Abstract
Background: The role of oxidative stress in endosulfan-induced reproductive toxicity has been implicated. This study was performed to evaluate the possible protective effect of vitamins E and C, against endosulfan-induced reproductive toxicity in rats.
Methods: Fifty adult male Sprague–Dawley rats were randomly divided into five groups (n=10 each). The groups included a control receiving vehicle, a group treated with endosulfan (10 mg/kg/day) alone, and three endosulfan-treated group receiving vitamin C (20 mg/kg/day), vitamin E (200 mg/kg/day), or vitamine C+vitamin E at the same doses. After 10 days of treatment, sperm parameters, plasma lactate dehydrogenase (LDH), plasma testosterone and malondialdehyde (MDA) levels in the testis were determined.
Results: Oral administration of endosulfan caused a reduction in the sperm motility, viability, daily sperm production (DSP) and increased the number of sperm with abnormal chromatin condensation. Endosulfan administration increased testis MDA and plasma LDH. Supplementation of vitamin C and vitamin E to endosulfan-treated rats reduced the toxic effect of endosulfan on sperm parameters and lipid peroxidation in the testis. Vitamin E was more protective than vitamin C in reducing the adverse effects of the endosulfan.
Conclusion: The findings data suggest that administration of vitamins C and E ameliorated the endosulfan-induced oxidative stress and sperm toxicity in rat. The effect of vitamin E in preventing endosulfan-induced sperm toxicity was superior to that of vitamin C.
Key Words: Endosulfan, spermatogenesis, oxidative stress, vitamin E, vitamin C
Introduction
Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benza-dioxathiepin-3-oxide) is a polycyclic chlorinated hydrocarbon insecticide. It has been classified as a moderately hazardous (class II) pesticide. However, it still continues to be used in agriculture and public health.1 Endosulfan toxicity has been demonstrated in various organs such as the brain,2 kidney,3 liver,4 heart,5 and reproductive system.
Reproductive toxicity of endosulfan has been shown in some studies. Endosulfan reduces circulating follicle stimulating hormone (FSH) and luteinising hormone (LH).6 It has also been associated with decrease in daily sperm production (DSP), sperm count, and increase in the sperm abnormalities in males.7,8
Endosulfan is a hydrophobic molecule that binds to biological membranes and enhances lipid peroxidation. The role of oxidative stress and lipid peroxidation in endosulfan toxicity has been shown in many organs including the brain,9 erythrocytes,10 peripheral blood mononuclear cells,11 liver and kidney,4 and testis.12 Oxidative stress occurs as a consequence of imbalance between cellular antioxidant system and production of free radicals. Hence, antioxidant compounds such as 5-aminosalicylic acid,13 N–acetyl cysteine,11 melatonin,14 vitamins E,5,15 and vitamins C,9,15 have been used to protect the cells from endosulfan-induced oxidative damages.
Vitamin E is a liposoluble antioxidant that inhibits free radical formation and lipid peroxidation in biological systems.16 On the other hand, vitamin C is a hydrophilic antioxidant that keeps the cellular compartment against water-soluble free radical. Vitamin C is also involved in the reduction and regeneration of oxidized vitamin E.17 In several studies, vitamin C and E have been used to reduce the oxidative stress induced by toxic substances in the testis.18-21 To our knowledge, the protective role of vitamin E supplementation against endosulfan-induced sperm dysfunction has not been studied. In this study, we compared the possible protective role of vitamins C and E against endosulfan-induced disorders in the sperm parameters of adult Sprague-Dawley rats.
Material and Methods
Material
Endosulfan 35% was purchased from Agroxir Chemical Industries Ltd (www.agroxir.com). Vitamin E (α-tocoferol acetate), was purchased from Osveh pharmaceutical Co., Iran. Vitamin C and thiobarbituric acid (TBA) were purchased from Sigma (St Louis, MO). Testosterone Kit was obtained from DRG Diagnostics, Germany. Other reagents were of analytical grade and obtained from Sigma Chemical Co. (St. Louis, MO).
