic effect of nerve agent exposure is hypoxic encephalopathy, which is one of the most important long-term neurologic sequels of nerve agents.6 Sensory nerve dysfunction of the lower extremities is more prevalent than motor nerves, which was predominantly a distal sensory deficit.68 Temporary psychological effects such as depression, fatigue, insomnia, irritability, nervousness and impairment of memory have been described after nerve agents exposure.69-71
An electroencephalogram (EEG) in a patient intoxicated with sarin showed considerable slowing with bursts of high voltage waves at a rate of five per second epileptic type changes of EEG 11 months after the exposure.72,73 Soman induced increasing cyclooxygenase-2 in damaged brain regions such as hippocampus, amygdale, piriform cortex and thalamus that was correlated with seizure intensity.74
Biochemical and Hematological Abnormalities
Acid-base and electrolyte disturbances are a common feature following severe OP poisoning. Nerve agent victims do not display a high anion gap metabolic acidosis that is observed in acute cyanide poisoning.6 Hypokalemia, hyperglycemia, elevation of serum amylase (an indicator for acute pancreatitis), transient elevation of liver enzymes, hematuria, leukocyturia, and proteinuria may occur. Arterial blood gas analysis and estimation of serum electrolytes, liver and kidney function tests, serum amylase, creatin phosphokinase (CPK) and lactate dehydrogenase (LDH), blood cell count and other hematological tests may be disturbed, and thus required to be performed for the management of patients.42
Death after nerve agent exposure and severe OP pesticides poisoning is mainly due to respiratory failure resulting from depression of the respiratory center, paralysis of respiratory muscles and obstruction caused by bronchospasm and bronchial secretions. Some animal studies suggest that lack of central drive is the major factor.51 Cardiomyopathy in soman and sarin-intoxicated rats has been reported, which may be a contributory cause of death.58
Status seizures occurred in animals after very high doses of sarin, soman, or VX despite early treatment with atropine and pralidoxime. Prolonged seizures may cause anoxia and morphological brain damage which induces more morbidity and mortality.75
Pretreatment in OP Nerve Agents Poisoning
Pretreatment with reversible carbamate AChE inhibitors, such as pyridostigmine and physostigmine, increases the efficacy of post-exposure treatment of soman because it can not reach AChE molecules which have already bounded by the carbamate. Pretreatment is not useful against sarin and VX poisoning, because physostigmine is toxic at the amounts required. Pyridostigmine is the drug of choice for pretreatment with the dosage of 30 mg orally twice daily.76
Medical Management of OP Poisonings
Primary Protection and Care
The first step in managing chemical victims is that the emergency responders must protect themselves to prevent contamination resulting from contact with casualties and the environment. Initial standard treatment of a nerve agent poisoning includes the administration of atropine to counteract muscarinic over-stimulation, an oxime to reactivate OP-inhibited AChE, and benzodiazepines to protect against central nervous system seizures.6 This should be done via an auto injector that is provided for the combatants.
Decontamination
Decontamination must be performed at the earliest opportunity to limit percutaneous absorption of the agent, and to prevent contamination of the rescuers. Complete decontamination is necessary before patients enter a health care center. Gastric aspiration and lavage is indicated in case of OP oral ingestion. If the eyes have been exposed, they should be irrigated as soon as possible with water and saline. Decontamination solutions, which are usually composed of strong alkaline chemicals, are used for efficient detoxification of chemical warfare agents.77 Some of the proposed decontaminants are aqueous mixtures (Sandia Foam, Decon Green), organic solutions (GD5, GD6F, and GDS2000) or sorbent powders (M100).77-80 Recombinant DNA-derived AChE represented a great improvement over wild-type AChE as bioscavengers. Using the cell immobilization technology, immobilized Escherichia coli with surface expressed OP hydrolase was made to detoxify nerve agents.81,82 By protein engineering techniques one BChE mutant G117H was made to hydrolyze V and G agents but reaction was too slowl.83 Organophosphate acid hydrolyses (OPAH) from two species of ateronomas were cloned and sequenced to detoxify G agents, which was effective.84 New polymers based on a dimethylacrylamide-methacrylate (DMAA-MA) co-polymer backbone are now available that support both chemical and biological agent decontamination.78 Recently, some decontaminants are dispersed in the form of fog, powder or aerosol produced that exhibit active degradation of VX, G agents and mustard gas (HD) to non-toxic products.85 In case of contact exposure with VX a simple and non-invasive application of cooling have been reported to be dramatically useful.86,87
In a recent systematic review,88 the authors suggested the following treatment protocol that include removal of contaminated clothes, washing the poisoned person, administering sodium bicarbonate, administering activated charcoal (single or multiple doses), gastric lavage, α2 adrenergic agonists, organophosphorous hydrolases, oximes, atropine, benzodiazepines, butyrylcholinesterase replacement, glycopyrronium bromide (glycopyrrolate), magnesium sulphate, N-methyl-D-aspartate receptor antagonists, and neuroprotective agents.
Antidotes
The famous antidotes of OPs are atropine and oximes. However, investigations over the recent years have introduced new adjunct therapy and cheap medications such as sodium bicarbonate and magnesium sulfate as well as antioxidants that should be considered for the management of OP poisoning.
Atropine Sulfate
Atropine sulfate blocks the effects of high concentrations of acetylcholine at muscarinic cholinergic synapses following OP inhibition of AChE, and is used as the drug of choice in acute OP poisoning. Atropine counteracts the muscarinic symptoms of OP poisoning including sweating, salivation, lacrimation, nausea, rhinorrhoea, vomiting and diarrhea, and can control cardiovascular problems. However, it is not effective on nicotinic receptor-medicated manifestations in such patients. However, The roles of atropine in OP poisoning are much more complex than in muscarinic blockade.89 It has been shown that atropine have anticonvulsant effects and inhibits brain damage caused by certain OPs.90 Other authors have demonstrated that atropine can only partly block convulsions following exposure to Ops, while GABA and glutamate are involved in cholinergic overstimulation in the CNS.91,92 In a study in rats it was revealed that atropine treatment reduced local use of cerebral glucose and brain damage during seizures induced with soman. Recent findings indicated that the dose of atropine given as antidotal therapy can significantly influence measures of nerve agent toxicity and responsiveness to anticonvulsant therapy.93
Atropine should be used to dry the secretions and improve cardiovascular and respiratory manifestations.87 Thus, no actual dose is determined for atropine. Atropine should be administered intravenously in doses that produce mild to moderate atropinization as soon as possible. Severity grading of OP intoxication can also be estimated based on the initial atropine dose required for atropinization as indicated in table 1. The same amount as the primary atropinization dose should be used in 500 mL dextrose 5% to sustain and repeat the atropinization, as required until the patient becomes asymptomatic. Atropine antagonizes the muscarinic and some of the CNS effects of OP poisoning, but is not as effective on skeletal muscle weakness, seizures, or unconsciousness.94 Aerosolized atropine can be administered quickly by inhalation that influences the lungs directly while being absorbed systemically.95
Oximes
Based on chemical structures, oximes can be divided in two groups including the monopyridinium and bispyridinium oximes. The only monopyridinium oxime that is used at present is pralidoxime (PAM-2). The most notable bispyridinium oximes consist of trimedoxime (TMB-4), obidoxime (LuH-6, Toxogonin) and asoxime (HI-6). The antidotal potency of pyridinium oximes is as a result of reactivation of the phosphorylated cholinesterases.96,97 Oximes can reactivate phosphorylated cholinesterases via replacing the phosphoryl moiety from the enzyme. Phosphorylated oximes are produced during this reaction and some of them seem to be potent inhibitors of AChE.98 The choice of oximes is based on the data presently available and may also be dependent on factors other than protection against lethality, such as cost and availability of the oximes and their side effects. Obidoxime (Toxogonin) is likely to cause more toxic effects than pralidoxime and HI6. asoxime is the least toxic, but is less unstable in solution and is not commercially available in many parts of the world.18 In soman-intoxicated guinea pigs, HI6 was therapeutically slightly more effective than HLo7, but was less effective than HLo7 against tabun intoxication.99
Pyridinium oximes are mostly used against OP-inhibited AChE in the peripheral nervous system and not as much in CNS. This is due to a limited penetration across the blood–brain barrier (BBB). However, it appears that the oximes penetrate BBB more than expected, since in soman poisoning oxime concentration in the brain was high.100 Recent studies in rats have shown that modulation of the BBB by a drug like tariquidar is of great value in enhancing the efficacy of oximes.101 The induction of local inflammatory processes and increase of brain–blood flow may also have some roles in enhancing the penetration of oximes through BBB. Sakurada et al have determined the amount of PAM-2 passing across the BBB at approximately 10% of the given dose. This amount may be effective in the reactivation of OP-inhibited AChE in the brain. A new class of amidine-oxime reactivators of organophosphate (OP)-inhibited cholinesterases (ChE) have been reported to have increased BBB penetration with greater reactivation rates for OP-BuChE than pralidoxime (2-PAM) and monoisonitrosoacetone, but lower rates for OP-AChE reactivation compared to 2-PAM.102
In another study, the authors demonstrated that purified human and rabbit serum paraoxonase1 significantly protected against sarin and soman exposure in guinea pigs.103 Newly developed oximes (K206, K269) are relatively effective in reducing cyclosarin-induced lethal toxic effects in mice. Their therapeutic efficacies exceed the therapeutic potency of obidoxime but not HI-6.104 Relative therapeutic effects of oximes in different OPs are presented in table 3.
