Abstract
The telomere-capping complex (shelterin) protects functional telomeres from initiating unwanted DNA damage response. Uncapped telomeres at the end of cellular replicative lifespan lose this protective mechanism and trigger DNA damage signaling to activate p53 and thereby induce replicative senescence. Here we identify a signaling pathway involving p53, Siah-1, a p53-inducible E3 ubiquitin ligase, and TRF2, a component of the shelterin complex. Endogenous Siah-1 and TRF2 were up- and down-regulated, respectively, at replicative senescence with activated p53. A series of experimental manipulations of p53 showed that p53 induced Siah-1 and repressed TRF2 protein levels. The p53-dependent ubiquitination and proteasomal degradation of TRF2 were attributed to the E3 ligase activity of Siah-1. Siah-1 knockdown stabilized TRF2 and delayed the onset of cellular replicative senescence, suggesting the role of Siah-1 and TRF2 in p53-regulated senescence. This study reveals that p53, a downstream effector of the telomere-initiated damage signaling, also functions upstream of the shelterin complex.
The tumor suppressor protein p53 signals the cellular responses initiated by endogenous or exogenous DNA damage and other stresses to induce cellular senescence, which functions as a tumor suppressor mechanism in vivo and may be involved in organismal aging1, 2. p53 may influence both aging and carcinogenesis in part by regulating self-renewal, genome stability and differentiation of normal and cancer stem cells3–5. Uncapped or dysfunctional telomeres, which are associated with the end stage of the replicative lifespan of normal human cells, are an endogenous DNA damage that activates p53 to induce cellular senescence2, 6–8. Telomere dysfunction also impairs the functional integrity of adult tissue stem cells3, 9, 10 and inhibits the reprogramming of differentiated cells to induced pluripotent stem (iPS) cells11. The telomere-capping protein complex (named “shelterin”) containing the single-stranded and double-stranded telomere binding proteins, including TRF2 (telomere repeat binding factor 2)12, functions to form and maintain the structure of functional telomeres and to inhibit unwanted DNA damage responses at chromosome ends13. Specifically, TRF2 is responsible for the formation and maintenance of “t-loop” structure14 and prevents ATM kinase from activating its downstream factors, including p53, and thereby from triggering DNA damage responses leading to cellular senescence15. Consistently, experimental inhibition of TRF2 induces cellular senescence through the ATM- and p53-mediated pathway8, 12, 16, 17. A recent report shows that TRF2 also inhibits another kinase in this pathway, Chk2, which is phosphorylated by ATM and phosphorylates p5318. These findings have established p53 as a downstream effector of the DNA damage signaling from uncapped, dysfunctional telomeres. However, it is unknown whether p53 also functions upstream to regulate a structural and/or functional component of the telomere-capping complex or the telomere DNA damage response machinery. This study reveals a proteolytic regulation of TRF2 by p53 through a p53-inducible E3 ubiquitin ligase, providing novel insight into p53-mediated telomere damage signaling to cellular senescence with significant implications in carcinogenesis, aging and stem cell biology.
RESULTS
Downregulation of TRF2 and upregulation of Siah-1 at replicative senescence
The endogenous expression of TRF2 protein, detected as ~65- and 69-kDa doublet bands in immunoblot as previously reported19, 20, was found to be diminished when normal human fibroblast strains (MRC-5 and WI-38) underwent replicative senescence (Fig. 1a), which is induced by DNA damage at critically shortened, uncapped telomeres (Supplementary Information, Fig. S1)8, 21, 22. The diminished TRF2 at replicative senescence was also confirmed by immunofluorescence staining (Supplementary Information, Fig. S2a). No change in TRF2 mRNA level was observed (Fig. 1b), suggesting a post-transcriptional regulation. The senescent state of these cells was associated with the activation of the p53 signaling pathway, as revealed by the increase in the phosphorylation of p53 at serine 15 (pS15-p53) and the upregulation of p21WAF1, while total amounts of p53 did not significantly change (Fig. 1a)23. Siah-1, an E3 ubiquitin ligase known to be transcripionally induced by p5324, 25, was upregulated at replicative senescence (Fig. 1a). Although endogenous Siah-1 was readily detectable when we used either nuclear extracts (Fig. 1a) or total protein lysates (Fig. 2a, for example) in the immunoblot analysis, the former generally gave better sensitivity of detection, which is explained by nuclear enrichment of Siah-1 protein (Supplementary Information, Fig. S3a). We thus hereafter use the nuclear extracts, whenever available, for detecting Siah-1 protein (denoted as “NE” in the figures). The upregulation of Siah-1 at replicative senescence was confirmed to occur at the mRNA level (Fig. 1c).
Figure 1.
Replicative cellular senescence is associated with decreased TRF2 and increased Siah-1. (a) Expressions of TRF2, p21WAF1, total p53, p53 phosphorylated at serine 15 (pS15-p53) and Siah-1 were examined by immunoblot in early-passage (Y) and senescent (S) human fibroblast strains MRC-5 and WI-38. The examined passage numbers were 30 (Y) and 65 (S) for MRC-5; and 30 (Y) and 58 (S) for WI-38.β-actin and histone H2B were loading controls. The top three panels used total protein lysates and the bottom three panels used nuclear extracts (NE). Three independent experiments gave reproducible results. (b) TRF2 mRNA levels are not changed in replicative senescence. The same set of cells as in a were examined for TRF2 mRNA expression by the real-time quantitative RT-PCR (qRT-PCR).β-2-microglobulin was a control. Data are mean ± s.d. from three independent experiments. (c) Siah-1 mRNA levels are increased in replicative senescence. The same set of cells as in a and b were examined for Siah-1 mRNA expression by qRT-PCR. β-2-microglobulin was a control. Data are mean ± s.d. from three independent experiments. * P < 0.01.
Figure 2.
p53 upregulates Siah-1 and downregulates TRF2. (a) Loss of wild-type p53 results in increased TRF2 and decreased Siah-1. Li-Fraumeni fibroblasts MDAH041 heterozygous (wt/−) and homozygous (−/−) for p53 frame-shift mutation were examined by immunoblot for p53, TRF2 and Siah-1. β-actin was a loading control. (b) shRNA knockdown of p53 results in increased TRF2 and decreased Siah-1. hTERT-immortalized human fibroblasts (hTERT/NHF) were transduced with the p53 shRNA retroviral vector (+) or the control vector (−) and examined by immunoblot for expressions of p53, TRF2, Siah-1 and Siah-2. NE, nuclear extracts. β-actin and histone H2B were loading controls. Three independent experiments gave reproducible results. (c) The same cells as in b were examined for TRF2 mRNA expression by qRT-PCR, as in Fig. 1b. Data are mean ± s.d. from three independent experiments. (d) Nutlin-3a activation of p53 results in decreased TRF2 and increased Siah-1. hTERT/NHF cells with (+) or without (−) p53 shRNA were treated with 10 μM of Nutlin-3a for the indicated time period and examined by immunoblot for p53, TRF2 and Siah-1. NE, nuclear extracts. β-actin and histone H2B were loading controls. (e) The same cells as in b and c were examined for Siah-1 mRNA expression by qRT-PCR, as in Fig. 1c. Data are mean ± s.d. from three independent experiments. * P < 0.01.