Animals and Treatments
Fifty adult male Sprague–Dawley rats (250±20 g) were obtained from Animal House, Paustor Institute (Tehran, Iran). The animals were kept in laboratory condition (12-h light/dark, 22±2˚C), and fed with standard pellet diet and water ad libitum. The use of animals and the experimental protocol were approved by the Animal Care and Use Committee, Shiraz University of Medical Sciences (Shiraz, Iran).
The animals were randomly divided into 5 groups (n=10 each). Animals in Group I served as controls. Rats in Group II to V received oral administration of 10 mg/kg/day of endosulfan for 10 days. Rats in group III to V were co-treated orally with 200 mg/kg/day Vitamin E (group III ), 20 mg/kg/day vitamin C (group IV) and 200 mg/kg/day vitamin E+20 mg/kg/day vitamin C (group V), respectively. The dose and duration of endosulfan exposure were selected based on previous studies in rats.17,22
Sperm Parameter Analysis
At the end of the treatment period, the animals were weighed and anesthetized with diethylether. Then, blood samples were collected via cardiac puncture, and their plasmas were separated and used to assay for testosterone and lactate dehydrogenase (LDH). The testes were removed, weighed, rinsed with in ice-cold saline. The relative weight of the testes was reported as a percentage of the body weight. A fraction of the testes of each animal was stored at -20°C for malondialdehyde (MDA) determination, while the remaining fraction was used to determine DSP. For determination of DSP, the testes were decapsulated and homogenized for 4 min in 50 mL of phosphate buffer saline (PBS) solution. The number of homogenization resistant sperm nuclei was counted using a hemocytometer. The numbers were then divided by 6.1 (the duration in days of spermatogenic cycle in rats) to determine DSP.23
To analyze the sperm motility and viability, the left epididymis was excised and placed in pre-warmed Petri dish. Caudal epididymes was minced in 4 ml of pre-warmed PBS at 37˚C. The minced tissue was placed in a 37˚C incubator for 5 min and then filtered through nylon mesh. To evaluate the sperm viability, a drop of the Eosin stain was added to the sperm suspension on the slide, kept for 5 min at 37˚C, and then observed under microscope. The head of the dead spermatozoa was stained with red color while the live spermatozoa unstained with Eosin stain. Sperm viability was expressed as the live sperm percentage of as the total sperm counted. For the analysis of sperm motility, one drop of sperm suspension was placed on a warmed microscope slide and a cover slip was placed over the droplet. At least 10 microscopic fields were observed at 400 X magnification under a microscope and the percentage of motile sperm was calculated.
The degree of sperm maturation was assessed by Aniline Blue (AB) staining. The protamine-rich nuclei of mature spermatozoa which contain abundant arginine and cysteine and low level of lysine react negatively with aniline blue stain and remain unstained whereas the histone-rich nuclei of immature spermatozoa with abundant lysine were stained by AB.24 To perform this staining, 5 µl of the sperm collected from the epididymis was smeared onto the glass slide and allowed to dry. The smears were fixed in 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 30 min. The slides were then stained with 5% aqueous AB mixed with 4% acetic acid (pH 3.5) for 5 min. On each slide 200 sperms were examined for the proportion of sperm with unstained head. Unstained or pale-blue stained and dark blue stained spermatozoa were considered as normal spermatozoa and abnormal spermatozoa, respectively.24
Biochemical Analyses
Lipid peroxidation in the testis was determined by the measurement of MDA using the method described by Draper and Hadley.25 Briefly, testis samples were homogenized in PBS (pH 7.4). The homogenate was centrifuged at 5000 g for 10 minutes, and the supernatant was used for MDA assays. For this purpose, 2.5 ml of TBA solution (100 g/L) was added to 0.5 ml supernatant in a test tube and the tubes were heated in boiling water for 15 min. After cooling, the tubes were centrifuged at 1000 g for 10 min, and 2 ml of the supernatant was added to 1 ml of TBA solution (6.7 g/L) in a test tube and the tube was placed in a boiling water bath for 15 min. The solution was then cooled and its absorbance was measured at 532 nm. The concentration of MDA was calculated by the absorbance coefficient of the MDA-TBA complex (absorbance coefficient ε=1.56×l05 cm−1.M−1). MDA is expressed as µg/mg protein. The protein content of the supernatant was determined using the method of Bradford.26 Plasma testosterone was measured by the use of the testosterone ELISA kit (DRG-Germany) following manufacturers instruction. Plasma levels of LDH were assayed using commercial kits (Parsazmoon Co., Karaj, Iran).