Table 3:
Relative therapeutic effects of different oximes in organophosphate nerve agents poisoning
| Oximes | GA Sarin | GB Soman | GD Tabun | GF | VX |
|---|---|---|---|---|---|
| Pralidoxime | +++ | ++ | + | + | +++ |
| Pyrimidoxime | ++ | ++ | ++ | + | ++ |
| Obidoxime | ++ | +++ | ++ | ++ | +++ |
| HI6 | +++ | ++++ | ++ | ++++ | +++ |
| HLo7 | ++++ | +++ | ++++ | ++++ | ++ |
| HGG12 | N/A | +++ | + | N/A | N/A |
NA: No data available; +:Least effective; ++: Partially effective; +++: Moderately effective; ++++: Most effective
G: German; GA: G agent A; GB: G agent B; GD: G agent D; GF: G agent F; VX: Victory agent X
Pralidoxime should be administered intravenously at 30 mg/kg initially over 30 min, followed by constant infusion of 8 mg/kg/hr in dextrose 5% solution.105 It could be continued until the full recovery or until atropine is required. Obidoxime was hepatotoxic at high recommended doses of 8 mg/kg initially, and 3 mg/kg/hr. It may be given at a dose not more than 500 mg initially and 750 mg/day. Liver function tests should be checked regularly during obidoxime therapy to avoid severe hepatotoxicity. Adverse effects of PAM-2 iodide include dizziness, blurred vision, occasional diplopia, impaired accommodation, nausea and headache. The use of PAM-2 iodide in conjunction with atropine and diazepam has been shown to be very useful. However, PAM-2 lacks the efficacy against tabun and soman and hence, can’t be considered as the drug of choice in nerve agent poisoning.106
Benzodiazepines
Benzodiazepines (BDZ) have several effects. Most importantly, they are CNS depressants, anxiolytics and muscle relaxants through action at the gamma-aminobutyric acid (GABA) receptors.72 The receptor for GABA, a major inhibitory neurotransmitter, is a ligand gated chloride ion channel, and contribute to nicotinic acetylcholine and glycine receptors. In a study on rat cerebral cortex, it was demonstrated that organophosphates in high dose inhibited GABA metabolism in synaptosomal preparations.107 The main effect of benzodiazepines in CNS is hyperpolarization of neurons resulting in less susceptibility to cholinergic depolarization. Benzodiazepines such as diazepam, alter GABA binding to its receptor allosterictly, but do not directly activate the receptors. Administration of atropine and diazepam at the same time is more efficient in decreasing mortality in soman poisoning than atropine or oxime alone. Diazepam enhances the efficacy of low doses of atropine, and decreases the synaptic release of ACh in the cholinergic nervous system.108
On the other hand, benzodiazepines have favorable effects on anxiety, restlessness, muscle fasciculation, seizures, apprehension and agitation, and decrease morbidity and mortality when used together with atropine and an oxime in nerve agents poisoning.109 Diazepam should be administered when convulsions or pronounced muscle fasciculation are present. In severe poisoning, it should be considered even before the occurrence of these complications.110 The dose of diazepam in OP poisoning is 5–10 mg intravenously in the absence of convulsions, and 10–20 mg intravenous bolus in its presence. Its use should be continued as required.111,112 The recommended dose of diazepam by World Health Organization (WHO) is 5–10 mg intravenously over a period of 3 min that can be repeated every 10–15 min in adult patients up to a maximum of 30 mg. For children, it is 0.2–0.3 mg/kg given intravenously over 3 minutes. The maximum dose for children up to 5 years old is 5 mg, while up to 10 mg can be used for children who are older than 5 years. Several authors have reported that compared with other benzodiazepines midazolam may stop the seizures faster and at lower blood concentrations when applied intramuscularly.113,114
Gacyclidine
Gacyclidine is an antiglutamatergic compound that was proved to be beneficial in conjunction with atropine, pralidoxime, and diazepam in nerve agents poisoning. Electroencephalogram findings demonstrated that gacyclidine inhibited seizures that were induced by soman. It also markedly enhanced clinical recovery of soman-challenged primates. Gacyclidine inhibited the neuropathology that occurred three weeks following soman exposure in animals. In the presence of severe nerve agent poisoning, gacyclidine can be a useful adjuvant therapy along with the present available polymedications of OP nerve agent poisonings.115
Sodium Bicarbonate
It has been suggested that intravenous infusion of sodium bicarbonate produces moderate alkalinization (blood pH between 7.45 and 7.55) in OP pesticide poisoning. Sodium bicarbonate was first used to correct the metabolic acidosis. Regarding its enhanced therapeutic effects, the infusion of higher doses of sodium bicarbonate (5 mEq/kg in 60 min followed by 5-6 mEq/kg/day) was shown to be useful.116 It may also be effective in nerve agents poisoning, and thus should be added to the treatment regimen. The alkalinization products of nerve agents such as soman are shown to be less toxic and hence, the IV infusion of sodium bicarbonate may even be more beneficial in nerve agents poisoning.
Magnesium Sulphate
Intravenous magnesium sulfate (4 g) given in the first day after admission have been shown to decrease hospitalization period and improve outcomes in patients with OP poisoning.117 Magnesium sulfate blocks calcium channels and thus reduce acetylcholine release. It also reduced CNS overstimulation resulting from NMDA receptor activation and reversed the neuroelectrophysiological defects.118
Adrenergic Agonists
The alpha2-adrenergic receptor agonistssuch as clonidine can reduce acetylcholine synthesis and release in presynaptic junctions. Although clonidine has been used successfully in animal models, the therapeutic effects of alpha2-adrenergic receptor agonists on human are not fully known.119
Antioxidants
Induction of reactive oxygen radicals and their contributors such as decreased total antioxidant capacity, and increased thiobarbituric reactive substances and lipid peroxidation occur in OP poisoning either as acute, subchronic or chronic exposure.120 Thus, antioxidants treatment may be beneficial in these patients. In a study on rats, vitamin E was reported to have therapeutic effects in dimethoate and malathion- induced oxidative stress in rat erythrocytes.121
New Treatments
Removal of OPs from blood using haemodialysis, haemoperfusion or haemofiltration is not clear. In a recent report, it was claimed that haemofiltration after dichlorvos poisoning had revealed beneficial therapeutic effects.122 Bio-scavengers such as fresh frozen plasma (FFP) or albumin has been recently suggested as a useful therapy through clearing of free organophosphates. In a non randomized controlled study of 12 patients and 21 control, authors found that FFP therapy may increase BuChE levels in OP poisoned patients and may prevent the development of intermediate syndrome and mortality rate.123 In another study, despite significant increase in BuChE concentrations with FFP, authors did not find considerable benefit following treatment with FFP or albumin.124
Some nerve agents such as tabun and cyclosarin are resistant to treatment. In a study, authors reported that modified cyclodextrin detoxified different nerve agents including the phosphoramidate tabun. More potent oximes have been proposed to be produced using 3D structure of the complexes between current oximes and OP-AChE conjugates based on molecular modeling.125
In a recent study, brain cell therapy and neuronal regeneration was employed as a valuable method for delayed treatment against OP intoxication. Results from soman-poisoned mice demonstrated that cytokine treatment induced migration and engrafting of stem cells in injured brain tissue that led to differentiation into functional neurons.126 However, this method does not ameliorate memory performance in soman-poisoned mice.79 Cytokine treatment has also enhanced neuronal regeneration in the hippocampus.127 More studies in this area are necessary to identify the potential role of gene and cell therapies in OP poisoning.