When three other shelterin components (RAP1, POT1 and TPP1) were examined by immunoblot, RAP1 was slightly decreased and POT1 and TPP1 were increased at replicative senescence (Supplementary Information, Fig. S4a), suggesting that senescence-associated downregulation is not common to all shelterin components.
p53 induces Siah-1 and represses TRF2
In a fibroblast strain derived from a Li-Fraumeni syndrome patient, the loss of wild-type p53 allele (−/−) increased TRF2 expression (Fig. 2a). The short hairpin RNA (shRNA) knockdown of endogenous p53 in human fibroblasts resulted in an increased amount of TRF2 protein in immunoblot (Fig. 2b) and immunofluorescence staining (Supplementary Information, Fig. S2b), which again was not due to a change in TRF2 mRNA level (Fig. 2c). The stabilization and activation of endogenous p53 by the small-molecule inhibitor of MDM2 (Nutlin-3a) resulted in a p53-dependent decrease in TRF2 expression (Fig. 2d). Notably, in these experiments, the expression of Siah-1 protein was inversely correlated with the expression of TRF2 protein: Siah-1 was decreased when TRF2 was increased with loss or knockdown of p53 (Fig. 2a, b); and Siah-1 was increased when TRF2 was decreased with Nutlin-3a activation of p53 (Fig. 2d). Decreased Siah-1 mRNA was also confirmed when p53 was knocked down (Fig. 2e). In contrast, the expression of the other Siah family protein, Siah-2, did not depend on p53 and did not show an inverse correlation with TRF2 expression (Fig. 2b). These results suggest that p53 transcriptionally induces Siah-1 and post-transcriptionally represses TRF2.
The p53 regulation of Siah-1 and TRF2 was further substantiated by an additional set of experimental manipulations of p53 expression and activity. The overexpression of wild-type p53 led to increased Siah-1 and decreased TRF2 (Supplementary Information, Fig. S5a). The overexpression of a dominant-negative isoform of p53 (Δ133p53)23, 26 led to decreased Siah-1 and increased TRF2 in normal human fibroblasts (Supplementary Information, Fig. S5b). In p53-deficient 293T cells, Δ133p53 had no effect on TRF2 protein levels by itself but abrogated the wild-type p53-induced downregulation of TRF2 (Supplementary Information, Fig. S5c). Consistently, small interfering RNA (siRNA)-mediated knockdown of endogenous Δ133p5323 resulted in increased Siah-1 and decreased TRF2 in normal human fibroblasts (Supplementary Information, Fig. S5d).
Inhibition of Siah-1 stabilizes TRF2 protein
To examine whether endogenous Siah-1 regulates TRF2 protein levels, siRNA-mediated knockdown of Siah-1 was performed. Two independent siRNA oligonucleotides resulted in increased amounts of TRF2, showing a correlation between Siah-1 knockdown efficiency and TRF2 increase (Fig. 3a). The inhibition of protein synthesis by cycloheximide, followed by immunoblot at different time points, showed that Siah-1 knockdown (using siRNA#2 with higher knockdown efficiency) remarkably extended the half-life of TRF2 protein (≥8 hours, compared with <4 hours in the control) (Fig. 3b, c). These results suggest that endogenous Siah-1 functions to limit TRF2 protein levels. Any of the other shelterin components examined (RAP1, POT1 and TPP1) was not altered in expression by Siah-1 knockdown (Supplementary Information, Fig. S4b), suggesting a specific regulation of TRF2 by Siah-1.
Figure 3.
Siah-1 knockdown stabilizes TRF2. (a) siRNA knockdown of Siah-1 results in increased TRF2. MRC-5 fibroblasts were transfected with each of two independent siRNA oligonucleotides against Siah-1 (#1 and #2) or a control oligonucleotide (Cont) and examined by immunoblot for Siah-1 and TRF2. β-actin was a loading control. Signal intensities of Siah-1 and TRF2 were normalized to β-actin. Relative expression levels of Siah-1 and TRF2 are shown below the images. (b) MRC-5 fibroblasts transfected with control oligonucleotide or Siah-1 siRNA (#2) were treated with 100 μg/ml of cycloheximide (CHX) at indicated time periods and examined for TRF2 expression in immunoblot. (c) Quantitative analysis of TRF2 stability. Relative TRF2 expression levels were from densitometric analysis of b. The value at 0 h was defined as 100% for each. Two independent experiments gave reproducible results.
The accumulation of TRF2 was observed with the inhibition of endogenous Siah-1 activity by the overexpression of a dominant-negative Siah-1 mutant lacking the RING finger domain (FLAG-Siah1-ΔRING)27 (Supplementary Information, Fig. S5e). The overexpression of a stabilized form of Siah-1 (FLAG-Siah1-Δ6)25, similar to that of wild-type p53, resulted in the downregulation of TRF2 (Supplementary Information, Fig. S5f). These results from the overexpression experiments provide additional support for the Siah-1-mediated downregulation of TRF2 protein.
TRF2 is subject to proteasomal degradation and ubiquitinated in p53- and Siah-1-dependent manners
When normal human fibroblasts (MRC-5) were treated with a proteasome inhibitor MG132, TRF2 protein levels were significantly increased both at early passage and at replicative senescence (Fig. 4a). p53 was also increased with MG132 (Fig. 4a), as expected from its regulation by proteasomal degradation28. Similar results were also obtained using WI-38 fibroblasts (data not shown). Thus, in contrast to the above-mentioned observations, increased p53 was not coincident with decreased TRF2 when proteasomal degradation was blocked. The proteasome inhibition by MG132 in hTERT-immortalized fibroblasts (hTERT/NHF) also resulted in increased amount of TRF2 protein, which was comparable with that when p53 activity was inhibited by Δ133p53 or shRNA knockdown (Fig. 4b). Notably, MG132 treatment did not lead to an additional increase in TRF2 in these p53-inhibited cells (Fig. 4b). These findings suggest that TRF2 is degraded through a proteasome-mediated mechanism, which is activated by p53 and enhanced as cells approach replicative senescence.
Figure 4.