Statistical Analysis
All statistical analyses were performed using SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean±SEM Differences among the groups were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey’s test as a post hoc for multiple comparisons. A P value of ≤0.05 was considered as statistically significant.
Results
There was no significant difference between the body weight, weight of testes or weight of testes normalized to body weight of control group, endosulfan-treated group, vitamin E-treated group, vitamin C-treated group and vitamineE+Vitamin C-treated group. (table 1).
Table 1:
The values (mean±SEM, n=10 each) of body and testes weights of control rats, and rats treated with endosulfan, endosulfan+vitamin C, endosulfan+vitamin E, or endosulfan+vitamin C and vitamin E
| Initial body weight (g) | Final body weight (g) | Testis weight. (g) | Testis wt / Body weight (%) | |
|---|---|---|---|---|
| Control | 264±9 | 273±8 | 1.5±0.1 | 0.51±0.01 |
| Endo | 267±7 | 276±12 | 1.5±0.1 | 0.54±0.02 |
| Endo+vit C | 263±10 | 272±9 | 1.4±0.2 | 0.48±.02 |
| Endo+vit E | 283±11 | 295±11 | 1.4±0.1 | 0.53±.02 |
| Endo+vit C +vit E | 298±9 | 298±12 | 1.4±0.1 | 0.49±.01 |
Values represent mean±SEM; n=10
The effect of endosulfan on some of the sperm parameters is summarized in table 2. Group treated with endosulfan alone had a significantly lower sperm viability, sperm motility and DSP/g tissue compared to that of the control group. However, endosulfan-treated groups receiving supplementation of vit C, vit E, or vit C+vit E had a significantly higher sperm viability, sperm motility and DSP/g tissue compared to that of the group treated with endosulfan alone. Group treated with endosulfan alone had a significantly higher AB-positive sperms compared to that of the control group. However, endosulfan-treated groups receiving supplementation of vit E or vit C+vit E, but not vit C, had a significantly lower AB-positive sperm compared to that of the group treated with endosulfan alone.
Table 2:
The values (mean±SEM, n=10 each) of sperm parameters of control rats, and rats treated with endosulfan, endosulfan+vitamin C, endosulfan+vitamin E, or endosulfan+vitamin C and vitamin E
| Treatment | Viability (%) | Sperm motility (%) | DSP (No.×106/gr testis) | AB+sperm (%) |
|---|---|---|---|---|
| Control | 83.0±1.5 | 71.9±1.9 | 14.8±1.2 | 11.7±4.4 |
| Endo | 26.8±3.0* | 18.0±1.45* | 7.5±0.8* | 42.4±7.9* |
| Endo+vit C | 77.8±1.5♦ | 78.1±2.1♦ | 11.4±0.9* | 27.6±9.2* |
| Endo+vit E | 75.4±2.2♦ | 66.4±2.7♦ | 12.9±0.5♦ | 12.0±3.4♦ |
| Endo+vit C & vit E | 86.2±1.1♦ | 64.3±2.5♦ | 18.3±1.6♦ | 14.4±5.6♦ |