The safe short-induction vectors, and recombinant bacterial phosphotriesterases and hydrolases that are able to transfer OP-degrading enzymes are very promising in delayed treatment of OP poisoning.128 They exert their protective actions via break down of OP pesticides. Genomics and proteomics research has targeted newer therapeutic modalities by increasing our knowledge in the toxicity of OP compounds.129 Recently, encapsulation of drugs or enzymes in nanocarriers has been proposed to enhance the BBB crossing.130 It is thus hoped that more effective treatment will soon be available for severe neurotoxic effects of human OP pesticides and the nerve agents poisonings.
Advanced Neuroprotective Drugs
Delayed medical management of convulsion and neuroprotection in OP poisoning needs newer agents since BZD are shown to have few therapeutic effects after the onset of seizure.131 Neuroprotection can be implemented via anticholinergic and antiglutamatergic agents, since the CNS toxic effects results from increased excitory release of glutamate. New pharmacological agents such as huperzine A, which is a reversible ChE inhibitor with imidazenil, 1 is a GABA A receptor modulator and have shown beneficial in animal studies.132 Huperzine A has also revealed useful effects in post-exposure treatment to prevent seizures and status epilepticus by blocking NMDA-induced excitation toxicity.133 Anti-muscarinic drugs that show antiglutamatergic properties such as aprophen, benactizyne and caramiphen in adjunction with oximes and atropine may protect nervous injury.134
Ketamine, a noncompetitive NMDA receptor antagonist, can be used until one hour following nerve agent-induced seizures specially, when administered in combination with midazolam or diazepam.135 In a recent study, Tezampanel, another glutamate receptor antagonist, which is specific for kainate sub-type receptors, was reported to be useful against soman-induced seizures and neuropathy in patients exposed to nerve agents.136
For intermediate syndrome, which is resistant to the standard treatment, supportive therapy and consideration of artificial respiration are recommended. For OPIDN, standard therapy should be accompanied with neuroprotective drugs like corticosteroids. Protease inhibitors have been useful in protecting the NTE and preventing the establishment of delayed neuropathy.131 However, further studies are required both experimentally and clinically to find out effective treatments for severe OP poisonings.
Prevention
Prevention of OP poisonings is vital, and should be implemented in developing countries where OP pesticides are readily available and may be used for self-poisoning.1-4 Prevention of nerve agents poisonings need different strategies, as they are used for chemical war and terrorist attacks. Organization for prohibition of chemical weapons (OPCW), which was established in 1997 in the Hague, the Netherlands has been very effective so far as an international organization on implementation of chemical weapons convention. It is hoped that no more chemical war or terrorism by the nerve agents will occur in the future.
Conclusions and Recommendations
Organophosphorous compounds, either as pesticides or nerve agents, have caused a considerable morbidity and mortality in the recent decades in some countries including Iran, Sri Lanka and Japan. Organophosphorous pesticides are still available in most developing countries, and may cause occupational, accidental, and intentional poisonings. Recent investigations have revealed more understanding on the basic principles of treatment, and more new medications are now available for the management of OP poisonings. However, further studies are required to find out more effective treatments for the severe OP poisonings.
Appropriate legislations and pesticides control, particularly OPs, which are the most commonly-used pesticides, are recommended for the developing countries, especially those with poor regulations and controls. It is hoped that OPCW continue to prevent chemical war and terrorism using the nerve agents in the future as well.
Conflict of Interest: None declared
References
- 1.Afshari R, Majdzadeh R, Balali-Mood M. Pattern of acute poisonings in Mashhad, Iran 1993-2000. J Toxicol Clin Toxicol. 2004;42:965–75. doi: 10.1081/clt-200042550. PubMed PMID: 15641642. [DOI] [PubMed] [Google Scholar]
- 2.Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiol Paris. 1998;92:375–8. doi: 10.1016/S0928-4257(99)80008-4. PubMed PMID: 9789840. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, Gunnell D, Karunaratne A, de SilvaD, Sheriff MH, Buckley NA. Epidemiology of intentional self-poisoning in rural Sri Lanka. Br J Psychiatry. 2005;187:583–4. doi: 10.1192/bjp.187.6.583. doi: 10.1192/bjp.187.6.583. PubMed PMID: 16319413; PubMed Central PMCID: PMC1475924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. doi: 10.1093/qjmed/93.11.715. PubMed PMID: 11077028. [DOI] [PubMed] [Google Scholar]
- 5.Pohanka M, Musilek K, Kuca K. Progress of biosensors based on cholinesterase inhibition. Curr Med Chem. 2009;16:1790–8. doi: 10.2174/092986709788186129. doi: 10.2174/092986709788186129. PubMed PMID: 19442145. [DOI] [PubMed] [Google Scholar]
- 6.Newmark J. Nerve agents. Neurologist. 2007;13:20–32. doi: 10.1097/01.nrl.0000252923.04894.53. doi: 10.1097/01.nrl. 0000252923.04894.53. PubMed PMID: 17215724. [DOI] [PubMed] [Google Scholar]
- 7.Newmark J. Nerve agents: pathophysiology and treatment of poisoning. Semin Neurol. 2004;24:185–96. doi: 10.1055/s-2004-830906. doi: 10.1055/s-2004-830906. PubMed PMID: 15257516. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson CH, Lehmann CR, Sidell FR, Jabbari B. Nerve agents: a review. Neurology. 1992;42:946–50. doi: 10.1212/wnl.42.5.946. PubMed PMID: 1315942. [DOI] [PubMed] [Google Scholar]
- 9.Vale A. What lessons can we learn from the Japanese sarin attacks? Przegl Lek. 2005;62:528–32. PubMed PMID: 16225116. [PubMed] [Google Scholar]
- 10.Okumura T, Takasu N, Ishimatsu S, Miyanoki S, Mitsuhashi A, Kumada K, et al. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28:129–35. doi: 10.1016/s0196-0644(96)70052-5. doi: 10.1016/S0196-0644(96)70052-5. PubMed PMID: 8759575. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. doi: 10.1016/j.jns.2006.06.007. PubMed PMID: 16962140. [DOI] [PubMed] [Google Scholar]
- 12.Jeyaratnam J, Maroni M. Organophosphorous compounds. Toxicology. 1994;91:15–27. doi: 10.1016/0300-483x(94)90236-4. doi: 10.1016/0300-483X(94)90236-4. PubMed PMID: 8052981. [DOI] [PubMed] [Google Scholar]
- 13.Newmark J. Nerve agents. Neurol Clin. 2005;23:623–41. doi: 10.1016/j.ncl.2004.12.013. doi: 10.1016/j.ncl. 2004.12.013. PubMed PMID: 15757800. [DOI] [PubMed] [Google Scholar]