TRF2 is subject to proteasomal degradation and ubiquitinated in vivo. (a) Proteasome inhibition increases TRF2. MRC-5 fibroblasts (the same set as in Fig. 1a) were incubated in the presence (+) or absence (−) of 10 μM of MG132 for 5 h and examined for TRF2 and p53 protein levels. (b) Proteasome-mediated regulation of TRF2 depends on functional p53. hTERT/NHF cells with control vector, Δ133p53 overexpression vector23 or p53 shRNA vector (as in Fig. 2b) were treated with MG132 and examined for TRF2 and p53 protein levels, as in a. As indicated by full blots in Supplementary Information, Fig. S9, all the data shown for each antibody were from the same blot. (c) TRF2-containing protein complex is ubiquitinated in vivo. Protein lysates from WI-38 fibroblasts incubated with (+) or without (−) MG132 (15μM for 6 h) were used in immunoprecipitation (IP) with anti-TRF2 antibody or control IgG. Immunoprecipitated proteins were analyzed by immunoblot (IB) using anti-polyubiquitin antibody (Poly-Ub). Immunoblot with anti-TRF2 antibody confirmed the efficiency and specificity of the immunoprecipitation. The data are composite images, but all three samples were on the same blot, as shown in Supplementary Information, Fig. S9. (d) TRF2-associated ubiquitination depends on Siah-1 and p53. MRC-5 fibroblasts were either transfected with siRNA against Siah-1 (#2) or control oligonucleotide (Cont), or transduced with p53 shRNA vector or control vector. Protein lysates prepared after 6 h treatment with 15μM of MG132 (+), as well as those from untreated cells (−) as negative controls, were used in immunoprecipitation with anti-TRF2 antibody, followed by immunoblot with anti-Poly-Ub or anti-TRF2 antibody. Smear signals were quantitated and expressed as relative values to cells without siRNA or shRNA (knockdown, −) after MG132 treatment (+) (defined as 100%). The effectiveness of Siah-1 siRNA and p53 shRNA was confirmed by immunoblot using total protein lysates (before IP). The experiment was repeated three times with reproducible results.
Immunoprecipitation with anti-TRF2 antibody and subsequent immunoblot with anti-polyubiquitin antibody indicated that TRF2 itself and/or its interacting protein(s) were polyubiquitinated in vivo in normal human fibroblasts (Fig. 4c; 4d, leftmost lane). To examine whether endogenous amounts of Siah-1 and p53 are involved in the TRF2-associated polyubiquitination in vivo, endogenous Siah-1 or p53 was knocked down and the immunoprecipitation-immunoblot experiment was performed (Fig. 4d). The knockdown of Siah-1 resulted in ~50% decrease in the polyubiquitination signals. The knockdown of p53 also abrogated the polyubiquitination, as shown by a ~70% decrease in the smear signals. In a p53-proficient colon cancer cell line RKO, the dominant-negative inhibition of endogenous Siah-1 activity (by FLAG-Siah1-ΔRING)27 reduced TRF2-associated polyubiquitination by approximately 50% (Supplementary Information, Fig. S6a). Doxorubicin treatment enhanced the polyubiquitination, likely through DNA damage-induced p53 activation, which was also inhibited by the dominant-negative inhibition of endogenous Siah-1 to a similar level to that observed without doxorubicin (Supplementary Information, Fig. S6a). These data suggest that endogenous p53 and Siah-1 positively regulate in vivo ubiquitination of TRF2 and/or its interacting protein(s).
Siah-1 ubiquitinates TRF2 in vitro and in vivo
All the data described above, as well as the presence in TRF2 of a Myb DNA-binding domain12, which is known to be targeted by Siah-1 for protein degradation25, prompted us to examine whether Siah-1 is an E3 ubiquitin ligase that directly ubiquitinates TRF2. When a GST-Siah-1 fusion protein and a His-tagged TRF2 protein were synthesized by in vitro transcription and translation (Supplementary Information, Fig. S7a, b) and used in an immunoprecipitation-immunoblot experiment, Siah-1 was shown to interact directly with TRF2 in vitro (Fig. 5a). For an in vitro ubiquitination assay, wild-type Siah-1 and RING domain-deleted and -mutated Siah-1 [Siah-1-ΔRING and Siah-1-H59W29, respectively] were produced as GST fusion proteins in E. coli, followed by thrombin cleavage and purification (Supplementary Information, Fig. S7c). A GST-TRF2 fusion protein, as the ubiquitination substrate, was also produced in E. coli and purified (Supplementary Information, Fig. S7d). In the presence of rabbit reticulocyte lysates, wild-type Siah-1, but not Siah-1-ΔRING or Siah-1-H59W, was able to produce the smear signal extending above from the GST-TRF2 fusion protein, but not from GST alone (Fig. 5b). The complete dependence of this signal on the intact RING finger domain of Siah-1, the presence of TRF2 polypeptide sequences in the substrate, and the inclusion of ubiquitin in the assay (Fig. 5b) validated that this in vitro assay specifically detected the ability of the Siah-1 E3 ubiquitin ligase to add polyubiquitin chains to TRF2. We thus conclude that Siah-1 interacts with and ubiquitinates TRF2 in vitro. Mass spectrometry analysis identified the lysine residue at amino acid position 173 as the major ubiquitination site of TRF2, with a concomitant ubiquitination of lysine residue at either position 180 or 184 (Supplementary Information, Fig. S8).
Figure 5.
Siah-1 interacts with and ubiquitinates TRF2. (a) Siah-1 interacts with TRF2 in vitro. His6-TRF2 was immobilized on Ni-NTA magnetic agarose beads (lanes 3–5) and incubated with GST-Siah-1 (lane 4), GST alone (lane 5) or no additional protein (lane 3). In lane 2, GST-Siah-1 was incubated with Ni-NTA magnetic agarose beads without His6-tagged TRF2. After extensive washing, the beads were boiled in SDS sample buffer and the eluted proteins were analyzed by immunoblot using anti-GST and anti-TRF2 antibodies. In lanes 1, 7 and 8, His6-TRF2, GST-Siah-1 and GST alone were run directly as input controls. A molecular weight marker was in lane 6 (MW). (b) Siah-1 ubiquitinates TRF2 in vitro in a RING finger-dependent manner. Rabbit reticulocyte lysates (RRL), ubiquitin, an E3 ubiquitin ligase (wild-type Siah-1, Siah-1-H59W or Siah-1-ΔRING) and a substrate (GST-TRF2 or GST) were added to the in vitro ubiquitination reaction as indicated. After reaction, glutathione-Sepharose 4FF-purified substrates were analyzed by immunoblot with anti-GST antibody. The position of non-ubiquitinated GST-TRF2 is indicated. Poly-ubiquitinated GST-TRF2 showed a smear signal (bracket) with the disappearance of non-ubiquitinated GST-TRF2. The experiment was repeated twice with reproducible results. (c) Siah-1 is essential to TRF2 ubiquitination in vivo. Myc-tagged TRF2, HA-tagged ubiquitin (Ub) and full-length p53 were transiently expressed in 293T cells, as indicated, which were pre-treated with control siRNA (−) or Siah-1 siRNA (#1 and #2). After treatment with MG132, protein lysates were prepared, immunoprecipitated with anti-Myc antibody or control IgG, and then analyzed by immunoblot using anti-Myc antibody (upper) and anti-HA antibody (lower). The knockdown of Siah-1 protein expression by Siah-1 siRNA was confirmed by immunoblot using total protein lysates before immunoprecipitation.β-actin was a loading control. White brackets indicate smear signals showing poly-ubiquitination. The strong signals at the bottom of the upper image correspond to IgG heavy chains. In the lower image, asterisks indicate non-specific bands. The closed arrowhead corresponded to the frontline of the electrophoresis, which likely contained non-specific signals and TRF2-associated ubiquitinated proteins of smaller size. The open arrowhead indicates a ubiquitinated protein of currently unknown origin. The experiment was repeated twice with reproducible results.
