Abstract
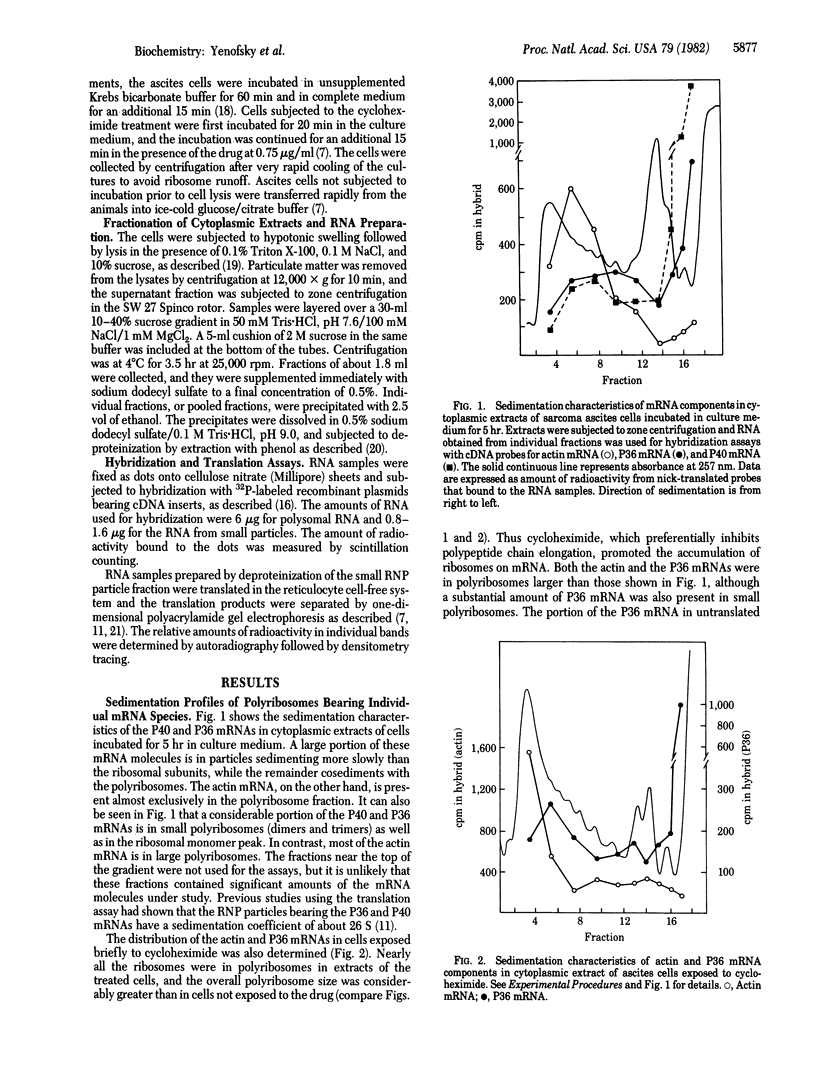
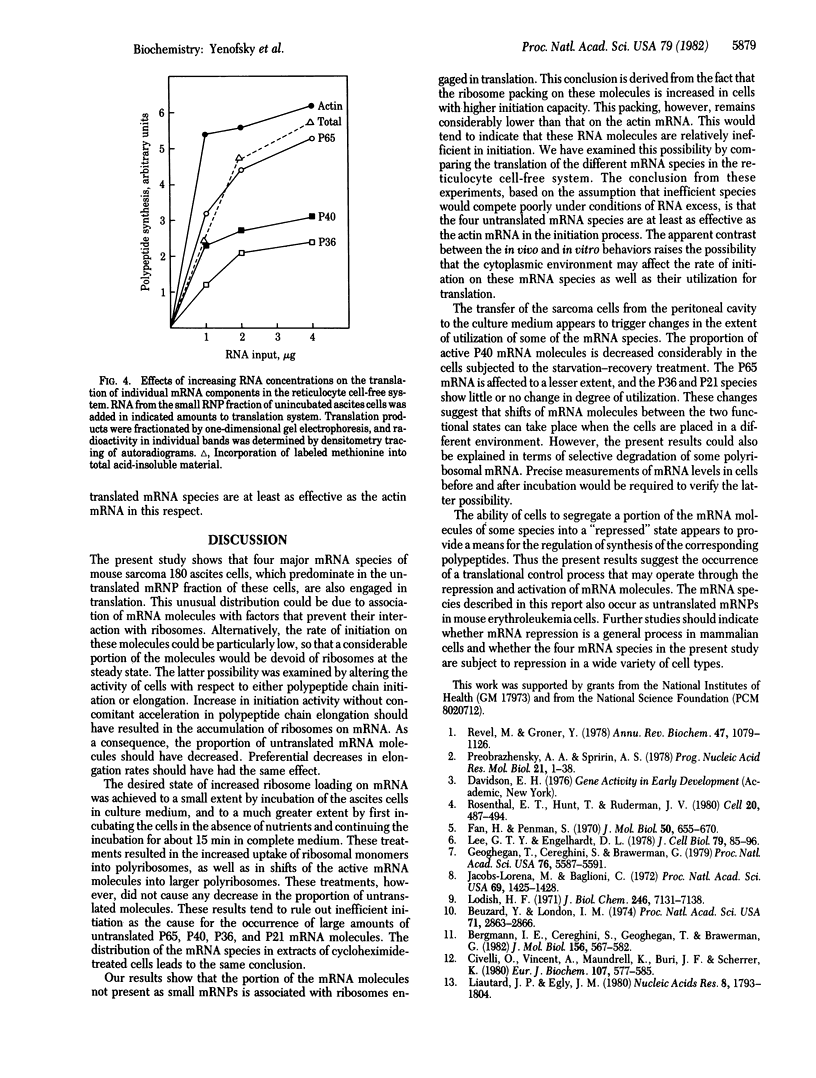
Four major mRNA species of mouse sarcoma ascites cells, coding for polypeptides designated P65, P40, P36, and P21, occur predominantly as untranslated messenger ribonucleoprotein particles. Cloned cDNA probes were used to study their distribution in cytoplasmic extracts of these cells. A considerable portion of the mRNA molecules sedimented as small particles, whereas the rest was present in polyribosomes. In contrast, the actin mRNA was present almost exclusively in polyribosomes. Incubation of the ascites cells in culture medium, particularly after a starvation treatment, caused an enhancement in polypeptide chain initiation relative to elongation in these cells, as evidenced by a shift of ribosomes into the polyribosome fraction and by an increase in polyribosome size. Exposure of the cells to a low concentration of cycloheximide, an inhibitor of the elongation step, had a similar effect. The actin mRNA and the active P65, P40, P36, and P21 mRNA molecules were shifted to larger polyribosomes in the treated cells, but no shift of molecules from small particles to polyribosomes was observed. The incubation in culture also led to considerable increases in the proportion of P65 and P40 mRNA molecules in the untranslated state. The results indicate that the untranslated state cannot be attributed to poor initiation efficiency. It is suggested that a portion of the mRNA molecules is maintained in a repressed state and that mRNA repression may represent an important translation control process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuzard Y., London I. M. The effects of hemin and double-stranded RNA on alpha and beta globin synthesis in reticulocyte and Krebs II ascites cell-free systems and the relationship of these effects to an initiation factor preparation. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2863–2866. doi: 10.1073/pnas.71.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Geoghegan T., Bergmann I., Brawerman G. Studies on the efficiency of translation and on the stability of actin messenger ribonucleic acid in mouse sarcoma ascites cells. Biochemistry. 1979 Jul 10;18(14):3153–3159. doi: 10.1021/bi00581a037. [DOI] [PubMed] [Google Scholar]