- 14.Leikin JB, Thomas RG, Walter FG, Klein R, Meislin HW. A review of nerve agent exposure for the critical care physician. Crit Care Med. 2002;30:2346–54. doi: 10.1097/00003246-200210000-00026. doi: 10.1097/00003246-200210000-00026. PubMed PMID: 12394966. [DOI] [PubMed] [Google Scholar]
- 15.Chemical casualties. Nerve agents. J R Army Med Corps. 2002;148:344–57. PubMed PMID: 12703422. [PubMed] [Google Scholar]
- 16.Worek F, Aurbek N, Wetherell J, Pearce P, Mann T, Thiermann H. Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: pig versus minipig acetylcholinesterase. Toxicology. 2008;244:35–41. doi: 10.1016/j.tox.2007.10.021. doi: 10.1016/j.tox.2007.10.021. PubMed PMID: 18054823. [DOI] [PubMed] [Google Scholar]
- 17.Jokanović M. Biotransformation of organophosphorus compounds. Toxicology. 2001;166:139–60. doi: 10.1016/s0300-483x(01)00463-2. doi: 10.1016/S0300-483X(01)00463-2. PubMed PMID: 11543910. [DOI] [PubMed] [Google Scholar]
- 18.Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107–15. doi: 10.1016/j.toxlet.2009.07.025. doi: 10.1016/j.toxlet.2009.07.025. PubMed PMID: 19651196. [DOI] [PubMed] [Google Scholar]
- 19.Hulet SW, McDonough JH, Shih TM. The dose-response effects of repeated subacute sarin exposure on guinea pigs. Pharmacol Biochem Behav. 2002;72:835–45. doi: 10.1016/s0091-3057(02)00761-x. doi: 10.1016/S0091-3057(02)00761-X. PubMed PMID: 12062573. [DOI] [PubMed] [Google Scholar]
- 20.Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett. 2002;128:215–28. doi: 10.1016/s0378-4274(01)00543-4. doi: 10.1016/S0378-4274(01)00543-4. PubMed PMID: 11869832. [DOI] [PubMed] [Google Scholar]
- 21.Mumford H, Troyer JK. Post-exposure therapy with recombinant human BuChE following percutaneous VX challenge in guinea-pigs. Toxicol Lett. 2011;206:29–34. doi: 10.1016/j.toxlet.2011.05.1016. doi: 10.1016/j.toxlet.2011.05.1016. PubMed PMID: 21620937; PubMed Central PMCID: PMC3159811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jokanović M. Current understanding of the mechanisms involved in metabolic detoxification of warfare nerve agents. Toxicol Lett. 2009;188:1–10. doi: 10.1016/j.toxlet.2009.03.017. doi: 10.1016/j.toxlet.2009.03.017. PubMed PMID: 19433263. [DOI] [PubMed] [Google Scholar]
- 23.Worek F, Eyer P, Aurbek N, Szinicz L, Thiermann H. Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis. Toxicol Appl Pharmacol. 2007;219:226–34. doi: 10.1016/j.taap.2006.10.001. doi: 10.1016/j.taap.2006.10.001. PubMed PMID: 17112559. [DOI] [PubMed] [Google Scholar]
- 24.Worek F, Koller M, Thiermann H, Szinicz L. Diagnostic aspects of organophosphate poisoning. Toxicology. 2005;214:182–9. doi: 10.1016/j.tox.2005.06.012. doi: 10.1016/j.tox.2005.06.012. PubMed PMID: 16051411. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima T, Sasaki K, Ozawa H, Sekjima Y, Morita H, Fukushima Y, et al. Urinary metabolites of sarin in a patient of the Matsumoto sarin incident. Arch Toxicol. 1998;72:601–3. doi: 10.1007/s002040050549. doi: 10.1007/s002040050549. PubMed PMID: 9806433. [DOI] [PubMed] [Google Scholar]
- 26.Jokanović M, Kosanović M, Maksimović M. Interaction of organophosphorus compounds with carboxylesterases in the rat. Arch Toxicol. 1996;70:444–50. doi: 10.1007/s002040050297. doi: 10.1007/s002040050297. PubMed PMID: 8740539. [DOI] [PubMed] [Google Scholar]
- 27.Black RM. An overview of biological markers of exposure to chemical warfare agents. J Anal Toxicol. 2008;32:2–9. doi: 10.1093/jat/32.1.2. PubMed PMID: 18269786. [DOI] [PubMed] [Google Scholar]
- 28.Lotti M, Moretto A. Cholinergic symptoms and Gulf War syndrome. Nat Med. 1995;1:1225–6. doi: 10.1038/nm1295-1225b. doi: 10.1038/nm1295-1225b. PubMed PMID: 7489390. [DOI] [PubMed] [Google Scholar]
- 29.Black RM. History and perspectives of bioanalytical methods for chemical warfare agent detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1207–15. doi: 10.1016/j.jchromb.2009.11.025. doi: 10.1016/j.jchromb.2009.11.025. PubMed PMID: 20018570. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Park JY, Min K, Cha HJ, Choi SS, Yoo YJ. A novel organophosphorus hydrolase-based biosensor using mesoporous carbons and carbon black for the detection of organophosphate nerve agents. Biosens Bioelectron. 2010;25:1566–70. doi: 10.1016/j.bios.2009.10.013. doi: 10.1016/j.bios.2009.10.013. PubMed PMID: 20093002. [DOI] [PubMed] [Google Scholar]
- 31.Mawhinney DB, Hamelin EI, Fraser R, Silva SS, Pavlopoulos AJ, Kobelski RJ. The determination of organophosphonate nerve agent metabolites in human urine by hydrophilic interaction liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:235–43. doi: 10.1016/j.jchromb.2007.01.023. doi: 10.1016/j.jchromb.2007.01.023. PubMed PMID: 17289448. [DOI] [PubMed] [Google Scholar]
- 32.Swaim LL, Johnson RC, Zhou Y, Sandlin C, Barr JR. Quantification of organophosphorus nerve agent metabolites using a reduced-volume, high-throughput sample processing format and liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2008;32:774–7. doi: 10.1093/jat/32.9.774. PubMed PMID: 19021934. [DOI] [PubMed] [Google Scholar]
- 33.van derMeerJA, Trap HC, Noort D, van derSchansMJ. Comprehensive gas chromatography with Time of Flight MS and large volume introduction for the detection of fluoride-induced regenerated nerve agent in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1320–5. doi: 10.1016/j.jchromb.2010.02.019. PubMed PMID: 20308021. [DOI] [PubMed] [Google Scholar]
- 34.Polhuijs M, Langenberg JP, Benschop HP. New method for retrospective detection of exposure to organophosphorus anticholinesterases: application to alleged sarin victims of Japanese terrorists. Toxicol Appl Pharmacol. 1997;146:156–61. doi: 10.1006/taap.1997.8243. doi: 10.1006/taap.1997.8243. PubMed PMID: 9299607. [DOI] [PubMed] [Google Scholar]
- 35.Fidder A, Hulst AG, Noort D, de RuiterR, van derSchansMJ, Benschop HP, et al. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–90. doi: 10.1021/tx0101806. doi: 10.1021/tx0101806. PubMed PMID: 11952345. [DOI] [PubMed] [Google Scholar]
- 36.Read RW, Riches JR, Stevens JA, Stubbs SJ, Black RM. Biomarkers of organophosphorus nerve agent exposure: comparison of phosphylated butyrylcholinesterase and phosphylated albumin after oxime therapy. Arch Toxicol. 2010;84:25–36. doi: 10.1007/s00204-009-0473-4. doi: 10.1007/s00204-009-0473-4. PubMed PMID: 19862504. [DOI] [PubMed] [Google Scholar]
- 37.Schier JG, Ravikumar PR, Nelson LS, Heller MB, Howland MA, Hoffman RS. Preparing for chemical terrorism: stability of injectable atropine sulfate. Acad Emerg Med. 2004;11:329–34. doi: 10.1111/j.1553-2712.2004.tb01447.x. doi: 10.1197/j.aem.2003.06.014. PubMed PMID: 15064203. [DOI] [PubMed] [Google Scholar]