To further examine whether Siah-1 is the E3 ligase responsible for TRF2 ubiquitination in vivo, a transient expression-based assay was performed in which exogenously expressed p53, TRF2 (Myc-tagged) and ubiquitin (HA-tagged) induced de novo TRF2 ubiquitination detected by anti-Myc antibody or anti-HA antibody in p53-deficient 293T cells (Fig. 5c). The inhibition of endogenous Siah-1 by pretreatment with the siRNA oligonucleotides nearly completely abrogated the p53-dependent signals detected by anti-Myc antibody (corresponding to the ubiquitination of Myc-tagged TRF2 alone) and by anti-HA antibody [probably including the ubiquitination of the interacting proteins, such as auto-ubiquitinated Siah-130] (Fig. 5c). This finding indicates that Siah-1 is essential to p53-induced TRF2 ubiquitination in vivo, in agreement with its in vitro catalytic activity on TRF2.
Siah-1 and TRF2 regulate cellular replicative lifespan in vitro and are associated with cellular senescence in vivo
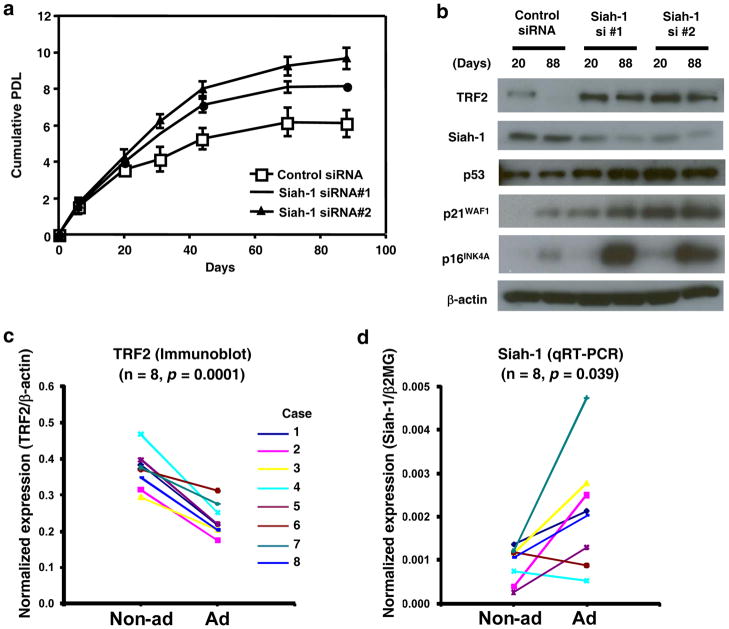
Overexpressed TRF2 was previously shown to delay the onset of replicative senescence31. To investigate the roles of endogenously expressed Siah-1 and TRF2 in the regulation of replicative senescence, MRC-5 human fibroblasts approaching replicative senescence were transfected with siRNA against Siah-1 or control siRNA every 4 days and the replicative lifespan of the cells was determined as cumulative population doubling levels (PDL) (Fig. 6a). The knockdown of endogenous Siah-1 and the resulting increase in endogenous TRF2 by two independent Siah-1 siRNA oligonucleotides were confirmed both when the cells were growing (at day 20) and when they were growth-arrested (at day 88) (Fig. 6b). The Siah-1 knockdown cells showed increased proliferation rate in the early phase of the experiment and underwent an extended replicative lifespan, which was ~2 or 3 PDL longer than control cells (Fig. 6a). This is consistent with our previous finding that Δ133p53 overexpression, which downregulated endogenous Siah-1 and upregulated endogenous TRF2 (Supplementary Information, Fig. S5b), resulted in accelerated cell proliferation and extended replicative lifespan in human fibroblasts23. However, whereas the dominant-negative inhibition of p53 by overexpressed Δ133p53 led to the repression of p21WAF1 as previously described23, the Siah-1 knockdown cells expressed increased levels of p21WAF1, with a slight increase in p53, even when they were proliferating (Fig. 6b). Our data thus show that endogenously expressed Siah-1 and TRF2, as the downstream effectors in a p53 signaling pathway, contribute to the regulation of replicative senescence, and suggest that elevated levels of endogenous TRF2 function to extend cellular replicative lifespan either in cooperation with the repression of p21WAF1 by p53 inhibition23 or in a manner that is resistant to increased p21WAF1 (Fig. 6a, b). The Siah-1 knockdown cells eventually ceased proliferation with remarkable induction of p16INK4A (Fig. 6b), the other major effector of cellular senescence in human cells32, 33. Notably, the TRF2 upregulation-associated extension of replicative lifespan irrespective of p21WAF1 expression is in contrast to the TRF2 inhibition-induced cellular senescence through the p53-p21WAF1 signaling16, 17.
Figure 6.
Roles of Siah-1 and TRF2 in cellular senescence in vitro and in vivo. (a) Siah-1 knockdown extends cellular replicative lifespan in vitro. MRC-5 fibroblasts at passage number 51 were transfected with Siah-1 siRNA#1, Siah-1 siRNA#2 or control siRNA every 4 days. The cumulative population doubling levels (PDL) were calculated and plotted to days after the first transfection. Data are mean ± s.d from three independent experiments. (b) Immunoblot analysis of TRF2, Siah-1, p53, p21WAF1 and p16INK4A. The same set of cells as in a at days 20 and 88 were examined. (c) Cellular senescence in vivo is associated with decreased expression of TRF2. Eight pairs of matched colon adenoma (Ad) and non-adenoma (Non-ad) tissues were analyzed in immunoblot, as shown in Supplementary Fig. S5g. These 8 cases were previously described23 and the adenoma tissues showed senescent phenotypes, such as positive staining for senescence-associated β-galactosidase and increased expression of p16INK4A and IL-823. Normalized expression levels (TRF2/β-actin) were from densitometric measurement and quantitative analysis of the immunoblot data. Paired Student’s t-test was performed. (d) Cellular senescence in vivo is associated with increased expression of Siah-1. RNA samples were isolated from the same 8 cases as in c and examined by qRT-PCR analysis for Siah-1 expression. Siah-1 mRNA levels were normalized to β-2-microglobulin (β2MG) levels. Paired Student’s t-test was performed.
