Abstract
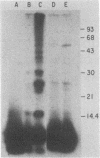
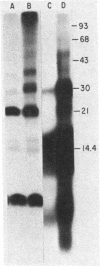
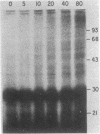
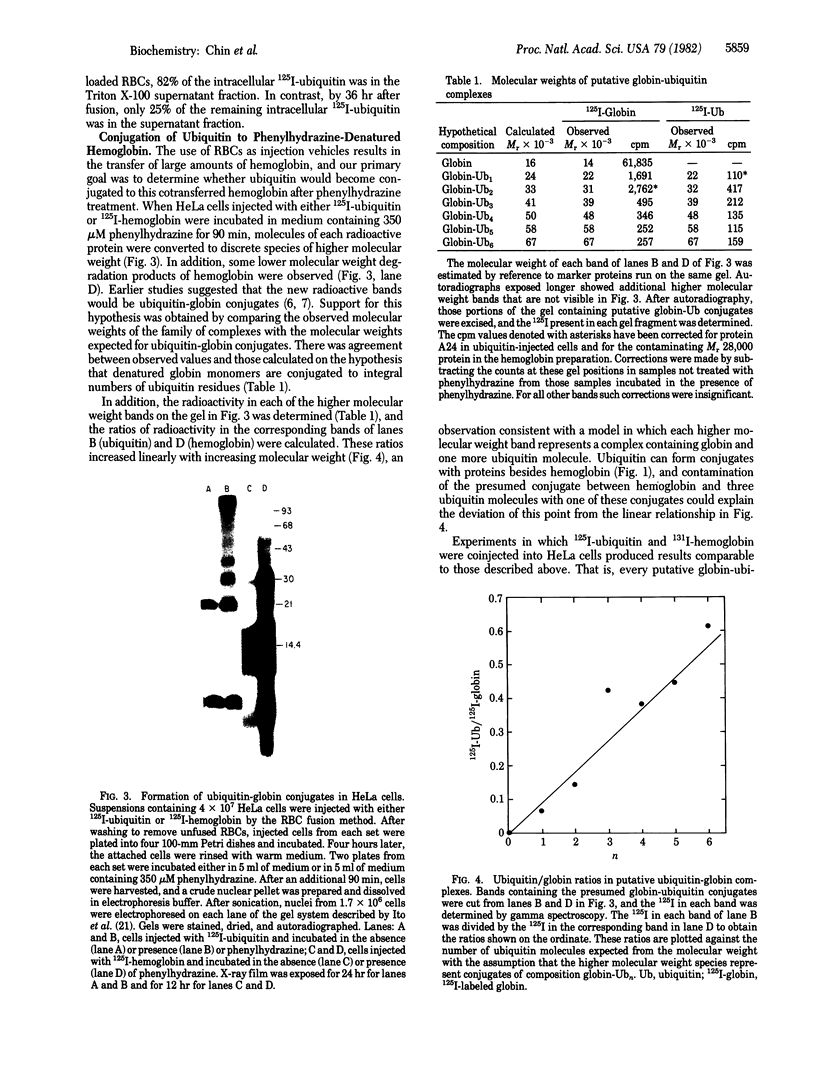
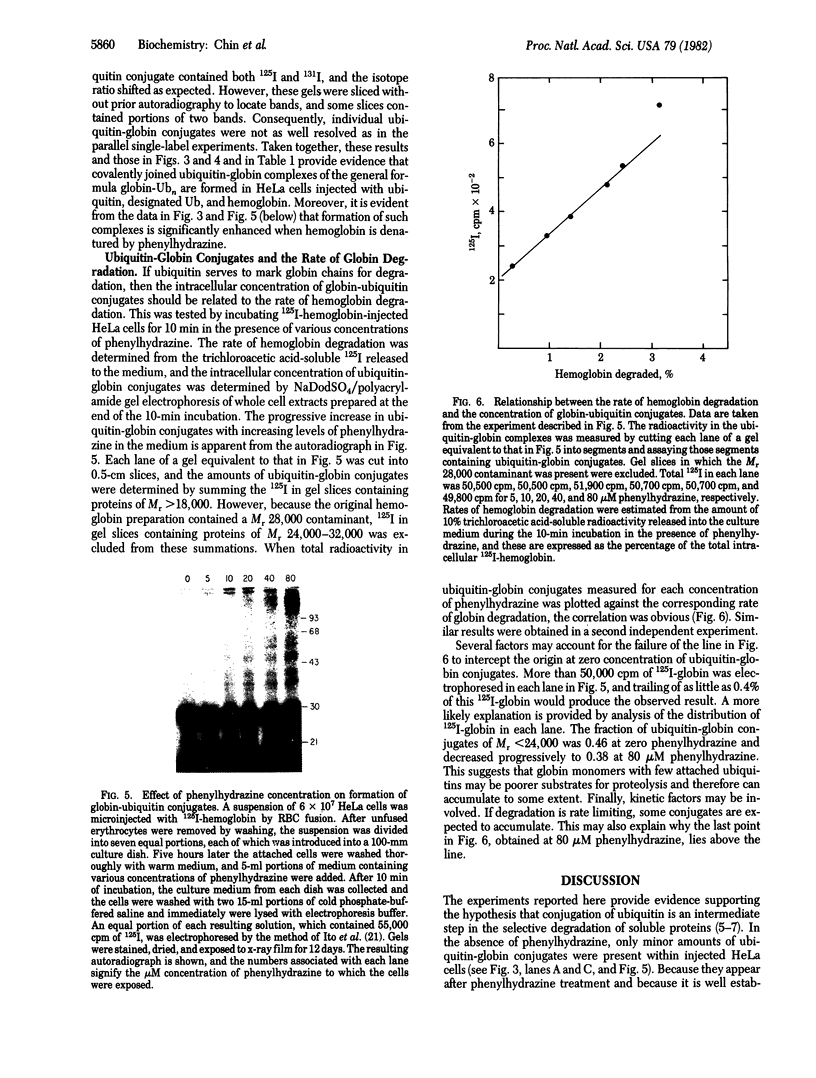
Ubiquitin was radioiodinated and introduced into HeLa cells by the erythrocyte-mediated fusion procedure. Fractionation of injected HeLa cells and subsequent NaDodSO4/polyacrylamide gel electrophoresis showed that HeLa nuclei contained two major labeled proteins: ubiquitin and the histone H2A-ubiquitin conjugate, protein A24. HeLa cytosol contained ubiquitin and a series of ubiquitin-protein conjugates of diverse molecular weights. When injected HeLa cells were treated with phenylhydrazine to denature the cotransferred hemoglobin, a series of prominent ubiquitin-globin conjugates appeared. The identity of these conjugates was established by microinjection experiments in which both proteins were labeled. At low doses of phenylhydrazine, the intracellular concentration of globin-ubiquitin conjugates was proportional to the rate of hemoglobin degradation. This result, together with the observation that ubiquitin conjugation to globin is markedly enhanced by phenylhydrazine-induced denaturation of hemoglobin, provides support for the hypothesis that the covalent attachment of ubiquitin to proteins signals proteolysis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Ballal N. R., Goldknopf I. L., Busch H. Protein A24 lyase activity in nucleoli of thioacetamide-treated rat liver releases histone 2A and ubiquitin from conjugated protein A24. Biochemistry. 1981 Mar 3;20(5):1100–1104. doi: 10.1021/bi00508a009. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Busch H., Goldknopf I. L. Ubiquitin - protein conjugates. Mol Cell Biochem. 1981 Nov 13;40(3):173–187. doi: 10.1007/BF00224611. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Goldberg A. L. Identification and partial purification of an ATP-stimulated alkaline protease in rat liver. J Biol Chem. 1979 May 25;254(10):3712–3715. [PubMed] [Google Scholar]