- 38.Anzueto A, Berdine GG, Moore GT, Gleiser C, Johnson D, White CD, et al. Pathophysiology of soman intoxication in primates. Toxicol Appl Pharmacol. 1986;86:56–68. doi: 10.1016/0041-008x(86)90399-6. doi: 10.1016/0041-008X(86)90399-6. PubMed PMID: 3764936. [DOI] [PubMed] [Google Scholar]
- 39.Huang YJ, Lundy PM, Lazaris A, Huang Y, Baldassarre H, Wang B, et al. Substantially improved pharmacokinetics of recombinant human butyrylcholinesterase by fusion to human serum albumin. BMC Biotechnol. 2008;8:50. doi: 10.1186/1472-6750-8-50. doi: 10.1186/1472-6750-8-50. PubMed PMID: 18485214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams NH, Harrison JM, Read RW, Black RM. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch Toxicol. 2007;81:627–39. doi: 10.1007/s00204-007-0191-8. doi: 10.1007/s00204-007-0191-8. PubMed PMID: 17345062. [DOI] [PubMed] [Google Scholar]
- 41.Satoh T, Hosokawa M. Organophosphates and their impact on the global environment. Neurotoxicology. 2000;21:223–7. PubMed PMID: 10794404. [PubMed] [Google Scholar]
- 42.Balali-Mood M, Balali-Mood K. Neurotoxic disorders of organophosphorus compounds and their managements. Arch Iran Med. 2008;11:65–89. PubMed PMID: 18154426. [PubMed] [Google Scholar]
- 43.Karalliedde L. Organophosphorus poisoning and anaesthesia. Anaesthesia. 1999;54:1073–88. doi: 10.1046/j.1365-2044.1999.01061.x. doi: 10.1046/j.1365-2044.1999.01061.x. PubMed PMID: 10540097. [DOI] [PubMed] [Google Scholar]
- 44.Tokuda Y, Kikuchi M, Takahashi O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation. 2006;68:193–202. doi: 10.1016/j.resuscitation.2005.05.023. doi: 10.1016/j.resuscitation.2005.05.023. PubMed PMID: 16325985. [DOI] [PubMed] [Google Scholar]
- 45.GROB D. The manifestations and treatment of poisoning due to nerve gas and other organic phosphate anticholinesterase compounds. AMA Arch Intern Med. 1956;98:221–39. doi: 10.1001/archinte.1956.00250260095010. doi: 10.1001/archinte.1956.00250260095010. PubMed PMID: 13354016. [DOI] [PubMed] [Google Scholar]
- 46.Jamal GA. Neurological syndromes of organophosphorus compounds. Adverse Drug React Toxicol Rev. 1997;16:133–70. PubMed PMID: 9512762. [PubMed] [Google Scholar]
- 47.Rastogi SK, Tripathi S, Ravishanker D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J Occup Environ Med. 2010;14:54–7. doi: 10.4103/0019-5278.72242. doi: 10.4103/0019-5278.72242. PubMed PMID: 21120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinbroum AA. Pathophysiological and clinical aspects of combat anticholinesterase poisoning. Br Med Bull. 2005;72:119–33. doi: 10.1093/bmb/ldh038. doi: 10.1093/bmb/ldh038. PubMed PMID: 15845747. [DOI] [PubMed] [Google Scholar]
- 49.Sidell FR. Soman and sarin: clinical manifestations and treatment of accidental poisoning by organophosphates. Clin Toxicol. 1974;7:1–17. doi: 10.3109/15563657408987971. doi: 10.3109/15563657408987971. PubMed PMID: 4838227. [DOI] [PubMed] [Google Scholar]
- 50.Paudyal BP. Organophosphorus poisoning. JNMA J Nepal Med Assoc. 2008;47:251–8. PubMed PMID: 19079407. [PubMed] [Google Scholar]
- 51.Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicology. 1986;7:225–36. PubMed PMID: 3714123. [PubMed] [Google Scholar]
- 52.Worek F, Kleine A, Falke K, Szinicz L. Arrhythmias in organophosphate poisoning: effect of atropine and bispyridinium oximes. Arch Int Pharmacodyn Ther. 1995;329:418–35. PubMed PMID: 8546540. [PubMed] [Google Scholar]
- 53.Chacko J, Elangovan A. Late onset, prolonged asystole following organophosphate poisoning: a case report. J Med Toxicol. 2010;6:311–4. doi: 10.1007/s13181-010-0095-5. doi: 10.1007/s13181-010-0095-5. PubMed PMID: 20532843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perkins MW, Pierre Z, Rezk P, Sabnekar P, Kabra K, Chanda S, et al. Acute respiratory toxicity following inhalation exposure to soman in guinea pigs. Toxicol Appl Pharmacol. 2010;245:171–8. doi: 10.1016/j.taap.2010.02.016. doi: 10.1016/j.taap.2010.02.016. PubMed PMID: 20206646. [DOI] [PubMed] [Google Scholar]
- 55.Vijayakumar S, Fareedullah M, Ashok KumarE, Mohan RaoK. A prospective study on electrocardiographic findings of patients with organophosphorus poisoning. Cardiovasc Toxicol. 2011;11:113–7. doi: 10.1007/s12012-011-9104-4. doi: 10.1007/s12012-011-9104-4. doi: 10.1007/s12012-011-9104-4. PubMed PMID: 21336997. [DOI] [PubMed] [Google Scholar]
- 56.Shadnia S, Okazi A, Akhlaghi N, Sasanian G, Abdollahi M. Prognostic value of long QT interval in acute and severe organophosphate poisoning. J Med Toxicol. 2009;5:196–9. doi: 10.1007/BF03178266. doi: 10.1007/BF03178266. PubMed PMID: 19876851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yurumez Y, Yavuz Y, Saglam H, Durukan P, Ozkan S, Akdur O, et al. Electrocardiographic findings of acute organophosphate poisoning. J Emerg Med. 2009;36:39–42. doi: 10.1016/j.jemermed.2007.08.063. doi: 10.1016/j.jemermed.2007.08.063. PubMed PMID: 18296005. [DOI] [PubMed] [Google Scholar]
- 58.Singer AW, Jaax NK, Graham JS, McLeod CGJr. Cardiomyopathy in Soman and Sarin intoxicated rats. Toxicol Lett. 1987;36:243–9. doi: 10.1016/0378-4274(87)90192-5. doi: 10.1016/0378-4274(87)90192-5. PubMed PMID: 3590220. [DOI] [PubMed] [Google Scholar]
- 59.Guadarrama-Naveda M, de CabreraLC, Matos-Bastidas S. Intermediate syndrome secondary to ingestion of chlorpiriphos. Vet Hum Toxicol. 2001;43 PubMed PMID: 11205075. [PubMed] [Google Scholar]
- 60.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc. 2007;70:467–72. doi: 10.1016/S1726-4901(08)70043-1. doi: 10.1016/S1726-4901(08)70043-1. PubMed PMID: 18063499. [DOI] [PubMed] [Google Scholar]
- 61.Jokanović M, Kosanović M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ Toxicol Pharmacol. 2010;29:195–201. doi: 10.1016/j.etap.2010.01.006. doi: 10.1016/j.etap.2010.01.006. PubMed PMID: 21787602. [DOI] [PubMed] [Google Scholar]
- 62.De BleeckerJL. Intermediate Syndrome in Organophosphate Poisoning. In: Ramesh CG, editor. Toxicology of Organophosphate & Carbamate Compounds. Burlington: Academic Press; 2006. p. 371. Burlington: Academic Press; 2006. pp. 371–80. [Google Scholar]
- 63.De BleeckerJ, Vogelaers D, Ceuterick C, Van DenNeuckerK, Willems J, De ReuckJ. Intermediate syndrome due to prolonged parathion poisoning. Acta Neurol Scand. 1992;86:421–4. doi: 10.1111/j.1600-0404.1992.tb05110.x. PubMed PMID: 1455989. [DOI] [PubMed] [Google Scholar]
- 64.McLeod CGJr, Singer AW, Harrington DG. Acute neuropathology in soman poisoned rats. Neurotoxicology. 1984;5:53–7. PubMed PMID: 6542190. [PubMed] [Google Scholar]