Endo: endosulfan; vit C: vitamin C; vit E: vitamin E; DSP: daily sperm production (DSP); AB+: Aniline Blue positive; *Indicate significant difference from the control group; ♦Indicate significant difference from treated with Endo alone
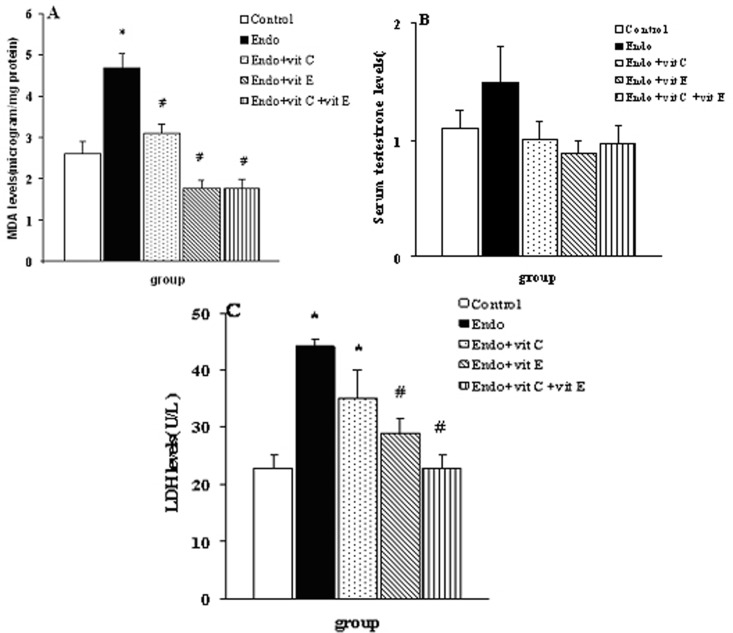
Group treated with endosulfan alone had a significantly (P<0.001) higher MDA levels compared to that of the control group. However, endosulfan-treated groups receiving supplementation of vit C, vit E or vit C+vit E had a significantly lower MDA levels compared to that of the group treated with endosulfan alone (figuer 1A). There was no significant difference among the testestrone of the control group, endosulfan-treated group, and endosulfan-treated group supplemented with vit C, vit E, or vita C+vit E (figure 1B). Group treated with endosulfan alone had a significantly (P<0.001) higher LDH levels compared to that of the control group. However, endosulfan-treated groups receiving supplementation of vit E or vit C+vit E had a significantly lower LDH levels compared to that of the group treated with endosulfan alone (figure 1C).
Figure 1:
Concentrations (mean±SEM, n=10) of Malondialdehyde (MDA) (A), serum testosterone (B), serum lactate dehydrogenase (LDH) levels (C) in the control rats and rats treated with endosulfan (Endo), endosulfan+vitamin C (vit C), edosulfan+vitamin E (vit E) or endosulfan+vitamin C and vitamin E. *indicate significant difference from the control group; #indicate significant difference from the group receiving endosulfan alone
Discussion
Exposure to pesticides could cause male infertility by causing a significant decrease in sperm quality and quantity.27 The results of the present study clearly indicate that endosulfan at a daily dose of 10 mg/kg significantly reduces the quality and quantity of sperm production. The result also shows the protective role of vitamin E and C on endosulfan–induced sperm toxicity by decreasing lipid peroxidation, as shown by biochemical examination and further proved by improvements in qualitative and quantitative sperm parameters in vitamin-treated rats compared to endosulfan treated ones.
Several studies have suggested that lipid peroxidation is involved in endosulfan toxicity.7,8 In this study, oral administration of endosulfan at 1/8 of the LD50,28 for 10 days increased MDA levels, as a marker of lipid per oxidation, in the testis. Lipid peroxidation of membrane polyunsaturated fatty acids disrupts the membrane integrity and results in the leakage of cellular enzyme into the systemic circulation.29 The increase in the level of LDH observed in the endosulfan-treated rats may be attributed to the excessive lipid peroxidation in the cell membrane that might have caused cell membrane damage.