Evidence that the p53- and Siah-1-mediated downregulation of TRF2 occurs in vivo was obtained from the analysis of human colon adenoma tissues, a premalignant tumor associated with p53-mediated cellular senescence23, 34. The eight cases of colon adenoma examined were previously confirmed to show senescent phenotypes, including senescence-associated β-galactosidase activity and increased expression of p16INK4A and IL-823. TRF2 expression was downregulated in all of these adenoma tissues compared with adjacent non-adenoma tissues (Fig. 6c and Supplementary Information, Fig. S5g). Consistent with our in vitro data, adenoma tissues expressed higher amounts of Siah-1 at a statistically significant level (Fig. 6d).
DISCUSSION
This study reveals a novel molecular mechanism of the regulation of TRF2, which involves the p53 tumor suppressor and the p53-induced E3 ubiquitin ligase Siah-1. Significantly, p53 is not only activated by the DNA damage signaling from uncapped telomeres6–8, but also in turn controls an essential component of the telomere-capping protein complex, TRF2. We thus propose that the p53-Siah-1-TRF2 pathway takes an integral part in orchestrating the telomere-initiated DNA damage response. Since loss or functional inhibition of TRF2 at uncapped telomeres allows ATM to initiate DNA damage signaling and activate the downstream effectors such as p5315–17, a feedback regulatory loop involving TRF2, ATM and p53 functions to amplify telomere dysfunction-induced, p53-mediated cellular responses: activated p53 results in, through the Siah-1-mediated ubiquitination and degradation, further inhibition of TRF2, which in turn leads to further enhanced activity of p53. The TRF2-ATM-p53 positive feedback loop may also cooperate with the negative feedback loops involving p53, MDM2, Wip1 and ATM to generate sustained oscillations of p53 activity35, 36. The ATM-dependent phosphorylation of Siah-1 leads to disruption of the complex between Siah-1 and its target HIPK237. It remains to be examined whether the phosphorylation of Siah-1 adds a further complexity to the TRF2-ATM-p53 feedback regulation.
While both TRF2 and Siah-1 are mainly localized in the nucleus (Supplementary Information, Fig. S3a), ubiquitinated TRF2 was found mostly in the cytoplasm (Supplementary Information, Fig. S3b). This is not surprising because some proteins mainly localized in the nucleus, such as p27Kip1 (ref. 38) and cyclin D1 (ref. 39), are found to be ubiquitinated in the cytoplasm. The nuclear-cytoplasmic shuttling of β-catenin, another target of Siah-1 for ubiquitination and degradation, plays an important role in the regulation of its turnover40, 41. It should be noted that, while total and nuclear TRF2 amounts were remarkably decreased in replicatively senescent cells (Fig. 1a and Supplementary Information, Fig. S3a), cytoplasmic TRF2 amounts were increased in these senescent cells (Supplementary Information, Fig. S3a). Thus, the dynamic regulation of subcellular localization of TRF2 may be coupled with its Siah-1-directed ubiquitination and degradation. It remains to be examined whether p53 also regulates a factor that facilitates the nuclear-cytoplasmic shuttling of TRF2.
Although this study mostly used human fibroblasts, the p53-Siah-1-TRF2 pathway seems to function in other cell types as well. The DNA damage by doxorubicin treatment induced Siah-1-mediated ubiquitination of TRF2 in RKO colon cancer cells (Supplementary Information, Fig. S6a). Doxorubicin treatment resulted in p53-dependent reduction of TRF2 in HCT116 colon cancer cells and TK6 lymphoblast cells (Supplementary Information, Fig. S6b, c). These findings also suggest that not only telomere damage but also other non-telomeric DNA damage can activate the p53-Siah-1-TRF2 pathway. However, this pathway is unlikely to be conserved in mice because the mouse Siah-1a and Siah-1b genes (mice have two Siah-1 genes) were not induced by p53 activation and mouse embryonic fibroblasts lacking those Siah-1 genes displayed normal p53 responses, including p53-mediated cellular senescence42. These findings suggest another fundamental difference in telomere biology between humans and mice43.
This study has significant implications in carcinogenesis. The negative regulation of TRF2 by p53 provides a mechanistic basis for the aberrant upregulation of TRF2 in human cancer44, 45. The identification of TRF2 as a novel substrate of Siah-1 suggests that the regulation of telomeres and cellular senescence is a previously unknown mechanism by which Siah-1 contributes to tumor suppression and reversion46, 47. The oncogenic activity of transgenically overexpressed Trf2 in a mouse model45 may be attributed, at least in part, to its ability to extend cellular replicative lifespan. p53 inactivation abrogates the TRF2-ATM-p53 feedback regulation, leading to a cancer-promoting condition where dysfunctional telomeres with supra-physiological amounts of TRF2 are susceptible to chromosomal instability45, 48 and normal senescence checkpoint is impaired. Our finding of the remarkable induction of p16INK4A associated with the end of extended replicative lifespan (Fig. 6b) suggests that p16INK4A inactivation could further extend the duration of this cancer-promoting condition during human carcinogenesis.
Lastly, with a growing body of evidence that telomere-initiated and/or p53-mediated DNA damage signaling and cellular senescence play critical roles in stem cell functions and aging processes in vivo1, 3–5, 9–11, our findings are relevant to stem cell-based regenerative medicine for aging-related degenerative disorders. Although diminished p53 activity enhanced the self-renewing divisions in normal and cancer stem cells4 and the efficiency of reprogramming to iPS cells5, it also resulted in chromosomal instability3, 5. An impairment of the p53- and Siah-1-mediated degradation of TRF2 might in part contribute to the chromosomal instability. An appropriate level of p53 activity for efficient self-renewal and pluripotency without chromosomal instability, as well as for efficient and precise differentiation of stem cells, may be influenced by the other factors involved in the TRF2-ATM-p53 positive feedback loop and the previously known negative feedback loops35. Monitoring and manipulation of TRF2 expression, in combination with those of p53 activity, may improve our understanding and methodology of stem cell biology.
