Abstract
Background: Nausea and vomiting are common complications of anesthesia and surgery. Patients undergoing tympanoplasty are exposed to a higher risk of postoperative nausea vomiting (PONV). These complications may alter the results of reconstruction and anatomical alignments. Numerous antiemetics have been studied to prevent and treat PONV in patients undergoing tympanoplasty. The aim of this study was to compare the effect of intravenous ondansetron and dexamethasone on post-tympanoplasty PONV.
Methods: In a double-blind randomized controlled clinical trial, 219 patients were divided into three groups including one receiving ondansetron, one receiving dexamethazone, and one receiving distilled water. All patients were subjected to tympanoplasty type I. The patients in the first group received ondansetron (4 mg IV), second group received oexamethasone (8 mg IV), and third group received distilled water prior to induction of anesthesia. Using Bellivelle’s scoring system, the incidence of PONV and its severity during the 24-hour period after surgery were measured and compared.
Results: There was no significant difference among PONV in the three groups in the first two hours after the surgery. However, in 2-8, 8-16 and 16-24 hours after the surgery the PONV in ondansetron and dexamethasone groups were significantly lower than that in the control group.
Conclusion: Ondansetron and dexamethasone were more effective than placebo in controlling PONV after tympanoplasty surgeries. Moreover, dexamethasone was more effective than ondansetron in preventing PONV.
Trial Registration Number: IRCT201106154005N4
Key Words: Postoperative, Ondansetron, Dexamethasone, vomiting, tympanoplasty
Introduction
Postoperative nausea and vomiting (PONV) are defined as the occurrence of nausea and vomiting in patients after surgical operations, starting from post-anesthesia care unit (PACU) to the early hours of transferring the patient to the ward, without any clear reasons like hypotension.1 Postoperative nausea and vomiting rate has been reported to vary (20% to 30%) in various surgical operations and in different methods of anesthesia, and constitutes the second most common complaint reported.2 It results in patient dissatisfaction, delayed discharge from hospital, unexpected hospitalization, and administration of various treatment modalities.
Different methods and medications have been used to treat PONV.2 For example, the use of dropridol at 10-20 ∞g/kg reduces its incidence to 60%. Ondansetron is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic following chemotherapy. Its effects are thought to be on both peripheral and central nerves. Ondanestron reduces the activity of the vagus nerve, which deactivates the vomiting center in the medulla oblongata, and blocks serotonin receptors in the chemoreceptor trigger zone. However, it is expensive and has some dangerous side effects such as headaches and high blood pressure that can lead to serious complications, especially in susceptible and hypertensive patients.1 Dexamethasone, which is used frequently in the patients undergoing ear, throat and nose surgical operations, is cheap and has no serious side effects. If dexamethasone is given, orally or parenterally, over a period of more than a few days, side-effects common to systemic glucocorticoids may occur.4 General anesthesia with inhaled anesthetics can give rise to post-operative nausea and vomiting, the rate of which has been reported to be 20-30%.3 It seems that PONV has multiple causes and is influenced by a number of factors including anesthetics, surgery and individual risk factors like smoking, anxiety and age. After the age of 50 years, the incidence of PONV decreases to about 13% in every 10 years.5
The emergence of 5-HT3 receptor antagonists in the 1990s revolutionized the antiemetic therapy. Their effect to prevent PONV is significant. Ondansetron is the first medicine introduced in this group. Ondansetron is one of the derivatives of Carbazolin, which is structurally the same as serotonin, but does not have any significant effects on the activity of dopaminergic, histamine, adrenergic, and cholinergic receptors. The most important side effect of this medicine is hypersensitivity reactions. Other side effects include headaches, lightheadedness, dizziness, obstruction of the intravenous line, temporary increase in liver transaminase levels, feeling of heat in the epigastrium, and constipation. Cardiac disrhythmias have been reported during the injection of this drug. The clinical dose of the drug (4-8 mg) usually does not have any side effects.1
The antiemetic effects of glucocorticoids (dexamethasone and methylprednisolone) are known; however, their mechanism is not fully understood. Although dexamethasone has been traditionally useful in preventing and treating nausea in the patients undergoing chemotherapy, it is widely used in preventing PONV. It has been shown that given inravenously one dose (8-10 mg) of this drug is effective in preventing PONV. It has been recommended that the use dexamethasone as a prophylactic agent against PONV should be combined with other drugs.6 Postoperative nausea and vomiting, however, remain a significant problem and the issue of the best prevention or treatment method is still under consideration. This problem prompted us to compare the efficacy of dexamethasone and ondansetron in the prevention of post-tympanoplasty nausea and vomiting.