Plasma membranes of the sperms have a high content of polyunsaturated fatty acid; hence, they are highly sensitive to oxidative stress and lipid peroxidation.30 Lipid peroxidation has been shown to be associated with reduction in sperm mobility, viability and count.31 In this study, as might be expected, enhancement of lipid peroxidation by endosulfan is accompanied by a noticeable decrease in sperm viability, motility and DSP. Our data confirm the findings of other studies which reported that endosulfan administration induced decreases in the sperm parameters.7,8
Sperm chromatin condensation is another valuable index of sperm quality that is essential for the capacity of the sperm to fertilize the ovum.24 We evaluated the effect of endosulfan administration on chromatin condensation by AB staining method. This staining discriminates histone-rich chromatin of the immature sperm from protamine-rich chromatin of the mature sperm.24 In agreement with the results of a more recent study,32 we found a high percentage of AB-positive sperm (incomplete or defective sperm DNA condensation) in endosulfan-treated animals compared to the normal control animals. This suggests that a negative effect on chromatin condensation of the sperm could be another mechanism by which endosulfan exposure leads to reproductive toxicity. It is well established that reactive oxygen species impair the sperm chromatin condensation.33 Endosulfan exposure is associated with an increase in free radical generation and lipid peroxidation.9-12 This might explain a part of the mechanism of the impaired chromatin condensation. On the other hand, a reduction in androgen levels in the lumen of cauda epididymis is correlated with a decreased number of disulfide bonds between protamine molecules, and defect in chromatin condensation.34 Endosulfan exposure is associated with the reduction in testosterone concentration in the testis.6 This could represent another mechanism by which endosulfan exposure leads to reduced sperm chromatin condensation.
In the present study, endosulfan-treated rats showed an insignificant increase in plasma testosterone compared to control rats. This finding does not agree with those reported by Singh and Pandey,6 that showed endosulfan increased the level of serum testosterone. They suggested that such an effect of endosulfan may result from a direct toxic effect of endosulfan on the structure of the leydig cells. The cause of this discrepancy might be due to short duration of endosulfan administration in our study. Furthermore, endosulfan has been shown to increase urinary clearance of testosterone.35 Hence, activation of homeostatic mechanism might lead to no appreciable change in serum testosterone levels.
Vitamins C and E are known antioxidants that are effective in preventing oxidative stress-induced testicular damages.18,19 In this study, treatment with vitamin E and C reduced lipid peroxidation and sperm parameter changes induced by endosulfan. These observations indicate the role of lipid peroxidation and oxidative stress in endosulfan reproductive toxicity. Contradictory results have been reported regarding the effectiveness of vitamin C for the prevention of endosulfan-induced sperm toxicity. While a study failed to demonstrate a significant protective effect of vitamin C,36 others suggested that vitamin C ameliorated sperm parameters changes induced by endosulfan.37,38 Here, we firstly showed the protective effects of vitamin C in neutralizing the spermotoxic effect of endosulfan. Furthermore, results of MDA analysis indicated that reduction of lipid peroxidation might be an underlying mechanism of vitamin C protective effects.
The present study show for the first time that vitamin E ameliorated the spermotoxic effect of endosulfan. Vitamin E administration reduced lipid peroxidation in endosulfan-treated rats. The effect of vitamin E in reducing lipid peroxidation was two-fold greater than that of vitamin C (62.8% vs.34.5%, respectively), indicating that vitamin E had a higher impact in preventing of membrane lipid peroxidation. This might be related to high lipid solubility of vitamin E that allow it to localize in the cell membrane, whereas vitamin C is found primarily in the cytosol. Vitamin E supplementation also resulted in significant protection of cell membrane damage with decreased serum LDH levels. The protective mechanism of vitamin E is probably through its capacity to scavenge lipid peroxyl radicals. Furthermore, vitamin E can also normalize the level of glutathione, which is an important for intracellular free radical scavenging system, thus reducing the degree of oxidative damage.19 Likewise, the effect of vitamin E in the improvement of daily sperm production and amelioration of sperm chromatin condensation abnormality induced by endosulfan was superior to vitamin C. The higher protective properties of vitamin E may probably be attributed to the lipophilic nature of vitamin E, which facilitates its free distribution in the cell membrane, while vitamin C is water soluble and functions better in an aqueous environment. In agreement with the current results, a recent study,9 showed that vitamin E and C with their antioxidant properties protected the brain from oxidative stress induced by endosulfan. This is also supported by other studies which showed the protective role of vitamin E,5,15 and other antioxidant compounds such as melatonin,14 and 5-aminosalicic acid,13 in endosulfan–induced oxidative stress in other experimental system.