METHODS
Cells and reagents
Normal human fibroblast strains (MRC-5 and WI-38), RKO and 293T were obtained from American Type Culture Collection (Manassas, VA). Fibroblasts from a Li-Fraumeni syndrome patient (MDAH041)49 were kindly provided by Dr. Michael Tainsky (Case Western Reserve University, Cleveland, OH). HCT116 and HCT116 p53−/− were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). TK6 (p53 wild-type) and NH32 (p53-null) lymphoblast cell lines were previously described50. hTERT/NHF, an hTERT (human telomerase reverse transcriptase)-immortalized human fibroblast cell line, was previously described51. Nutlin-3a52 was purchased from Cayman Chemical (Ann Arbor, MI). MG132 and doxorubicin were from Sigma-Aldrich (St. Louis, MO).
Plasmid constructs
The retroviral vectors for overexpressing full-length p53 (pQCXIN-p53) and Δ133p53 (pQCXIN-FLAG-Δ133p53)23 and the retroviral shRNA construct for p53 knockdown53 were previously described. The retroviral construct pLPC-Myc-TRF2 was a gift from Dr. Titia de Lange (Rockefeller University, NY). To generate a retroviral expression vector of the dominant-negative mutant of Siah-1 (FLAG-Siah1-ΔRING)27, the human Siah-1 cDNA fragment (corresponding to amino acid residues 70 to 282) was PCR-amplified using a 5′ primer with FLAG tag sequence and cloned into pBABE-puro. For a retroviral vector driving FLAG-tagged Siah1-Δ6 (a stable form of Siah-1)25, the human Siah-1 cDNA fragment (corresponding to amino acid residues 6 to 282) was amplified and processed in the same way. To generate E. coli expression constructs of GST-Siah-1 fusion proteins, the full-length Siah-1 cDNA and the Siah1-ΔRING cDNA fragment (amino acid residues 70 to 282, as above) were cloned into pGEX-KG (American Type Culture Collection). For Siah-1-H59W, the histidine residue at position 59 in the full-length Siah-1 was mutated to tryptophan using QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The full-length TRF2 cDNA was inserted into pGEX-4T2 vector (Amersham Biosciences, Piscataway, NJ) to produce GST-TRF2 fusion protein. For in vitro protein synthesis, the full-length TRF2 cDNA was cloned into pEXP1-DEST vector (Invitrogen, Carlsbad, CA) to produce a polyhistidine tagged TRF2 (His6-TRF2). The cDNA fragment for GST-Siah-1 fusion protein (full-length, wild-type Siah-1; as in pGEX-KG above) was also cloned in pEXP1-DEST. pRK5-HA-Ub for overexpression of HA-tagged ubiquitin was previously described54. All constructs were verified by DNA sequencing. Retroviral vector transduction was performed as previously described23. Transient transfection of plasmid DNA was performed using Lipofectamine 2000 transfection reagent (Invitrogen).
siRNA oligonucleotides
A stealth siRNA duplex oligoribonucleotide targeting Siah-1 mRNA (Siah-1 #2 mixture, 5′-CAG GAA ACA GUU GCA UGU AGU AAC A-3′ and 5′-GAA GCC AUG GU UCC AGA AAG UAA A-3′), its scrambled control, and a standard siRNA duplex oligoribonucleotide targeting Siah-1 mRNA (Siah-1 #1 mixture, 5′-AAU GUA ACU AUU UCC AUG UGU [dT][dT]-3′ and 5′-GCU GAU AGG AAC ACG CAA GCA [dT][dT]-3′) were synthesized at Invitrogen. siRNA oligonucleotides against Δ133p53 (Δ133si-1 and Δ133si-2) were described previously23. These siRNA oligonucleotides were transfected at the final concentration of 12 nM using Lipofectamine RNAiMAX transfection reagent (Invitrogen).
Immunoblot and immunoprecipitation
Total protein lysates were prepared as previously described23. Nuclear and cytoplasmic extracts were prepared as follows. Cells were harvested, suspended in four pellet volumes of hypotonic homogenizing buffer (200 mM HEPES at pH 7.9, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% NP-40, 1 mM DTT, 10 mM NaF, 2 mM NaVO3, 25 mM β-glycerophosphate, supplemented with complete protease inhibitors from Roche), incubated on ice for 10 min and centrifuged at 500g for 5 min at 4°C. The supernatants were used as cytoplasmic fractions. For nuclear extracts, the pellets were resuspended in 0.5 M NaCl, incubated on ice for 30 min and centrifuged for 30 min at 20,000g at 4°C, followed by the collection of supernatants. For immunoblot analysis, SDS-PAGE, transfer to membranes, and incubation with antibodies were performed as described previously23. Signal detection was performed using ECL detection (Amersham Biosciences) or SuperSignal West Dura Extended Duration system (Pierce Biotechnology, Rockford, IL). Immunoprecipitation followed the standard procedures as previously described55. The quantitative image analysis was performed using the ImageJ 1.40g software (http://rsb.info.nih.gov/ij/).
In vitro binding assay
In vitro protein synthesis of His6-tagged TRF2, GST and GST-Siah-1 fusion protein was performed using Expressway cell-free E. coli expression system (Invitrogen), followed by the purification of His6-tagged TRF2 using Ni-NTA magnetic agarose beads (Qiagen, Valencia, CA) and of GST and GST-Siah-1 using glutathione-Sepharose 4FF (GE Healthcare, Piscataway, NJ). The purified His6-tagged TRF2 (150 ng) bound with Ni-NTA magnetic agarose beads was incubated with 250 ng of purified GST-Siah-1 or GST in 300 μl of the binding buffer [50 mM NaPO4 (pH 8.0), 300 mM NaCl, 15 μM MG132 supplemented with complete protease inhibitors] for 3 h at 4°C. The beads were washed four times with the wash buffer [50 mM NaPO4 (pH 8.0), 300 mM NaCl, 20 mM imidazole, 0.005% Tween-20, 15 μM MG132 supplemented with complete protease inhibitors], followed by boiling in 20 μl of SDS-PAGE sample buffer. The eluted proteins were analyzed by immunoblot as described above.
In vitro ubiquitination assay
All recombinant proteins were expressed as GST fusion proteins in BL21 (DE3) (Invitrogen) and purified using glutathione-Sepharose 4FF (GE Healthcare). After cleavage by thrombin (GE Healthcare), wild-type Siah-1, Siah-1-H59W and Siah-1-ΔRING were purified using Benzamidine-Sepharose 4FF (GE Healthcare). The in vitro ubiquitination assay was performed as described by Chang et al.56 with slight modifications: the reaction mix contained 6.6 μl of rabbit reticulocyte lysate (Promega, Madison, WI), 1 μM ubiquitin aldehyde (Boston Biochem, Cambridge, MA), 121 μM methyl ubiquitin (Boston Biochem), 1× energy solution (Boston Biochem), 150 μM ubiquitin (Boston Biochem) and 20 μM MG132 in 11.59 μl of total volume. The reaction mix was preincubated at 37°C for 5 min to inhibit deubiquitinating enzymes. GST alone or GST-TRF2 as substrate (175 ng) and wild-type Siah-1, Siah-1-H59W or Siah-1-ΔRING as enzyme (220 ng) were added to the reaction mix (final volume 20 μl) and incubated at 30°C for 2 h. The reaction was terminated by addition of stop buffer (Boston Biochem), followed by the purification of the substrate using glutathione-Sepharose 4FF (Amersham). Immunoblot was performed using anti-GST antibody as described above.