- Civelli O., Vincent A., Maundrell K., Buri J. F., Scherrer K. The translational repression of globin mRNA in free cytoplasmic ribonucleoprotein complexes. Eur J Biochem. 1980 Jun;107(2):577–585. doi: 10.1111/j.1432-1033.1980.tb06066.x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. E., Sonenshein G. E., Brawerman G. Characteristics and polyadenylate content of the actin messenger RNA of mouse sarcoma-180 ascites cells. Biochemistry. 1978 Oct 3;17(20):4200–4207. doi: 10.1021/bi00613a014. [DOI] [PubMed] [Google Scholar]
- Geoghegan T., Cereghini S., Brawerman G. Inactive mRNA-protein complexes from mouse sarcoma-180 ascites cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5587–5591. doi: 10.1073/pnas.76.11.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaranis A. S., Heywood S. M. Cytoplasmic utilization of liposome-encapsulated myosin heavy chain messenger ribonucleoprotein particles. During muscle cell differentiation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6898–6902. doi: 10.1073/pnas.78.11.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Baglioni C. Messenger RNA for globin in the postribosomal supernatant of rabbit reticulocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1425–1428. doi: 10.1073/pnas.69.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Growth-related fluctuation in messenger RNA utilization in animal cells. J Cell Biol. 1978 Oct;79(1):85–86. doi: 10.1083/jcb.79.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Initiation of polysome formation in mouse sarcoma 180 ascites cells. Utilization of cytoplasmic messenger ribonucleic acid. Biochemistry. 1971 Mar 2;10(5):895–900. doi: 10.1021/bi00781a026. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Egly J. M. In vitro translation studies of the cytoplasmic nonpolysomal particles containing messenger RNA. Nucleic Acids Res. 1980 Apr 25;8(8):1793–1804. doi: 10.1093/nar/8.8.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky A. A., Spirin A. S. Informosomes and their protein components: the present state of knowledge. Prog Nucleic Acid Res Mol Biol. 1978;21:1–38. doi: 10.1016/s0079-6603(08)60265-2. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sonenshein G. E., Brawerman G. Entry of mRNA into polyribosomes during recovery from starvation in mouse sarcoma 180 cells. Eur J Biochem. 1977 Feb 15;73(1):307–312. doi: 10.1111/j.1432-1033.1977.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Cloned complementary deoxyribonucleic acid probes for untranslated messenger ribonucleic acid components of mouse sarcoma ascites cells. Biochemistry. 1982 Aug 17;21(17):3909–3913. doi: 10.1021/bi00260a001. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]