The combination of two vitamins provided more potent protection than either vitamin alone in some parameters. This could be attributed to regeneration of vitamin E by vitamin C.39
Conclusions
The results of this study demonstrate that endosulfan administration causes oxidative stress in the testis by increasing lipid peroxidation and concomitantly impairs spermatogenesis and epididymal sperm physiology. Vitamin E and C have a protective role against endosulfan-induced alteration in the adult rat spermatogenesis by reducing lipid peroxidation. In comparison to vitamin C, vitamin E was more protective against sperm damage and oxidative stress induced by endosulfan.
Acknowledgment
This work was financially supported by Vice Chancellor for Research of Shiraz University of Medical Science.
Conflict of Interest: None declared
References
- 1.WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. Geneva: World Health Organization; 2000. p. 2. [Google Scholar]
- 2.Silva MH, Gammon D. An assessment of the developmental, reproductive, and neurotoxicity of endosulfan. Birth Defects Res B Dev Reprod Toxicol. 2009;86:1–28. doi: 10.1002/bdrb.20183. doi: 10.1002/bdrb.20183. PubMed PMID: 19243027. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Pandey RS. Toxicity of endosulfan on kidney of male rats in relation to drug metabolizing enzymes and microsomal lipid peroxidation. Indian J Exp Biol. 1989;27:725–8. PubMed PMID: 2633982. [PubMed] [Google Scholar]
- 4.Choudhary N, Sharma M, Verma P, Joshi SC. Hepato and nephrotoxicity in rat exposed to endosulfan. J Environ Biol. 2003;24:305–8. PubMed PMID: 15259607. [PubMed] [Google Scholar]
- 5.Kalender S, Kalender Y, Ogutcu A, Uzunhisarcikli M, Durak D, Açikgoz F. Endosulfan-induced cardiotoxicity and free radical metabolism in rats: the protective effect of vitamin E. Toxicology. 2004;202:227–35. doi: 10.1016/j.tox.2004.05.010. doi: 10.1016/j.tox.2004.05.010. PubMed PMID: 15337585. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, Pandey RS. Effect of sub-chronic endosulfan exposures on plasma gonadotrophins, testosterone, testicular testosterone and enzymes of androgen biosynthesis in rat. Indian J Exp Biol. 1990;28:953–6. PubMed PMID: 2279768. [PubMed] [Google Scholar]
- 7.Chitra KC, Latchoumycandane C, Mathur PP. Chronic effect of endosulfan on the testicular functions of rat. Asian J Androl. 1999;1:203–6. PubMed PMID: 11225895. [PubMed] [Google Scholar]
- 8.Sinha N, Narayan R, Shanker R, Saxena DK. Endosulfan-induced biochemical changes in the testis of rats. Vet Hum Toxicol. 1995;37:547–9. PubMed PMID: 8588293. [PubMed] [Google Scholar]
- 9.Zervos IA, Nikolaidis E, Lavrentiadou SN, Tsantarliotou MP, Eleftheriadou EK, Papapanagiotou EP, et al. Endosulfan-induced lipid peroxidation in rat brain and its effect on t-PA and PAI-1: ameliorating effect of vitamins C and E. J Toxicol Sci. 2011;36:423–33. doi: 10.2131/jts.36.423. PubMed PMID: 21804306. [DOI] [PubMed] [Google Scholar]
- 10.Saxena R, Garg P, Jain DK. In Vitro Anti-oxidant Effect of Vitamin E on Oxidative Stress Induced due to Pesticides in Rat Erythrocytes. Toxicol Int. 2011;18:73–6. doi: 10.4103/0971-6580.75871. PubMed PMID: 21430928; PubMed Central PMCID: PMC3052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed T, Tripathi AK, Ahmed RS, Das S, Suke SG, Pathak R, et al. Endosulfan-induced apoptosis and glutathione depletion in human peripheral blood mononuclear cells: Attenuation by N-acetylcysteine. J Biochem Mol Toxicol. 2008;22:299–304. doi: 10.1002/jbt.20240. PubMed PMID: 18972393. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Xin-Qiang, Zheng Yi-Fan, Zhang Qun-Wei, Jiang Huai, Huang Xin-Shu. [Effects of endosulfan on the spermatogenesis and oxidative damage in rats]. CJPT. 2002;16:391–5. [In Japanese] [Google Scholar]