Patients and Methods
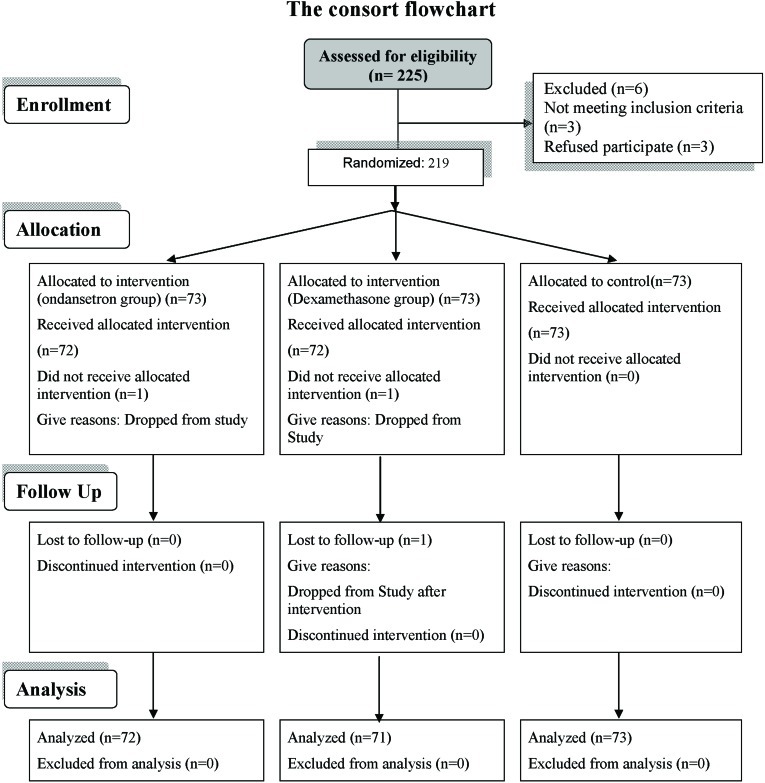
The study is a double-blind randomized controlled clinical trial performed at Imam Reza Hospital, Tabriz, Iran over a period of one year. Two hundred and nineteen patients with physical conditions of ASA (American Society of anesthesiologists) I or II undergoing tympanoplasty type I were divided into three groups of 73 patients to receive ondansetron, dexamethasone, or distilled water before the operation (figure 1). There were no simultaneous ossiculoplasties and mastoid surgeries. Simple randomized sampling procedure was carried out on the basis of study criteria using the “Random Allocation Software” program (practical solution for randomization in clinical trial with different types of formatted output platform windows, version: 1.0, filesize: 6.33 MB). The patients, those administering the drug, and those registering the signs and symptoms of the patients were unaware of the medicine used in each group. Patients with digestive problems, a history of treatment with antiemetics and nausea in the preceding 24 hours, or obesity (BMI>40) were excluded from the study. A written consent was obtained from all the patients. The study was approved by the Ethics Committee, Tabriz University of Medical Sciences.
Figure 1:
The Flowchart of the design and the protocol of the study.
Before the induction of anesthesia, 4 mg of ondansetron, 8 mg of dexamethasone or distilled water were administered intravenously to respective groups. The volume of the administered drug was 3 ml in all the three groups. In each group, premedication was given using midazolam at 0.15 mg/kg and fentanyl at 1-2 ∞g/kg. Induction was carried out with propofol (1-2.5 mg/kg) and atracurium (0.5 mg/kg). Anesthesia maintenance for both groups was performed using Total Intravenous Anesthesia method and through propofol (10-20 ∞g/kg/min) and remifentanyl (0.5 ∞g/kg/min). Administration of anesthetics for maintenance continued until the last stitch of the operation. Extubation was performed after creating inhaling power of 20 cm of water, and all the patients were dismissed from PACU provided that they had acquired at least a score of 9. During the anesthetic maintenance, no inhalation anesthetic drugs and N2O were used, and ventilation was carried out with 100% oxygen.