In vivo ubiquitination assay
293T cells were transfected with Siah-1 siRNA (#1 or #2) or control siRNA for 24 h and then transfected with pLPC-Myc-TRF2, pRK5-HA-Ub and pQCXIN-p53 plasmids. After 24 h, the cells were treated with 15 μM of MG132 for 8 h and lysed in the buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 % glycerol, 10 μM MG132, 100 mM NaF, 1 mM NaVO3, 25 mM β-glycerophosphate and complete protease inhibitors. Immunoprecipitation and immunoblot were performed as described above.
Antibodies
Following antibodies were used: anti-TRF2 (1:1,000; 4A794, Upstate, Lake Placid, NY); anti-TRF2 (1:2,000; H-164, Santa Cruz Biotechnology, Santa Cruz, CA); anti-Siah-1 (1:1,000; ab2237, Abcam, Cambridge, MA); anti-p53 (1:2,000; DO-1, Santa Cruz Biotechnology); anti-pS15-p53 (1:1,000; #9284, Cell Signaling Technology, Danvers, MA); anti-p21WAF1 (1:500; EA10, Calbiochem, San Diego, CA); anti-p16INK4A (1:1,000; G175-1239, BD Pharmingen, San Diego, CA); anti-RAP1 (1:1,000; Bethyl Laboratories, Montgomery, TX); anti-POT1 (1:1,000; Novus Biologicals, Littleton, CO); anti-TPP1 (1:1,000; Sigma-Aldrich); anti-Siah-2 (1:500; Novus Biologicals); anti-HSP90 (1:2,000; BD Biosciences); anti-Lamin A/C (1:500; Upstate); anti-FLAG tag (1:10,000; M2, Sigma-Aldrich); anti-Myc tag (1:5,000; Invitrogen); anti-HA tag (1:1,000; 3F10, Roche, Indianapolis, IN); anti-GST (1:500; B14, Santa Cruz Biotechnology); anti-His tag (1:3,000; BMG-His-1, Roche); anti-histone H2B (1:2,000; #07-371, Upstate); anti-polyubiquitin (1:500; P4D1, Santa Cruz Biotechnology); anti-β-catenin (1:1,000; BD Biosciences); anti-α-tubulin (1:5,000; Sigma-Aldrich) and anti-β-actin (1:10,000; AC-15, Sigma-Aldrich). Anti-Δ133p53 antibody (1:5,000; MAP4) was previously described23. Horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies (Santa Cruz Biotechnology) were used as secondary antibodies in immunoblot analyses.
Real-time quantitative RT-PCR
TRF2 mRNA levels were quantitated using the TRF2 primers purchased from Roche Applied Science (cat. no. 04689038001). β-2-microglobulin (cat. no. 04688015001, Roche Applied Science) was a control for quantification. Siah-1 mRNA levels were quantitated using the Taqman gene expression assay (Hs00361785_m1, Applied Biosystems, Foster City, CA). β-2-microglobulin (4310886E, Applied Biosystems) was a control. The procedures and quantitative data analysis were as previously described23.
Examination of cellular replicative lifespan
Cell numbers were counted at each passage of siRNA-transfected cells. The number of population doubling levels (PDL) achieved between passages were determined by log2 (number of cells obtained/number of cells inoculated)57. Data were mean ± s.d. from three independent experiments.
Mass spectrometry
Siah-1-dependent in vitro ubiquitination reactions containing either His6-tagged TRF2 or GST-TRF2 as substrate (as described above) were separated on a 4–12% Bis-Tris gel and stained with Colloidal Coomassie (Invitrogen). The band corresponding to unmodified tagged-TRF2 and multiple bands at higher molecular weight were cut from the gel and subjected to in-gel trypsin digestion as described58. Briefly, the cysteines were reduced with DTT and alkylated with iodoacetamide. The protein was then digested with 13 ng/μl trypin (Applied Biosystems) in 10 mM ammonium bicarbonate for 16 h at 30 °C. The peptides were then extracted and dried by vacuum-evaporation using a Vacufuge (Eppendorf). The dried peptides were resuspended in water containing 2% acetonitrile, 0.5% acetic acid. They were then injected onto a 0.2 × 50 mm Magic C18AQ reverse phase column (Michrom Bioresources, Inc.) using the Paradigm MS4 HPLC (Michrom Bioresources, Inc.). Peptides were separated at a flow rate of 2 nL/min followed by online analysis by tandem mass spectrometry using an LTQ ion trap mass spectrometer (Thermo Scientific) equipped with an ADVANCE CaptiveSpray ion source (Michrom Bioresources, Inc.). Peptides were eluted into the mass spectrometer using a linear gradient from 95% mobile phase A (2% acetonitrile, 0.5% acetic acid, 97.5% water) to 65% mobile phase B (10% water, 0.5% formic acid, 89.5% acetonitrile) over 20 minutes followed by 95% mobile phase B over 5 minutes. Peptides were detected in positive ion mode using a data-dependent method in which the nine most abundant ions detected in an initial survey scan were selected for MS/MS analysis. The MS/MS spectra were searched against the human IPI database (v 3.72) using TurboSEQUEST in BioWorks v. 3.3.1 SP1 (ThermoElectron, San Jose, CA). The search parameters included: precursor mass tolerance: ±1.5 amu; fragment mass tolerance: ±0.8 amu; a static modification of +57.02 on cysteine; a variable modification of +15.99 for methionine oxidation; a variable modification of +114.04 on lysine for GG modification that remains after trypsin cleavage of ubiquitin modification59.
Statistical analyses
Statistical analysis was carried out with paired or unpaired Student’s t-test as appropriate.
Supplementary Material
Acknowledgments
We thank M. Tainsky, B. Vogelstein and T. de Lange for cells and reagents. We also thank K. Kumamoto for Nutlin-3a treatment, A. Robles for doxorubicin treatment of lymphoblast cells, M. Yoneda for technical assistance, and E. Spillare for continuous support. This research was supported in part by the Intramural Research Program of the NIH, NCI. B.V. was supported by the grants from the Grant Agency of the Czech Republic (number 301/08/1468) and the Internal Grant Agency of Health of Czech Republic (number NS/9812-4). J.C.B. was supported by Breast Cancer Campaign, Cancer-Research UK and the Institut National de la Sante et de la Recherche Medicale. D.L. is a Gibb fellow of Cancer-Research UK.