- 13.Jaiswal A, Parihar VK, Sudheer KumarM, Manjula SD, Krishnanand BR, Shanbhag R, et al. 5-Aminosalicylic acid reverses endosulfan-induced testicular toxicity in male rats. Mutat Res. 2005;585:50–9. doi: 10.1016/j.mrgentox.2005.04.010. doi: 10.1016/j.mrgentox.2005.04.010. PubMed PMID: 16002328. [DOI] [PubMed] [Google Scholar]
- 14.Omurtag GZ, Tozan A, Sehirli AO, Sener G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J Pineal Res. 2008;44:432–8. doi: 10.1111/j.1600-079X.2007.00546.x. doi: 10.1111/j.1600-079X.2007.00546.x. PubMed PMID: 18205731. [DOI] [PubMed] [Google Scholar]
- 15.Pal R, Ahmed T, Kumar V, Suke SG, Ray A, Banerjee BD. Protective effects of different antioxidants against endosulfan-induced oxidative stress and immunotoxicity in albino rats. Indian J Exp Biol. 2009;47:723–9. PubMed PMID: 19957884. [PubMed] [Google Scholar]
- 16.Mukai K, Morimoto H, Okauchi Y, Nagaoka S. Kinetic study of reactions between tocopheroxyl radicals and fatty acids. Lipids. 1993;28:753–6. doi: 10.1007/BF02535999. [Google Scholar]
- 17.Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725–31. doi: 10.1139/y93-109. doi: 10.1139/y93-109. PubMed PMID: 8313238. [DOI] [PubMed] [Google Scholar]
- 18.Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol Reprod. 1995;52:262–6. doi: 10.1095/biolreprod52.2.262. doi: 10.1095/biolreprod52.2.262. PubMed PMID: 7711198. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamoorthy G, Venkataraman P, Arunkumar A, Vignesh RC, Aruldhas MM, Arunakaran J. Ameliorative effect of vitamins (alpha-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm. Reprod Toxicol. 2007;23:239–45. doi: 10.1016/j.reprotox.2006.12.004. PubMed PMID: 17267175. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar SD, Maiti R, Ghosh D. Management of fluoride induced testicular disorders by calcium and vitamin-E co-administration in the albino rat. Reprod Toxicol. 2006;22:606–12. doi: 10.1016/j.reprotox.2006.05.001. PubMed PMID: 16769200. [DOI] [PubMed] [Google Scholar]
- 21.Acharya UR, Mishra M, Patro J, Panda MK. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol. 2008;25:84–8. doi: 10.1016/j.reprotox.2007.10.004. doi: 10.1016/j.reprotox.2007.10.004. PubMed PMID: 18065194. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK, Pandey RS. Gonadal toxicity of short term chronic endosulfan exposure to male rats. Indian J Exp Biol. 1989;27:341–6. PubMed PMID: 2553591. [PubMed] [Google Scholar]
- 23.Fernandes GS, Arena AC, Fernandez CD, Mercadante A, Barbisan LF, Kempinas WG. Reproductive effects in male rats exposed to diuron. Reprod Toxicol. 2007;23:106–12. doi: 10.1016/j.reprotox.2006.09.002. doi: 10.1016/j.reprotox.2006.09.002. PubMed PMID: 17070669. [DOI] [PubMed] [Google Scholar]
- 24.Auger J, Mesbah M, Huber C, Dadoune JP. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13:452–62. doi: 10.1111/j.1365-2605.1990.tb01052.x. doi: 10.1111/j.1365-2605.1990.tb01052.x. PubMed PMID: 1710607. [DOI] [PubMed] [Google Scholar]