Using a questionnaire, all instances of nausea and vomiting were recorded carefully every few hours for 24 hours until the patient was discharged to the ward. The intensity of vomiting was evaluated through the Bellville scoring scale (lack of nausea and vomiting=0, nausea=1, nausea with belching=2, and vomiting=3).
Data were collected on the type of the surgical operation, age, NPO duration, ASA, induction and duration of anesthesia, duration of the operation, blood pressure before and after the operation, respiratory rats before the operation, saturation of peripheral oxygen (SPO2) before the operation, body temperature before the operation, duration of recovery, blood pressure five minutes after induction and after extubation, SPO2 five minutes after extubation, SPO2 at discharge from recovery, presence and the intensity of nausea or vomiting at 0-2, 2-8, 16-24 hours after the operation. Data, presented as Mean±SD or frequency and percentage, were analyzed using SPSS (Version 15, Chicago, IL, USA) statistical program. The quantitative variables were compared using paired t test or one-way ANOVA followed by Tukey test for pairwise comparisons. The comparison of qualitative variables was performed using contingency tables, chi-square, or Fisher's exact test. A p value of ≤0.05 was considere statistically significant.
Results
There was no significant difference between the three groups in terms of demographic data or physical condition of ASA (table 1).
Table 1:
Demographic data and physical condition (ASA score) of groups receiving ondanesteron (group O), dexamethasone (group D) or distilled water (group DW)
| Characteristics | Group O | Group D | Group DW | P value |
|---|---|---|---|---|
| Male Female |
19 54 |
24 49 |
18 55 |
0.494 |
| Age (years) | 44±15 | 46±12 | 50±9 | 0.398 |
| ASA | I (61) II (12) |
I (64) II (9) |
I (63) II (10) |
0.796 |
Moreover, there were no statistically significant differences between the three groups in terms of systolic and diastolic pressure, duration of operation, duration of recovery, SPO2 before induction or SPO2 at emergence from recovery (%) (table 2).The average systolic or diastolic blood pressure measured before the induction in the three groups were not significantly different.
Table 2:
Blood pressure (mmHg),saturation of peripheral oxygen (SPO2), duration of operation, duration of recovery of groups receiving ondanesteron (group O), dexamethasone (group D) or distilled water (group DW)
| Group O | Group D | Group DW | |
|---|---|---|---|
| Systolic blood pressure before induction | 120±18.5 | 118±15.4 | 118.2±12.9 |
| Diastolic blood pressure before induction | 76/3±12 | 73.5±10.9 | 74.8±9.8 |
| Systolic blood pressure at emergence from recovery | 123.2±11.3 | 124.4±13.7 | 124.1±12.3 |
| Diastolic blood pressure at emergence from recovery | 77.9±12.5 | 78.9±12.1 | 78.6±10 |
| SPO2 before induction (%) | 97.4±2.6 | 96.3±2.2 | 97.7±1.6 |
| SPO2 at emergence from recovery (%) | 98.5±1.2 | 98.5±1.1 | 98.6±0.9 |
| Duration of operation (min) | 119±27 | 121±27 | 124±30 |
| Duration of recovery (min) | 36.6±11 | 38.2±11 | 38.4±12 |
There was no significant difference among PONV in the three groups in the first two hours after the surgery. However, in 2-8, 8-16 and 16-24 hours after the surgery the PONV in O group and D groups were significantly lower than that in the control group. Moreover, PONV at 8-16 and 16-24 hours after the surgery was significantly lower in D group compared to that of O group (Table 3).
Table 3:
The number and percentage of nausea and vomiting at various postoperative intervals in groups receiving ondanesteron (group O), dexamethasone (group D) or distilled water (group DW)
| Group | 0-2 hours after operation | 2-8 hours after operation | 8-16 hours after operation | 16-24 hours after operation |
|---|---|---|---|---|
| O | (17.8%) 13 | (49.3%) 36 | (53.4%) 39 | (9.5%) 7 |
| D | (23/28%) 17 | (54.7% )40 | (21.9% )16 | (2.7%) 2 |
| DW | (21/9%) 16 | (75.3%) 55 | (100%) 73 | (27.3) 20 |
| P=0.193 | P=0.003 | P<0.001 | P<0.001 |
Table 4 shows the intensity of nausea and vomiting according to Bellville scoring scale. During 0-2 hours after the operation there were only few cases of nausea, which were not significantly different among the three groups. During 2-8 and 8-16 hours post operation, there were cases of nausea and nausea with belching, but no vomiting. The incidence of nausea and nausea with belching in O or D group was significantly higher than those in the control groups. During 16-24 hours nausea, but nausea with belching or vomiting, occurred in all groups. The incidence of nausea was significantly lower in O and D groups than that in the control group.