Footnotes
AUTHOR CONTRIBUTIONS
K.F., I.H., A.M.M. and L.M.M.J. performed experiments. B.V., J.-C.B. and D.P.L. provided essential reagents and suggestions. K.F., I.H., E.A., L.M.M.J. and C.C.H. coordinated the study and wrote the manuscript. C.C.H. was responsible for the overall project. All authors discussed the results and commented on the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begus-Nahrmann Y, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet. 2009;41:1138–1143. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 4.Cicalese A, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Marion RM, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosme-Blanco W, et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 2007;8:497–503. doi: 10.1038/sj.embor.7400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 9.Ju Z, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 10.Nalapareddy K, Jiang H, Guachalla Gutierrez LM, Rudolph KL. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Exp Gerontol. 2008;43:998–1004. doi: 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Marion RM, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141–154. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 12.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 13.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 14.Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 16.Lechel A, et al. The cellular level of telomere dysfunction determines induction of senescence or apoptosis in vivo. EMBO Rep. 2005;6:275–281. doi: 10.1038/sj.embor.7400352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagno D’Alcontres M, Mendez-Bermudez A, Foxon JL, Royle NJ, Salomoni P. Lack of TRF2 in ALT cells causes PML-dependent p53 activation and loss of telomeric DNA. J Cell Biol. 2007;179:855–867. doi: 10.1083/jcb.200703020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buscemi G, et al. The shelterin protein TRF2 inhibits Chk2 activity at telomeres in the absence of DNA damage. Curr Biol. 2009;19:874–879. doi: 10.1016/j.cub.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 19.Bilaud T, et al. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. doi: 10.1038/ng1097-236. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- 21.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 22.Stewart SA, et al. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet. 2003;33:492–496. doi: 10.1038/ng1127. [DOI] [PubMed] [Google Scholar]
- 23.Fujita K, et al. p53 isoforms Δ133p53 and p53β are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–1142. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 25.Tanikawa J, et al. p53 suppresses the c-Myb-induced activation of heat shock transcription factor 3. J Biol Chem. 2000;275:15578–15585. doi: 10.1074/jbc.M000372200. [DOI] [PubMed] [Google Scholar]
- 26.Bourdon JC, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu G, Fearon ER. Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol Cell Biol. 1999;19:724–732. doi: 10.1128/mcb.19.1.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez MS, Desterro JM, Lain S, Lane DP, Hay RT. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol Cell Biol. 2000;20:8458–8467. doi: 10.1128/mcb.20.22.8458-8467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh S, et al. High precision NMR structure and function of the RING-H2 finger domain of EL5, a rice protein whose expression is increased upon exposure to pathogen-derived oligosaccharides. J Biol Chem. 2003;278:15341–15348. doi: 10.1074/jbc.M210531200. [DOI] [PubMed] [Google Scholar]
- 30.Lorick KL, et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14:2302–2308. doi: 10.1016/j.cub.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 35.Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer. 2009;9:371–377. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T, Brazhnik P, Tyson JJ. Exploring mechanisms of the DNA-damage response: p53 pulses and their possible relevance to apoptosis. Cell Cycle. 2007;6:85–94. doi: 10.4161/cc.6.1.3705. [DOI] [PubMed] [Google Scholar]
- 37.Winter M, et al. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat Cell Biol. 2008;10:812–824. doi: 10.1038/ncb1743. [DOI] [PubMed] [Google Scholar]
- 38.Kamura T, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 39.Lin DI, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-αB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dimitrova YN, et al. Direct ubiquitination of β-catenin by Siah-1 and regulation by the exchange factor TBL1. J Biol Chem. 2010;285:13507–13516. doi: 10.1074/jbc.M109.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 42.Frew IJ, et al. Normal p53 function in primary cells deficient for Siah genes. Mol Cell Biol. 2002;22:8155–8164. doi: 10.1128/MCB.22.23.8155-8164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 44.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 45.Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet. 2005;37:1063–1071. doi: 10.1038/ng1633. [DOI] [PubMed] [Google Scholar]
- 46.Roperch JP, et al. SIAH-1 promotes apoptosis and tumor suppression through a network involving the regulation of protein folding, unfolding, and trafficking: identification of common effectors with p53 and p21Waf1. Proc Natl Acad Sci USA. 1999;96:8070–8073. doi: 10.1073/pnas.96.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Telerman A, Amson R. The molecular programme of tumour reversion: the steps beyond malignant transformation. Nat Rev Cancer. 2009;9:206–216. doi: 10.1038/nrc2589. [DOI] [PubMed] [Google Scholar]
- 48.Munoz P, Blanco R, Blasco MA. Role of the TRF2 telomeric protein in cancer and ageing. Cell Cycle. 2006;5:718–721. doi: 10.4161/cc.5.7.2636. [DOI] [PubMed] [Google Scholar]
- 49.Bischoff FZ, et al. Spontaneous abnormalities in normal fibroblasts from patients with Li-Fraumeni cancer syndrome: aneuploidy and immortalization. Cancer Res. 1990;50:7979–7984. [PubMed] [Google Scholar]
- 50.Robles AI, et al. Schedule-dependent synergy between the heat shock protein 90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin and doxorubicin restores apoptosis to p53-mutant lymphoma cell lines. Clin Cancer Res. 2006;12:6547–6556. doi: 10.1158/1078-0432.CCR-06-1178. [DOI] [PubMed] [Google Scholar]
- 51.Sengupta S, et al. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J. 2003;22:1210–1222. doi: 10.1093/emboj/cdg114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buolamwini JK, et al. Small molecule antagonists of the MDM2 oncoprotein as anticancer agents. Curr Cancer Drug Targets. 2005;5:57–68. doi: 10.2174/1568009053332672. [DOI] [PubMed] [Google Scholar]
- 53.Yang Q, et al. Functional diversity of human protection of telomeres 1 isoforms in telomere protection and cellular senescence. Cancer Res. 2007;67:11677–11686. doi: 10.1158/0008-5472.CAN-07-1390. [DOI] [PubMed] [Google Scholar]
- 54.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Q, Zheng YL, Harris CC. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol. 2005;25:1070–1080. doi: 10.1128/MCB.25.3.1070-1080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang W, Dynek JN, Smith S. TRF1 is degraded by ubiquitin-mediated proteolysis after release from telomeres. Genes Dev. 2003;17:1328–1333. doi: 10.1101/gad.1077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 59.Jeram SM, Srikumar T, Pedrioli PG, Raught B. Using mass spectrometry to identify ubiquitin and ubiquitin-like protein conjugation sites. Proteomics. 2009;9:922–934. doi: 10.1002/pmic.200800666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.