- 25.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. PubMed PMID: 2233309. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. doi: 10.1016/0003-2697(76)90527-3. PubMed PMID: 942051. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasa J, Maxim P, Urban J, D’Souza A. Effects of pesticides on male reproductive functions. Iran J Med Sci. 2005;30:153–9. [Google Scholar]
- 28.Gupta PK, Chandra SV. Toxicity of endosulfan after repeated oral administration to rats. Bull Environ Contam Toxicol. 1977;18:378–84. doi: 10.1007/BF01683436. doi: 10.1007/BF01683436. PubMed PMID: 907863. [DOI] [PubMed] [Google Scholar]
- 29.Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997;3:203–13. doi: 10.1093/molehr/3.3.203. doi: 10.1093/molehr/3.3.203. PubMed PMID: 9237246. [DOI] [PubMed] [Google Scholar]
- 30.Lenzi A, Gandini L, Maresca V, Rago R, Sgrò P, Dondero F, et al. Fatty acid composition of spermatozoa and immature germ cells. Mol Hum Reprod. 2000;6:226–31. doi: 10.1093/molehr/6.3.226. doi: 10.1093/molehr/6.3.226. PubMed PMID: 10694269. [DOI] [PubMed] [Google Scholar]
- 31.Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008;89:1183–90. doi: 10.1016/j.fertnstert.2007.05.029. doi: 10.1016/j.fertnstert.2007.05.029. PubMed PMID: 17669405. [DOI] [PubMed] [Google Scholar]
- 32.Selvaraju S, Nandi S, Gupta PS, Ravindra JP. Effects of heavy metals and pesticides on buffalo (Bubalus bubalis) spermatozoa functions in vitro. Reprod Domest Anim. 2011;46:807–13. doi: 10.1111/j.1439-0531.2010.01745.x. doi: 10.1111/j.1439-0531.2010.01745.x. PubMed PMID: 21241381. [DOI] [PubMed] [Google Scholar]
- 33.Said TM, Agarwal A, Sharma RK, Thomas AJJr, Sikka SC. Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 2005;83:95–103. doi: 10.1016/j.fertnstert.2004.06.056. PubMed PMID: 15652893. [DOI] [PubMed] [Google Scholar]
- 34.Huang HF, Nieschlag E. Alteration of free sulphydryl content of rat sperm heads by suppression of intratesticular testosterone. J Reprod Fertil. 1984;70:31–8. doi: 10.1530/jrf.0.0700031. doi: 10.1530/jrf.0.0700031. PubMed PMID: 6363692. [DOI] [PubMed] [Google Scholar]
- 35.Wilson VS, LeBlanc GA. Endosulfan elevates testosterone biotransformation and clearance in CD-1 mice. Toxicol Appl Pharmacol. 1998;148:158–68. doi: 10.1006/taap.1997.8319. doi: 10.1006/taap.1997.8319. PubMed PMID: 9465275. [DOI] [PubMed] [Google Scholar]
- 36.Khan PK, Sinha SP. Vitamin C mediated amelioration of pesticide genotoxicity in murine spermatocytes. Cytobios. 1994;80:199–204. PubMed PMID: 7774290. [PubMed] [Google Scholar]
- 37.Rao M, Narayana K, Benjamin S, Bairy KL. L-ascorbic acid ameliorates postnatal endosulfan induced testicular damage in rats. Indian J Physiol Pharmacol. 2005;49:331–6. PubMed PMID: 16440852. [PubMed] [Google Scholar]
- 38.Ata A, Hatipoglu FS, Yildiz-Gulay O, Gulay MS. Protective role of ascorbic acid on subacute sperm toxicity in male New Zealand white rabbits treated with endosulfan. Drug Chem Toxicol. 2007;30:181–95. doi: 10.1080/01480540701374896. doi: 10.1080/01480540701374896. PubMed PMID: 17613005. [DOI] [PubMed] [Google Scholar]
- 39.Doba T, Burton GW, Ingold KU. Antioxidant and co-antioxidant activity of vitamin C The effect of vitamin C either alone or in the presence of vitamin E or a water-soluble vitamin E analogue upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985;835:298–302. doi: 10.1016/0005-2760(85)90285-1. doi: 10.1016/0005-2760(85)90285-1. PubMed PMID: 4005285. [DOI] [PubMed] [Google Scholar]