Table 4:
Intensity of nausea and vomiting according to Bellville scoring scale (lack of nausea and vomiting=0, nausea=1, nausea with belching=2, and vomiting=3) at various postoperative intervals in groups receiving ondanesteron (group O), dexamethasone (group D) or distilled water (group DW)
| Group O | Group D | Group DW | P value | |
|---|---|---|---|---|
| 0-2 hours after the operation Lack of nausea and vomiting (0) |
60(%82/2) | 55(%76/72) | 57(%78/1) | O vs D=0.314 D vs DW=0.02 O vs DW=0.164 |
| Nausea (1) | 13(%17/8) | 17(%23/28) | 16(%21/9) | O vs DW=0.001 D vs DW=0.002 O vs D=0.016 |
| Nausea with belching (2) | - | - | - | - |
| Vomiting (3) | - | - | - | - |
| 2-8 hours After the operation Lack of nausea and vomiting (0) |
37(%50/7) | 33(%45/2) | 18(%24/7) | O vs D=0.388 D vs DW=0.005 O vs DW=0.0003 |
| Nausea (1) | 30(%41/1) | 36(%49/3) | 53(%72/6) | O vs D=0.878 D vs DW=0.92 O vs DW=0.976 |
| Nausea with belching (2) | 4(%5/5) | 4(%5/5) | 2(%2/7) | O vs D=0.358 D vs DW=0.131 O vs DW=0.328 |
| Vomiting (3) | - | - | - | - |
| 8-16 hours After the operation Lack of nausea and vomiting (0) |
34(%46/6) | 57(%78/1) | - | O vs D=0.057 D vs DW=0.128 O vs DW=0.4995 |
| Nausea (1) | 35(%47/9) | 14(%19/2) | 38(%52/1) | O vs D=00001 D vs DW=0.00 O vs DW=0.00 |
| Nausea with belching (2) | 4(%5/5) | 2(%2/7) | 35(%47/9) | O vs D=0.134 D vs DW=0.00 O vs DW=0.000 |
| Vomiting (3) | - | - | - | - |
| 16-24 hours After the operation Lack of nausea and vomiting (0) |
66(%90/4) | 71(%97/3) | 53(%72/6) | O vs D=0.758 D vs DW=0.23 O vs DW=0.37 |
| Nausea (1) | 7(%9/6) | 2(%217) | 20(%27/4) | O vs D=0.085 D vs DW=0.004 O vs DW=0.005 |
| Nausea with belching (2) | - | - | - | - |
| Vomiting (3) | - | - | - | - |
Discussion
In the present study, the effects of administration of ondansetron (4 mg IV) and dexamethasone (8 mg IV) before anesthetic induction on postoperative nausea and vomiting was evaluated in tympanoplasty type I surgical operations. The incidence rate of postoperative nausea and vomiting after tympanoplasty surgical operations has been reported to be significant.7 The high incidence of nausea and vomiting after tympanoplasty might be attributed to the complex innervation of this area by the cranial nerves V, VII, VIII and X, and cervical nerves II and III.8,9 Moreover, proximity of cranial surgical field to the semilunar ducts and vestibular system, and heat and vibration transmission at excision of the surgical field through stimulation of the ampulla can lead to postoperative nausea, dizziness, and vomiting. Therefore, post-operative nausea and vomiting are more common and these patients in these patients.10
In the present study, the incidence rates of PONV in the placebo, dexamethasone and ondansetron groups were 100%, 54.8% and 49.3%, respectively. The incidence rate and intensity of PONV in the dexamethasone and ondansetron groups were significantly lower than that in the control group. In the final stages of the study, incidence rate and intensity of PONV in the dexamethasone group was less than that in the ondansetron group.