- French J. K., Winterbourn C. C., Carrell R. W. Mechanism of oxyhaemoglobin breakdown on reaction with acetylphenylhydrazine. Biochem J. 1978 Jul 1;173(1):19–26. doi: 10.1042/bj1730019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Wilson G., Ballal N. R., Busch H. Chromatin conjugate protein A24 is cleaved and ubiquitin is lost during chicken erythropoiesis. J Biol Chem. 1980 Nov 25;255(22):10555–10558. [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. Conservation of histones in chromatin during growth and mitosis in vitro. J Mol Biol. 1969 Mar 28;40(3):457–466. doi: 10.1016/0022-2836(69)90165-x. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular degradation of hemoglobin transferred into fibroblasts by fusion with red blood cells. J Cell Physiol. 1980 Dec;105(3):449–460. doi: 10.1002/jcp.1041050309. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Itano H. A., Vedvick T. S. Oxidative degradation of haemoglobin by nitrosobenzene in the erythrocyte. Biochem J. 1978 Sep 15;174(3):693–697. doi: 10.1042/bj1740693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Thomas J. E., Lee G. R. Inhibition of methemoglobin formation from purified oxyhemoglobin by superoxide dismutase. Biochemistry. 1977 Oct 18;16(21):4563–4567. doi: 10.1021/bi00640a004. [DOI] [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., Bourret L., Miao P., Dice J. F. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981 Oct;91(1):184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Rechsteiner M. C. Red cell-mediated microinjection of macromolecules into mammalian cells. Methods Cell Biol. 1978;20:341–354. doi: 10.1016/s0091-679x(08)62026-9. [DOI] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2B can be modified by the attachment of ubiquitin. Nucleic Acids Res. 1980 Oct 24;8(20):4671–4680. doi: 10.1093/nar/8.20.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K. D., Audhya T. K. Stimulation of ATP-dependent proteolysis requires ubiquitin with the COOH-terminal sequence Arg-Gly-Gly. J Biol Chem. 1981 Sep 10;256(17):9235–9241. [PubMed] [Google Scholar]
- Wilkinson K. D., Urban M. K., Haas A. L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7529–7532. [PubMed] [Google Scholar]
- Wu R. S., Kohn K. W., Bonner W. M. Metabolism of ubiquitinated histones. J Biol Chem. 1981 Jun 10;256(11):5916–5920. [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]