- 65.Emerick GL, Peccinini RG, de OliveiraGH. Organophosphorus-induced delayed neuropathy: a simple and efficient therapeutic strategy. Toxicol Lett. 2010;192:238–44. doi: 10.1016/j.toxlet.2009.10.032. doi: 10.1016/j.toxlet.2009.10.032. PubMed PMID: 19914363. [DOI] [PubMed] [Google Scholar]
- 66.Johnson MK. The mechanism of delayed neuropathy caused by some organophosphorus esters: using the understanding to improve safety. J Environ Sci Health B. 1980;15:823–41. doi: 10.1080/03601238009372219. doi: 10.1080/03601238009372219. PubMed PMID: 7002990. [DOI] [PubMed] [Google Scholar]
- 67.Tan DH, Peng SQ, Wu YL, Wang YM, Lu CF, Yan CH. Chronic organophosphate (OP)-induced neuropsychiatric disorder is a withdrawal syndrome. Med Hypotheses. 2009;72:405–6. doi: 10.1016/j.mehy.2008.11.026. doi: 10.1016/j.mehy.2008.11.026. PubMed PMID: 19131175. [DOI] [PubMed] [Google Scholar]
- 68.Jalali N, Balali-Mood M, Jalali I, Shakeri MT. Electrophysiological changes in patients with acute organophosphorous pesticide poisoning. Basic Clin Pharmacol Toxicol. 2011;108:251–5. doi: 10.1111/j.1742-7843.2010.00652.x. doi: 10.1111/j.1742-7843.2010.00652.x. PubMed PMID: 21156031. [DOI] [PubMed] [Google Scholar]
- 69.Fullerton CS, Ursano RJ. Behavioral and psychological responses to chemical and biological warfare. Mil Med. 1990;155:54–9. PubMed PMID: 2106650. [PubMed] [Google Scholar]
- 70.Lallement G. [Overview on neurogenesis induced by organophosphate poisoning: results and perspectives]. Ann Pharm Fr. 2007;65:415–21. doi: 10.1016/s0003-4509(07)74201-3. PubMed PMID: 18079674. [DOI] [PubMed] [Google Scholar]
- 71.Riddle JR, Brown M, Smith T, Ritchie EC, Brix KA, Romano J. Chemical warfare and the Gulf War: a review of the impact on Gulf veterans’ health. Mil Med. 2003;168:606–13. PubMed PMID: 12943034. [PubMed] [Google Scholar]
- 72.Masson P. Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicol Lett. 2011;206:5–13. doi: 10.1016/j.toxlet.2011.04.006. doi: 10.1016/j.toxlet.2011.04.006. PubMed PMID: 21524695. [DOI] [PubMed] [Google Scholar]
- 73.Tang FR, Loke WK, Ling EA. Comparison of status epilepticus models induced by pilocarpine and nerve agents - a systematic review of the underlying aetiology and adopted therapeutic approaches. Curr Med Chem. 2011;18:886–99. doi: 10.2174/092986711794927720. PubMed PMID: 21182477. [DOI] [PubMed] [Google Scholar]
- 74.Angoa-Pérez M, Kreipke CW, Thomas DM, Van ShuraKE, Lyman M, McDonough JH, et al. Soman increases neuronal COX-2 levels: possible link between seizures and protracted neuronal damage. Neurotoxicology. 2010;31:738–46. doi: 10.1016/j.neuro.2010.06.007. doi: 10.1016/j.neuro.2010.06.007. PubMed PMID: 20600289; PubMed Central PMCID: PMC2974036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. doi: 10.1016/S0041-008X(03)00019-X. PubMed PMID: 12691725. [DOI] [PubMed] [Google Scholar]
- 76.Sharabi Y, Danon YL, Berkenstadt H, Almog S, Mimouni-Bloch A, Zisman A, et al. Survey of symptoms following intake of pyridostigmine during the Persian Gulf war. Isr J Med Sci. 1991;27:656–8. PubMed PMID: 1757241. [PubMed] [Google Scholar]
- 77.Koskela H, Hakala U, Vanninen P. Structural characterization of chemical warfare agent degradation products in decontamination solutions with proton band-selective (1)H-(31)P NMR spectroscopy. Anal Chem. 2010;82:5331–40. doi: 10.1021/ac100867x. PubMed PMID: 20507069. [DOI] [PubMed] [Google Scholar]
- 78.Amitai G, Murata H, Andersen JD, Koepsel RR, Russell AJ. Decontamination of chemical and biological warfare agents with a single multi-functional material. Biomaterials. 2010;31:4417–25. doi: 10.1016/j.biomaterials.2010.02.004. doi: 10.1016/j.biomaterials.2010.02.004. PubMed PMID: 20199807. [DOI] [PubMed] [Google Scholar]
- 79.Collombet JM. Nerve agent intoxication: recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicol Appl Pharmacol. 2011;255:229–41. doi: 10.1016/j.taap.2011.07.003. doi: 10.1016/j.taap.2011.07.003. PubMed PMID: 21791221. [DOI] [PubMed] [Google Scholar]
- 80.Kanugula AK, Repalle ER, Pandey JP, Sripad G, Mitra CK, Dubey DK, et al. Immobilization of organophosphate hydrolase on biocompatible gelatin pads and its use in removal of organophosphate compounds and nerve agents. Indian J Biochem Biophys. 2011;48:29–34. PubMed PMID: 21469599. [PubMed] [Google Scholar]
- 81.Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 2007;233:31–9. doi: 10.1016/j.tox.2006.11.066. doi: 10.1016/j.tox.2006.11.066. PubMed PMID: 17188793. [DOI] [PubMed] [Google Scholar]
- 82.Raveh L, Grunwald J, Marcus D, Papier Y, Cohen E, Ashani Y. Human butyrylcholinesterase as a general prophylactic antidote for nerve agent toxicity. In vitro and in vivo quantitative characterization. Biochem Pharmacol. 1993;45:2465–74. doi: 10.1016/0006-2952(93)90228-o. PubMed PMID: 8328984. [DOI] [PubMed] [Google Scholar]
- 83.Caranto GR, Waibel KH, Asher JM, Larrison RW, Brecht KM, Schutz MB, et al. Amplification of the effectiveness of acetylcholinesterase for detoxification of organophosphorus compounds by bis-quaternary oximes. Biochem Pharmacol. 1994;47:347–57. doi: 10.1016/0006-2952(94)90026-4. doi: 10.1016/0006-2952(94)90026-4. PubMed PMID: 8304979. [DOI] [PubMed] [Google Scholar]
- 84.Saxena A, Maxwell DM, Quinn DM, Radić Z, Taylor P, Doctor BP. Mutant acetylcholinesterases as potential detoxification agents for organophosphate poisoning. Biochem Pharmacol. 1997;54:269–74. doi: 10.1016/s0006-2952(97)00180-9. doi: 10.1016/S0006-2952(97)00180-9. PubMed PMID: 9271331. [DOI] [PubMed] [Google Scholar]
- 85.Mizrahi DM, Saphier S, Columbus I. Efficient heterogeneous and environmentally friendly degradation of nerve agents on a tungsten-based POM. J Hazard Mater. 2010;179:495–9. doi: 10.1016/j.jhazmat.2010.03.030. doi: 10.1016/j.jhazmat.2010.03.030. PubMed PMID: 20363072. [DOI] [PubMed] [Google Scholar]
- 86.Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, et al. Immobilization of Russian VX skin depots by localized cooling: implications for decontamination and medical countermeasures. Toxicol Lett. 2011;206:47–53. doi: 10.1016/j.toxlet.2011.05.1047. doi: 10.1016/j.toxlet.2011.05.1047. PubMed PMID: 21704135. [DOI] [PubMed] [Google Scholar]
- 87.Sawyer TW, Mikler J, Worek F, Reiter G, Thiermann H, Tenn C, et al. The therapeutic use of localized cooling in the treatment of VX poisoning. Toxicol Lett. 2011;204:52–6. doi: 10.1016/j.toxlet.2011.04.008. doi: 10.1016/j.toxlet.2011.04.008. PubMed PMID: 21530621. [DOI] [PubMed] [Google Scholar]
- 88.Blain PG. Organophosphorus poisoning (acute) Clin Evid . 2011;17 pii: 2102. PubMed PMID: 21575287. [PMC free article] [PubMed] [Google Scholar]
- 89. [Cases of poisoning from organophosphoric insecticides and verification method for their exposure]. Chudoku Kenkyu. 2008;21:133–40. PubMed PMID: 18516937. [PubMed] [Google Scholar]