Previous studies have shown that compared to distilled water, intravenous dexamethasone significantly reduced the rate and intensity of the PONV.11-13 Limited studies have compared the effects of dexamethasone and ondansetron on PONV, and their findings are contradictory. Erhan et al. conducted a comparative study on ondansetron (4 mg IV), granisteron (3 mg IV) and dexamethasone (8 mg IV) effects given before induction of anesthesia to prevent postoperative PONV in laparoscopic cholecystectomy. They showed that compared to placebo all the three drugs in similar manner significantly reduced the incidence rate of PONV.14 Lopez-Olaondo et al. reported that dexamethasone was as effective as ondansetron in reducing nausea and vomiting induced by chemotherapy.15 Gupta also concluded that intravenous dexamethasone and ondansetron had a similar effect on PONV prevention.16 Moreover, Munoz et al. showed that the effects of dexamethasone and ondansetron in preventing PONV were similar.17 However, in another study,18 it was shown that ondansetron was better than dexamethasone. Another study showed that dexamethasone was a little more effective than ondansetron in preventing post-tonsillectomy PONV.19 Also, a study of 60 patients undergoing laparoscopic cholecystectomy showed that the incidence rate of PONV in the dexamethasone group was significantly lower (20% versus 43.3%).20 The difference in the findings of the above studies might be related to wide range of differences in sample sizes, patients qualities, type of surgical operations and anesthetic techniques, the way that PONV was defined and studied, and most important of all the dosage of the antiemetic drugs and the timing of their administration.17,21
The present study showed that dexamethasone was more effective than ondansetron in preventing PONV; therefore, it may be more suitable to be administered in such a situation. In the studies in which no difference was reported between dexamethasone and ondansetron, the use of dexamethasone was preferred. This might be attributed to the lower cost of dexamethasone than that of ondansetron.14
The present study showed that neither dexamethasone nor ondansetron was associated with no significant side effects. The safety of these drugs has already been confirmed.14,22 Although dexamethasone was more effective than ondansetron in reducing PONV, the incidence PONV was still considerably high. Therefore, further studies using other common drugs would be helpful.
Conclusion
Both ondansetron and dexamethasone were more effective than placebo in preventing PONV in post-tympanoplasty operations. Dexamethasone was more effective, safer, and less expensive than ondansetron, therefore, it may be a better substitute for ondansetron.
Conflict of Interest: None declared
References
- 1.Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Miller’s Anesthesia. 6th ed. USA: Churchill Livingstone; 2009. pp. 2317–33. [Google Scholar]
- 2.Jabalameli M, Rouholamin S, Gourtanian F. A comparison of the effects of fentanyl and remifentanil on nausea, vomiting, and pain after cesarean section. Iran J Med Sci. 2011;36:183–7. [PMC free article] [PubMed] [Google Scholar]
- 3.McKean S, Kochilas X, Kelleher R, Dockery M. Use of intravenous steroids at induction of anaesthesia for adult tonsillectomy to reduce post-operative nausea and vomiting and pain: a double-blind randomized controlled trial. Clin Otolaryngol. 2006;31:36–40. doi: 10.1111/j.1749-4486.2006.01141.x. doi: 10.1111/j.1749-4486.2006.01141.x. PubMed PMID: 16441800. [DOI] [PubMed] [Google Scholar]
- 4.Watcha MF, White PF. Postoperative nausea and vomiting Its etiology treatment and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. PubMed PMID: 1609990. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109–18. doi: 10.1097/00000542-199907000-00018. doi: 10.1097/00000542-199907000-00018. PubMed PMID: 10422935. [DOI] [PubMed] [Google Scholar]
- 6.Ku CM, Ong BC. Postoperative nausea and vomiting: a review of current literature. Singapore Med J. 2003;44:366–74. PubMed PMID: 14620731. [PubMed] [Google Scholar]