- 90.McDonough JHJr, McLeod CGJr, Nipwoda MT. Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res. 1987;435:123–37. doi: 10.1016/0006-8993(87)91593-9. doi: 10.1016/0006-8993(87)91593-9. PubMed PMID: 3427447. [DOI] [PubMed] [Google Scholar]
- 91.Antonijevic B, Stojiljkovic MP. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin Med Res. 2007;5:71–82. doi: 10.3121/cmr.2007.701. doi: 10.3121/cmr.2007.701. PubMed PMID: 17456837; PubMed Central PMCID: PMC1855336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zilker T. Medical management of incidents with chemical warfare agents. Toxicology. 2005;214:221–31. doi: 10.1016/j.tox.2005.06.028. doi: 10.1016/j.tox.2005.06.028. PubMed PMID: 16098652. [DOI] [PubMed] [Google Scholar]
- 93.Shih TM, Rowland TC, McDonough JH. Anticonvulsants for nerve agent-induced seizures: The influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007;320:154–61. doi: 10.1124/jpet.106.111252. doi: 10.1124/jpet.106.111252. PubMed PMID: 17015638. [DOI] [PubMed] [Google Scholar]
- 94.Ohbu S, Yamashina A, Takasu N, Yamaguchi T, Murai T, Nakano K, et al. Sarin poisoning on Tokyo subway. South Med J. 1997;90:587–93. doi: 10.1097/00007611-199706000-00002. doi: 10.1097/00007611-199706000-00002. PubMed PMID: 9191733. [DOI] [PubMed] [Google Scholar]
- 95.Shockley LW. The use of inhaled nebulized atropine for the treatment of malathion poisoning. J Toxicol Clin Toxicol. 1989;27:183–92. doi: 10.3109/15563658909038582. doi: 10.3109/15563658909038582. PubMed PMID: 2810443. [DOI] [PubMed] [Google Scholar]
- 96.Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–90. doi: 10.2165/00139709-200322030-00004. doi: 10.2165/00139709-200322030-00004. PubMed PMID: 15181665. [DOI] [PubMed] [Google Scholar]
- 97.Dunn MA, Sidell FR. Progress in medical defense against nerve agents. JAMA. 1989;262:649–52. doi: 10.1001/jama.262.5.649. PubMed PMID: 2664236. [PubMed] [Google Scholar]
- 98.Ashani Y, Bhattacharjee AK, Leader H, Saxena A, Doctor BP. Inhibition of cholinesterases with cationic phosphonyl oximes highlights distinctive properties of the charged pyridine groups of quaternary oxime reactivators. Biochem Pharmacol. 2003;66:191–202. doi: 10.1016/s0006-2952(03)00204-1. doi: 10.1016/S0006-2952(03)00204-1. PubMed PMID: 12826262. [DOI] [PubMed] [Google Scholar]
- 99.Jokanović M, Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr Med Chem. 2009;16:2177–88. doi: 10.2174/092986709788612729. doi: 10.2174/092986709788612729. PubMed PMID: 19519385. [DOI] [PubMed] [Google Scholar]
- 100.Grange-Messent V, Bouchaud C, Jamme M, Lallement G, Foquin A, Carpentier P. Seizure-related opening of the blood-brain barrier produced by the anticholinesterase compound, soman: new ultrastructural observations. Cell Mol Biol (Noisy-le-grand) 1999;45:1–14. PubMed PMID: 10099835. [PubMed] [Google Scholar]
- 101.Joosen MJ, van derSchansMJ, van DijkCG, Kuijpers WC, Wortelboer HM, van HeldenHP. Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett. 2011;206:67–71. doi: 10.1016/j.toxlet.2011.05.231. doi: 10.1016/j.toxlet.2011.05.231. PubMed PMID: 21600273. [DOI] [PubMed] [Google Scholar]
- 102.Kalisiak J, Ralph EC, Zhang J, Cashman JR. Amidine-oximes: reactivators for organophosphate exposure. J Med Chem. 2011;54:3319–30. doi: 10.1021/jm200054r. doi: 10.1021/jm200054r. PubMed PMID: 21438612. [DOI] [PubMed] [Google Scholar]
- 103.Valiyaveettil M, Alamneh Y, Rezk P, Biggemann L, Perkins MW, Sciuto AM, et al. Protective efficacy of catalytic bioscavenger, paraoxonase 1 against sarin and soman exposure in guinea pigs. Biochem Pharmacol. 2011;81:800–9. doi: 10.1016/j.bcp.2010.12.024. doi: 10.1016/j.bcp.2010.12.024. PubMed PMID: 21219877. [DOI] [PubMed] [Google Scholar]
- 104.Kassa J, Karasova J, Musilek K, Kuca K, Bajgar J. An evaluation of reactivating and therapeutic efficacy of newly developed oximes (K206, K269) and commonly used oximes (obidoxime, HI-6) in cyclosarin-poisoned rats and mice. Clin Toxicol. 2009;47:72–6. doi: 10.1080/15563650802043652. doi: 10.1080/15563650802043652. PubMed PMID: 18686075. [DOI] [PubMed] [Google Scholar]
- 105.Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011;2:CD005085. doi: 10.1002/14651858.CD005085.pub2. PubMed PMID: 21328273. [DOI] [PubMed] [Google Scholar]
- 106.Kassa J, Kuca K, Cabal J. A comparison of the potency of trimedoxime and other currently available oximes to reactivate tabun-inhibited acetylcholinesterase and eliminate acute toxic effects of tabun. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:419–23. doi: 10.5507/bp.2005.072. PubMed PMID: 16601802. [DOI] [PubMed] [Google Scholar]
- 107.Shahroukhi A, Ghasemi A, Poorabdolhossein F, Asgari A, Khoshbaten A. The effect of paraoxon on GABA uptake in rat cerebellar synaptosomes. Med Sci Monit. 2007;13:194–9. PubMed PMID: 17767114. [PubMed] [Google Scholar]
- 108.Lewin L, Mattsson MO, Sellström A. Inhibition of transporter mediated gamma-aminobutyric acid (GABA) release by SKF 89976-A, a GABA uptake inhibitor, studied in a primary neuronal culture from chicken. Neurochem Res. 1992;17:577–84. doi: 10.1007/BF00968786. PubMed PMID: 1603264. [DOI] [PubMed] [Google Scholar]
- 109.Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006;249:86–94. doi: 10.1016/j.jns.2006.06.008. doi: 10.1016/j.jns.2006.06.008. PubMed PMID: 16945386. [DOI] [PubMed] [Google Scholar]
- 110.Marrs TC. The role of diazepam in the treatment of nerve agent poisoning in a civilian population. Toxicol Rev. 2004;23:145–57. doi: 10.2165/00139709-200423030-00002. doi: 10.2165/00139709-200423030-00002. PubMed PMID: 15862082. [DOI] [PubMed] [Google Scholar]
- 111.Johnson MK, Vale JA, Marrs TC, Meredith TJ. Pralidoxime for organophosphorus poisoning. Lancet. 1992;340:64. doi: 10.1016/0140-6736(92)92487-z. doi: 10.1016/0140-6736(92)92487-Z. PubMed PMID: 1351653. [DOI] [PubMed] [Google Scholar]
- 112.Kassa J. [Effect of diazepam on the effectiveness of antidote therapy in eliminating the acute lethal effects of soman in mice]. Cas Lek Cesk. 2001;140:497–9. PubMed PMID: 11678028. [PubMed] [Google Scholar]
- 113.Capacio BR, Byers CE, Merk KA, Smith JR, McDonough JH. Pharmacokinetic studies of intramuscular midazolam in guinea pigs challenged with soman. Drug Chem Toxicol. 2004;27:95–110. doi: 10.1081/dct-120030727. doi: 10.1081/DCT-120030727. PubMed PMID: 15198070. [DOI] [PubMed] [Google Scholar]
- 114.Gilat E, Kadar T, Levy A, Rabinovitz I, Cohen G, Kapon Y, et al. Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol Appl Pharmacol. 2005;209:74–85. doi: 10.1016/j.taap.2005.03.007. doi: 10.1016/j.taap.2005.03.007. PubMed PMID: 16271623. [DOI] [PubMed] [Google Scholar]
- 115.Lallement G, Clarençon D, Galonnier M, Baubichon D, Burckhart MF, Peoc’h M. Acute soman poisoning in primates neither pretreated nor receiving immediate therapy: value of gacyclidine (GK-11) in delayed medical support. Arch Toxicol. 1999;73:115–22. doi: 10.1007/s002040050595. doi: 10.1007/s002040050595. PubMed PMID: 10350192. [DOI] [PubMed] [Google Scholar]
- 116.Balali-Mood M, Ayati MH, Ali-Akbarian H. Effect of high doses of sodium bicarbonate in acute organophosphorous pesticide poisoning. Clin Toxicol (Phila) 2005;43:571–4. doi: 10.1081/clt-200068845. doi: 10.1081/CLT-200068845. PubMed PMID: 16255339. [DOI] [PubMed] [Google Scholar]
- 117.Pajoumand A, Shadnia S, Rezaie A, Abdi M, Abdollahi M. Benefits of magnesium sulfate in the management of acute human poisoning by organophosphorus insecticides. Hum Exp Toxicol. 2004;23:565–9. doi: 10.1191/0960327104ht489oa. doi: 10.1191/0960327104ht489oa. PubMed PMID: 15688984. [DOI] [PubMed] [Google Scholar]
- 118.Singh G, Avasthi G, Khurana D, Whig J, Mahajan R. Neurophysiological monitoring of pharmacological manipulation in acute organophosphate (OP) poisoning The effects of pralidoxime, magnesium sulphate and pancuronium. Electroencephalogr Clin Neurophysiol. 1998;107:140–8. doi: 10.1016/s0013-4694(98)00053-4. doi: 10.1016/S0013-4694(98)00053-4. PubMed PMID: 9751285. [DOI] [PubMed] [Google Scholar]
- 119.Liu WF. A symptomatological assessment of organophosphate-induced lethality in mice: comparison of atropine and clonidine protection. Toxicol Lett. 1991;56:19–32. doi: 10.1016/0378-4274(91)90086-l. PubMed PMID: 2017778. [DOI] [PubMed] [Google Scholar]
- 120.Ranjbar A, Solhi H, Mashayekhi FJ, Susanabdi A, Rezaie A, Abdollahi M. Oxidative stress in acute human poisoning with organophosphorus insecticides; a case control study. Environ Toxicol Pharmacol. 2005;20:88–91. doi: 10.1016/j.etap.2004.10.007. doi: 10.1016/j.etap.2004.10.007. PubMed PMID: 21783573. [DOI] [PubMed] [Google Scholar]
- 121.John S, Kale M, Rathore N, Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem. 2001;12:500–4. doi: 10.1016/s0955-2863(01)00160-7. doi: 10.1016/S0955-2863(01)00160-7. PubMed PMID: 11834209. [DOI] [PubMed] [Google Scholar]
- 122.Peng A, Meng FQ, Sun LF, Ji ZS, Li YH. Therapeutic efficacy of charcoal hemoperfusion in patients with acute severe dichlorvos poisoning. Acta Pharmacol Sin. 2004;25:15–21. PubMed PMID: 14704117. [PubMed] [Google Scholar]
- 123.Güven M, Sungur M, Eser B, Sari I, Altuntaş F. The effects of fresh frozen plasma on cholinesterase levels and outcomes in patients with organophosphate poisoning. J Toxicol Clin Toxicol. 2004;42:617–23. doi: 10.1081/clt-200026967. doi: 10.1081/CLT-200026967. PubMed PMID: 15462154. [DOI] [PubMed] [Google Scholar]
- 124.Pichamuthu K, Jerobin J, Nair A, John G, Kamalesh J, Thomas K, et al. Bioscavenger therapy for organophosphate poisoning - an open-labeled pilot randomized trial comparing fresh frozen plasma or albumin with saline in acute organophosphate poisoning in humans. Clin Toxicol. 2010;48:813–9. doi: 10.3109/15563650.2010.518970. doi: 10.3109/15563650.2010.518970. PubMed PMID: 20923392. [DOI] [PubMed] [Google Scholar]
- 125.Masson P. Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicol Lett. 2011;206:5–13. doi: 10.1016/j.toxlet.2011.04.006. doi: 10.1016/j.toxlet.2011.04.006. PubMed PMID: 21524695. [DOI] [PubMed] [Google Scholar]
- 126.Collombet JM, Four E, Burckhart MF, Masqueliez C, Bernabé D, Baubichon D, et al. Effect of cytokine treatment on the neurogenesis process in the brain of soman-poisoned mice. Toxicology. 2005;210:9–23. doi: 10.1016/j.tox.2005.01.013. doi: 10.1016/j.tox.2005.01.013. PubMed PMID: 15804454. [DOI] [PubMed] [Google Scholar]
- 127.Collombet JM, Béracochéa D, Liscia P, Piérard C, Lallement G, Filliat P. Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res. 2011;221:261–70. doi: 10.1016/j.bbr.2011.03.006. doi: 10.1016/j.bbr.2011.03.006. PubMed PMID: 21396966. [DOI] [PubMed] [Google Scholar]
- 128.Sogorb MA, Vilanova E, Carrera V. Future applications of phosphotriesterases in the prophylaxis and treatment of organophosporus insecticide and nerve agent poisonings. Toxicol Lett. 2004;151:219–33. doi: 10.1016/j.toxlet.2004.01.022. doi: 10.1016/j.toxlet.2004.01.022. PubMed PMID: 15177657. [DOI] [PubMed] [Google Scholar]
- 129.Everley PA, Dillman JF3rd. Genomics and proteomics in chemical warfare agent research: recent studies and future applications. Toxicol Lett. 2010;198:297–303. doi: 10.1016/j.toxlet.2010.08.003. doi: 10.1016/j.toxlet.2010.08.003. PubMed PMID: 20708669. [DOI] [PubMed] [Google Scholar]
- 130.Gaydess A, Duysen E, Li Y, Gilman V, Kabanov A, Lockridge O, et al. Visualization of exogenous delivery of nanoformulated butyrylcholinesterase to the central nervous system. Chem Biol Interact. 2010;187:295–8. doi: 10.1016/j.cbi.2010.01.005. doi: 10.1016/j.cbi.2010.01.005. PubMed PMID: 20060815; PubMed Central PMCID: PMC2998607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McDonough JH, McMonagle JD, Shih TM. Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol. 2010;33:279–83. doi: 10.3109/01480540903483417. doi: 10.3109/01480540903483417. PubMed PMID: 20429808. [DOI] [PubMed] [Google Scholar]
- 132.Husain K, Ansari RA, Ferder L. Pharmacological agents in the prophylaxis/treatment of organophosphorous pesticide intoxication. Indian J Exp Biol. 2010;48:642–50. PubMed PMID: 20929049. [PubMed] [Google Scholar]
- 133.Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, et al. [+]-Huperzine A treatment protects against N-methyl-d-aspartate-induced seizure/status epilepticus in rats. Chem Biol Interact. 2008;175:387–95. doi: 10.1016/j.cbi.2008.05.023. doi: 10.1016/j.cbi.2008.05.023. PubMed PMID: 18588864. [DOI] [PubMed] [Google Scholar]
- 134.Weissman BA, Raveh L. Therapy against organophosphate poisoning: the importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol. 2008;232:351–8. doi: 10.1016/j.taap.2008.07.005. PubMed PMID: 18680758. [DOI] [PubMed] [Google Scholar]
- 135.Dorandeu F, Baille V, Mikler J, Testylier G, Lallement G, Sawyer T, et al. Protective effects of S+ ketamine and atropine against lethality and brain damage during soman-induced status epilepticus in guinea-pigs. Toxicology. 2007;234:185–93. doi: 10.1016/j.tox.2007.02.012. doi: 10.1016/j.tox.2007.02.012. PubMed PMID: 17408839. [DOI] [PubMed] [Google Scholar]
- 136.Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. The GluK1 (GluR5) Kainate/{alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther. 2011;336:303–12. doi: 10.1124/jpet.110.171835. PubMed PMID: 20962029; PubMed Central PMCID: PMC3033714. [DOI] [PMC free article] [PubMed] [Google Scholar]