- 7.Isik B, Cekmen N, Arslan M, Ozsoylar O, Kordan AZ, Akcabay M. Comparison of the antiemetic effects of ondansetron and dexamethasone on middle ear surgery. Saudi Med J. 2006;27:646–51. doi:10.1097/00003643-200606001-00042. PMID: 16680254. [PubMed] [Google Scholar]
- 8.van denBergAA. A comparison of ondansetron and prochlorperazine for the prevention of nausea and vomiting after tympanoplasty. Can J Anaesth. 1996;43:939–45. doi: 10.1007/BF03011808. PubMed PMID: 8874912. [DOI] [PubMed] [Google Scholar]
- 9.Honkavaara P. Effect of transdermal hyoscine on nausea and vomiting during and after middle ear surgery under local anaesthesia. Br J Anaesth. 1996;76:49–53. doi: 10.1093/bja/76.1.49. doi: 10.1093/bja/76.1.49. PubMed PMID: 8672379. [DOI] [PubMed] [Google Scholar]
- 10.Liu YH, Li MJ, Wang PC, Ho ST, Chang CF, Ho CM, et al. Use of dexamethasone on the prophylaxis of nausea and vomiting after tympanomastoid surgery. Laryngoscope. 2001;111:1271–4. doi: 10.1097/00005537-200107000-00024. doi: 10.1097/00005537-200107000-00024. PubMed PMID: 11568553. [DOI] [PubMed] [Google Scholar]
- 11.Wang JJ, Ho ST, Liu YH, Lee SC, Liu YC, Liao YC, et al. Dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy. Br J Anaesth. 1999;83:772–5. doi: 10.1093/bja/83.5.772. doi: 10.1093/bja/83.5.772. PubMed PMID: 10690141. [DOI] [PubMed] [Google Scholar]
- 12.Wang JJ, Ho ST, Uen YH, Lin MT, Chen KT, Huang JC, et al. Small-dose dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy: a comparison of tropisetron with saline. Anesth Analg. 2002;95:229–32. doi: 10.1097/00000539-200207000-00042. doi: 10.1097/00000539-200207000-00042. PubMed PMID: 12088975. [DOI] [PubMed] [Google Scholar]
- 13.Nesek-Adam V, Grizelj-Stojcić E, Rasić Z, Cala Z, Mrsić V, Smiljanić A. Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2007;21:607–12. doi: 10.1007/s00464-006-9122-7. doi: 10.1007/s00464-006-9122-7. PubMed PMID: 17285386. [DOI] [PubMed] [Google Scholar]
- 14.Erhan Y, Erhan E, Aydede H, Yumus O, Yentur A. Ondansetron, granisetron, and dexamethasone compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy : A randomized placebo-controlled study. Surg Endosc. 2008;22:1487–92. doi: 10.1007/s00464-007-9656-3. PubMed PMID: 18027038. [DOI] [PubMed] [Google Scholar]
- 15.López-Olaondo L, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Sáez A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76:835–40. doi: 10.1093/bja/76.6.835. doi: 10.1093/bja/76.6.835. PubMed PMID: 8679359. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A. Evidence-based medicine in day surgery. Curr Opin Anaesthesiol. 2007;20:520–5. doi: 10.1097/ACO.0b013e3282f021c9. doi: 10.1097/ACO.0b013e3282f021c9. PubMed PMID: 17989543. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz HR, Ibacache ME, Mertz VF. [[Controlled trial of dexamethasone compared with droperidol and ondansetron for the treatment of postoperative nausea and vomiting]]. Rev Med Chil. 2006;134:697–702. doi: 10.4067/s0034-98872006000600004. PubMed PMID: 17130943. [DOI] [PubMed] [Google Scholar]
- 18.Schnaider TB, Vieira AM, Brandão AC. Comparative study of anti-emetics and their association, in the prevention of postoperative nausea and vomiting in patients undergoing gynecologic surgeries. Rev Bras Anestesiol. 2008;58:614–22. doi: 10.1590/s0034-70942008000600006. PubMed PMID: 19082408. [DOI] [PubMed] [Google Scholar]
- 19.Bolton CM, Myles PS, Nolan T, Sterne JA. Prophylaxis of postoperative vomiting in children undergoing tonsillectomy: a systematic review and meta-analysis. Br J Anaesth. 2006;97:593–604. doi: 10.1093/bja/ael256. doi: 10.1093/bja/ael256. PubMed PMID: 17005507. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu D, Mitre C, Leuke L, Bertianu C, Paskarenko G, Puia C, et al. [[Procedures for preventing postoperative nausea and vomiting after laparoscopic cholecystectomy: dexamethasone and ondansetron]]. Anesteziol Reanimatol. 2007;2:50–2. PubMed PMID: 17564002. [PubMed] [Google Scholar]
- 21.Cruz NI, Portilla P, Vela RE. Timing of ondansetron administration to prevent postoperative nausea and vomiting. P R Health Sci J. 2008;27:43–7. PubMed PMID: 18450232. [PubMed] [Google Scholar]
- 22.Aapro MS, Alberts DS. Dexamethasone as an antiemetic in patients treated with cisplatin. N Engl J Med. 1981;305:520. PubMed PMID: 7195983. [PubMed] [Google Scholar]